Summary
The melanocortin 1 receptor (MC1R) is a transmembrane Gs-coupled surface protein found on melanocytes that binds melanocyte stimulating hormone (MSH) and mediates activation of adenylyl cyclase and generation of the second messenger cAMP. MC1R regulates growth and differentiation of melanocytes and protects against carcinogenesis. Persons with loss-of-function polymorphisms of MC1R tend to be UV-sensitive (fair-skinned and with a poor tanning response) and are at high risk for melanoma. Mechanistic studies of the role of MC1R in melanocytic UV responses, however, have been hindered in part because Mc1r-defective primary murine melanocytes have been difficult to culture in vitro. Until now, effective growth of murine melanocytes has depended on cAMP stimulation with adenylyl cyclase activating or phosphodiesterase inhibiting agents. However, rescuing cAMP in the setting of defective MC1R signaling would be expected to confound experiments directly testing MC1R function on melanocytic UV responses. Here we report a novel method of culturing primary murine melanocytes in the absence of pharmacologic cAMP stimulation by incorporating conditioned supernatants containing stem cell factor (SCF) derived from primary keratinocytes. Importantly, this method seems to permit similar pigment expression by cultured melanocytes as that found in the skin of their parental murine strains. This novel approach will allow mechanistic investigation into MC1R’s role in protection against UV-mediated carcinogenesis and determination of the role of melanin pigment subtypes on UV-mediated melanocyte responses.
Keywords: melanocyte, keratinocyte, melanocortin 1 receptor (MC1R), pigmentation, pheomelanin, eumelanin, primary cell culture, stem cell factor (SCF)
Melanocytes are neural crest-derived cells responsible for pigment production in skin and hair (Westerhof 2006). The type of melanin made by melanocytes is regulated by various pigment-related genes. In general, there are two main melanin species found in skin and hair. The amount and ratio of the two main forms of melanin, black/brown eumelanin and red/blonde pheomelanin, are the primary determinants of hair and skin color (Rees 2004). Epidermal eumelanin protects the skin against ultraviolet radiation by absorbing harmful DNA damaging wavelengths of light and by functioning as a free radical scavenger (Hearing 2000; Kowalczuk et al. 2001; Kadekaro et al. 2003). In contrast, the red/blond pheomelanin pigment is a poor UV blocker and may even be pro-oxidative when exposed to UV (Wenczl et al. 1998; Hill and Hill 2000; Takeuchi et al. 2004). The Mc1r is the surface Gs-coupled receptor on melanocytes that binds melanocyte stimulating hormone (MSH) and mediates pigment enzyme activation via recruitment of adenylyl cyclase and generation of cAMP (Kadekaro et al. 2003; Abdel-Malek et al. 2008). In melanocytes, cAMP signaling through MSH-MC1R interaction results in downstream induction and activation of microphthalmia (Mitf), a myc-like transcription factor that up-regulates a variety of melanogenic synthetic enzymes and other mediators of differentiation and cell survival (Busca and Ballotti 2000; Widlund and Fisher 2003; Levy et al. 2006). Loss-of-function MC1R polymorphisms in humans correlate with the red hair color (RHC) phenotype, fair-skin, UV-sensitivity, a decreased ability to adaptively melanize (i.e., tan), and most importantly, a much-increased risk for developing skin cancers including melanoma (Valverde et al. 1995; Valverde et al. 1996; Han et al. 2006; Hauser et al. 2006; Stratigos et al. 2006; Fernandez et al. 2007; Abdel-Malek et al. 2008). In C57BL/6 mice, Mc1r mutation directly results in a fair complexion, UV-sensitivity red/blonde coat color (Fig. 1A), abrogated UV-dependent tanning, and an increase risk of UV-dependent skin cancer formation (Robbins et al. 1993; D’Orazio et al. 2006). Though several recent studies have directly implicated the MSH-MC1R signaling axis in the tanning response, apoptosis and repair of UV-induced DNA damage (D’Orazio et al. 2006; Cui et al. 2007), the mechanism by which MC1R protects melanocytes from malignant degeneration is not well understood.
Figure 1.
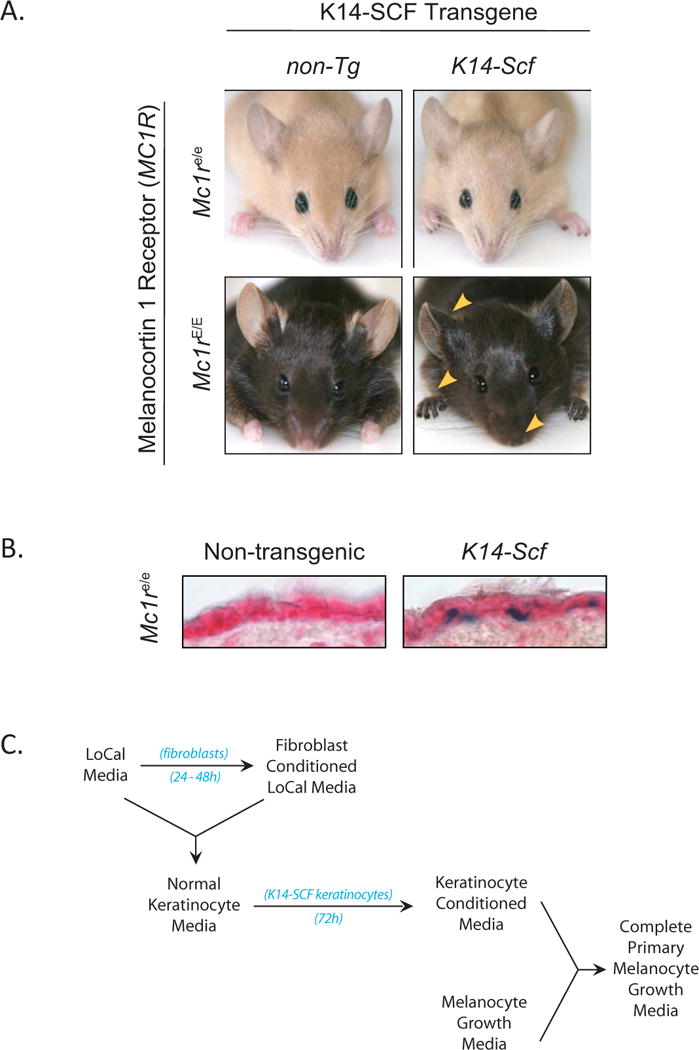
The K14-Scf transgene promotes epidermal localization of melanocytes but not rescue of dark pigmentation caused by defects in the melanocortin 1 receptor (Mc1r). A) Photographs of C57BL/6 murine strains from which primary keratinocytes and melanocytes were derived. Note the robust eumelanotic (darkly pigmented) phenotype in the setting of a functional Mc1r and the change to a pheomelanotic fair-skinned (red/blonde) phenotype in congenic animals defective at the Mc1r locus. Also note pigmentation of the epidermal skin itself in animals transgenic for K14-Scf (most clearly seen in Mc1rE/E animals; gold triangles). B) Epidermal melanocytes, identified by β-galactosidase blue staining, localize to the basal layer of the epidermis of dorsal skin of K14-Scf animals. For these experiments, skin biopsies were taken from young adult Mc1re/e animals harboring the DCT-LacZ transgene that localizes β-gal staining to melanocytes and their precursors (Mackenzie et al. 1997). C) Algorhythm of the protocol described in this report used to generate complete primary melanocyte growth media.
Primary murine melanocytes defective at the Mc1r locus have been historically difficult to culture in vitro (Tamura et al. 1987; Yoon and Hearing 2003; Hirobe et al. 2007). Their poor growth has been attributed to faulty MSH responsiveness and decreased survival. Accordingly, current protocols for culturing primary murine melanocytes rely on pharmacologic manipulation of intracellular cAMP with α-MSH (either in recombinant form or as bovine pituitary extract), dibutyryl cAMP, cholera toxin and/or IBMX (Boissy and Halaban 1985; Halaban et al. 1986; Abdel-Malek et al. 1992; Furuya et al. 1998) as well as stimulation of the protein kinase C pathway with phorbol esters (Kitano 1976; Pittelkow and Shipley 1989). Even with the use of these agents, however, the growth rate of Mc1r-defective melanocytes is delayed when compared to melanocytes with intact MC1R signaling suggesting that MC1R is involved in cell growth in ways other than by simply raising cytoplasmic cAMP levels (Abdel-Malek et al. 1995; Suzuki et al. 1996). In addition, cAMP-manipulating agents rescue eumelanin production in the setting of a defective Mc1r, further complicating attempts to isolate the consequences of pheomelanin expression on melanocyte physiology (Price et al. 1998; D’Orazio et al. 2006). Thus we sought to develop a method for successfully isolating and growing primary Mc1r-defective murine melanocytes in the absence of pharmacologic cAMP manipulation.
Stem cell factor (steel factor), the cognate agonist for the c-kit receptor tyrosine kinase, is another important growth and survival factor for melanocytes (Murphy et al. 1992; Hirobe et al. 2003). Inborn defects in c-kit directly lead to loss of melanocyte development and cause piebaldism (Grichnik et al. 1998). In humans, interfollicular epidermal melanocytes are maintained throughout life by low levels of stem cell factor constitutively produced from keratinocytes in the stratum basale. In the mouse, however, basal keratinocytes do not normally secrete stem cell factor. Thus, after a transient migration through the epidermis in the neonatal period, melanocytes migrate to the hair follicle and are lost from the epidermis itself, resulting in lack of pigmentation to the skin even in strains with highly pigmented fur such as C57BL/6 (Kunisada et al. 2001). The K14-Scf transgenic mouse constitutively produces SCF in basal keratinocytes and therefore maintains a life-long robust population of epidermal melanocytes (Fig. 1B). The original K14-Scf mouse was developed on the C57BL/6 genetic background and thus had an intensely eumelanotic phenotype with jet black fur as well as skin due to robust accumulation of epidermal eumelanin (Kunisada et al. 1998). When Mc1r defective extension C57BL/6 animals were crossed with C57BL/6 K14-Scf animals, resultant progeny maintained epidermal melanocytes but the skin accumulated pheomelanin instead of eumelanin (Fig. 1A) (D’Orazio et al. 2006). Since SCF could maintain Mc1r-defective melanocytes in the epidermis but did not rescue defective eumelanization caused by Mc1r mutation in vivo (Fig. 1B), we reasoned that SCF might similarly support the purification and growth of Mc1r defective melanocytes in vitro. Thus, we developed a method for culturing primary murine melanocytes independent of MC1R agonists or cAMP stimulants based on inclusion of SCF-containing keratinocyte-conditioned media (Fig. 1C).
Our methods for harvesting and purifying primary melanocytes and primary K14-Scf keratinocytes are based on previously published protocols (Halaban and Alfano 1984; Tamura et al. 1987). Briefly, whole skins are removed from euthanized 2–3 day old pups and the epidermis is separated from the dermis enzymatically using dispase (Invitrogen). Epidermal cells are isolated by trituration, and purification of keratinocytes is accomplished through growth in Normal Keratinocyte Media supplemented with 2 ng/ml epidermal growth factor, 0.4 Pg/μl hydrocortisone and 0.75 mM aminoguanidine nitrate (Yuspa et al. 1981; Gilchrest et al. 1982; Marcelo and Tomich 1983). Normal Keratinocyte Media is comprised of LoCal media (Modified Eagle’s Media with Earl’s BSS lacking calcium (Hyclone) with 8% chelexed fetal bovine serum, 60 μM CaCl2, penicillin, streptomycin, amphotericin-B (Invitrogen), and GlutaMAX (Invitrogen) mixed in equal parts with the very same media conditioned for 24–48 hours (35–50 cc per T175 tissue culture flask) by near-confluent cultures of dermal fibroblasts isolated from neonatal C57BL/6 pups. In this manner, primary keratinocytes are gradually selected over time and become homogeneous cultures (i.e., no pigmented cells or cells with fibroblast morphology) within 4–8 weeks. Once keratinocytes are pure and growing very well in culture (typically after several passages), they are then used them to make conditioned media for the purposes of growing primary melanocytes, as illustrated (Fig. 1C). To condition the media, keratinocytes are grown to near-confluence (35–50 cc per T175 tissue culture flask), fresh normal keratinocyte media is added and flasks are incubated for 48–72 h. Conditioned media is then collected, sterile filtered (0.22 μ cellulose acetate) and either used fresh or frozen (−80°C) for future use.
Purification of non-transgenic (i.e., no K14-Scf) primary melanocytes from our murine strains begins in much the same way as keratinocyte purification (i.e. with harvesting whole neonatal skins, enzymatic dissociation of epidermis and enzymatic and mechanical isolation of epidermal cells) as described above (Fritsch et al. 1979; Pittelkow and Shipley 1989). However, purification of melanocytes instead of keratinocytes from the epidermis is accomplished by growth in a mixture of equal parts melanocyte growth media and keratinocyte-conditioned media, as described above, and supplemented with 2×10−10 M endothelin-1 and 50 ng/ml TPA. The melanocyte growth media consists of Ham’s F12 medium (Cellgro) supplemented with 7% horse serum, 10% fetal bovine serum, penicillin, streptomycin, amphotericin-B, and GlutaMAX (Eisinger et al. 1983). In this manner, melanocytes grow preferentially over the next few weeks with media changes provided three times per week until purified populations are obtained within 1–2 months (Fig. 2A). In this manner, without the need for exogenous cAMP stimulation, we have successfully cultured both Mc1r-intact and Mc1r-defective primary melanocytes (Fig. 2A). In order to determine the role of SCF in supporting the growth of primary melanocytes by our method, we compared the growth of cultures of Mc1r-defective (extension) melanocytes grown in media containing conditioned supernatants from K14-Scf transgenic versus non-transgenic keratinocytes (each derived from our murine lines). We found that melanocytes grew better in media conditioned by K14-Scf transgenic keratinocytes than in either media conditioned by non-transgenic keratinocytes or unconditioned media (Fig. 2B). We assayed each media for SCF by ELISA (RayBiotech Systems) and found significantly higher levels of SCF in conditioned media from K14-Scf transgenic keratinocytes as compared to their non-transgenic counterparts (Fig. 2C). In addition, melanocytes grown in K14-Scf keratinocyte conditioned media exhibited roughly fifty percent more DNA synthesis than melanocytes grown in conditioned media from non-transgenic keratinocytes as measured by 3H-thymidine incorporation (Fig. 2D). Though not excluding the contribution of other keratinocyte-derived factors, these data argue that stem cell factor is a potent growth/survival factor for murine primary Mc1r-defective melanocytes.
Figure 2.
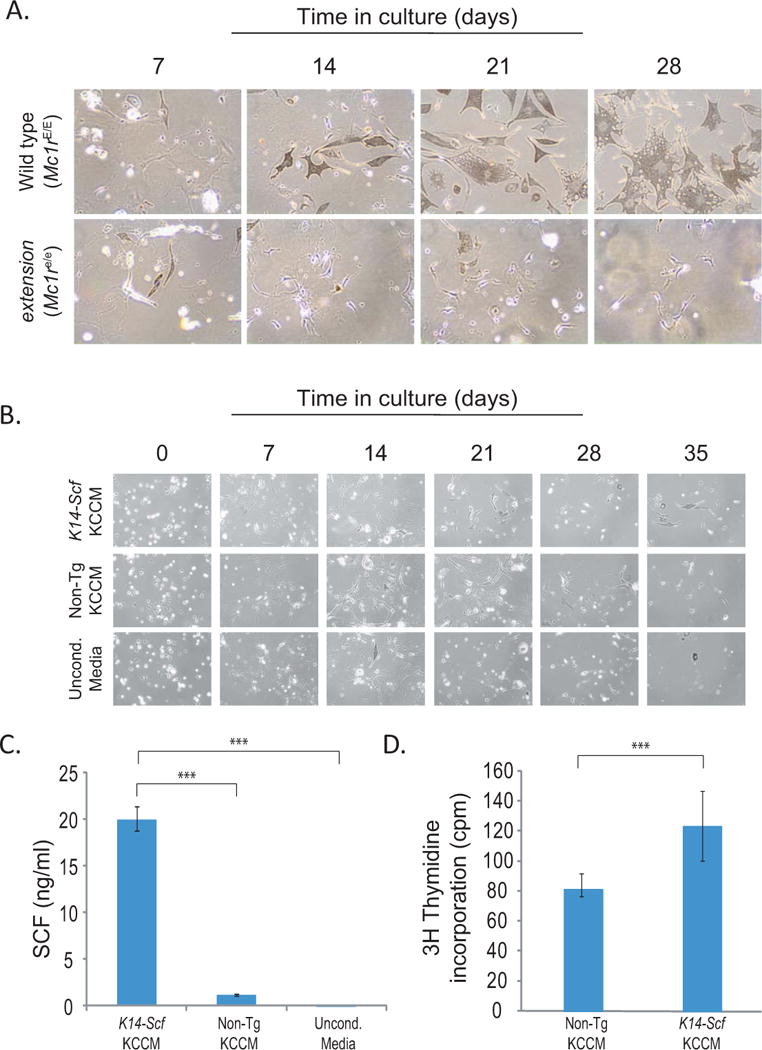
Progressive purification of primary melanocytes is facilitated by stem cell factor. A) Phase contrast photographs of primary melanocytes from Mc1r-intact (wild type) or –defective (extension) strains (magnification 100×). Note that cultures become pure after 3–4 weeks in culture, as determined by morphology (dendricity). Also note that melanocytes from Mc1r-intact (wild type) strains are much larger and more robustly pigmented than their Mc1r-defective counterparts. B) Comparison of cultures of Mc1r-defective (extension) melanocytes grown in complete primary melanocyte growth media made with media conditioned from either K14-Scf transgenic primary keratinocytes (upper panels) or non-transgenic primary keratinocytes (middle panels) or grown in unconditioned media (lower panels). Note that cells grew better with conditioned media from K14-Scf transgenic keratinocytes. C) Quantification of the amount of soluble SCF in Complete Primary Melanocyte Growth Media made with conditioned supernatants from K14-Scf transgenic primary keratinocytes, non-transgenic primary keratinocytes or unconditioned media as indicated. SCF quantification was performed by ELISA (RayBiotech Systems) and statistical significance (p <0.01) is indicated (***). D) 3H-thymidine incorporation of purified Mc1re/e (extension) melanocytes grown in complete primary melanocyte growth media made either with keratinocyte conditioned media from non-transgenic primary keratinocytes or K14-Scf transgenic keratinocytes as labeled. Melanocytes grown in media containing the K14-Scf conditioned media incorporated a significantly greater amount of 3H-thymidine than melanocytes grown in non-transgenic conditioned media (p < 0.018).
On visual inspection, wild type primary melanocytes appeared much larger and more heavily pigmented than their Mc1r-defective counterparts (Fig. 2A), suggesting that even without exogenous MSH added to the media, the presence of a functional MC1R is important to melanocyte differentiation. Notably, we cannot entirely rule out basal MC1R signaling entirely by this method since cells were grown in the presence of serum (which may have contained low levels of MSH or ACTH). However, the support of primary melanocytes without additional MC1R agonist (e.g. α-MSH or bovine pituitary extract) or cAMP analogue (e.g. dibutyryl cAMP) or adenylyl cyclase activator (e.g. forskolin) or phosphodiesterase inhibitor (e.g. IBMX) represents a novel and important advance. We reasoned that one advantage of culturing Mc1r-defective melanocytes in the absence of cAMP stimulants would be theoretical preservation of their native pheomelanotic pigment expression found in vivo. Being able to maintain pheomelanin expression in vitro would be particularly relevant for studies investigating contribution of various melanin pigments to melanocyte physiology and UV responses. Thus we next sought to characterize Mc1r-intact and Mc1r-defective melanocytes grown by this method.
First, we measured their total protein contents. An equivalent number (1 × 106) of viable wild type (Mc1rE/E) and extension (Mc1re/e) melanocytes were lysed and processed according to the bicinchoninic acid (BCA) protein assay (Thermo Fisher Scientific). Mc1r-intact wild type primary melanocytes had almost four times greater protein content than their Mc1r-defective extension counterparts, perhaps explained at least in part by their greater size (Fig. 2A). We next measured their melanin expression by direct quantification of eumelanin and pheomelanin by HPLC as described (Ito et al. 2000). Wild type (Mc1rE/E) melanocytes robustly expressed eumelanin overwhelmingly (eumelanin-to-pheomelanin ratio of 309:1). In contrast, melanocytes harvested from genetically identical C57BL/6 animals differing only at the Mc1r locus expressed roughly thirty-fold less eumelanin while maintaining near-equivalent pheomelanin expression (Fig. 3A). Importantly, these data paralleled the melanin phenotypes observed in the skin of the murine strains from which these primary melanocytes were derived. Specifically, when melanin levels of depillated epidermis of K14-Scf mice were quantified, we found extension animals had similar amounts of pheomelanin, but roughly 60-fold less eumelanin relative to their wild type counterparts (D’Orazio et al. 2006). Thus we conclude that the melanin expression patterns of melanocytes purified in this manner approximates that found in their native state. Next, we determined the ability of purified Mc1r-intact and Mc1r-defective primary melanocytes to respond physiologically to α-MSH, the cognate agonist ligand of MC1R whose binding initiates a well-characterized cAMP-mediated up-regulation/activation of the transcription factor microphthalmia (Mitf) (McGill et al. 2002; Newton et al. 2007). Purified wild type (Mc1r-intact) or extension (Mc1r-defective) melanocytes were incubated (4h, 37°C, 5% CO2) with either 100 nM α-MSH or 10 μM forskolin (a direct activator of adenylyl cyclase as a positive control) before being lysed and subjected to SDS-PAGE and western blotting using the C5 monoclonal anti-melanocytic Mitf antibody (generously provided by Dr. David Fisher, Harvard Medical School) or monoclonal anti-β-tubulin (Thermo Fisher Scientific) as a loading control. Mc1r-intact primary wild type melanocytes responded to both MSH and forskolin with increased levels of Mitf whereas Mc1re/e melanocytes exhibited increased Mitf levels to forskolin but not to α-MSH (Fig. 3B). These data suggest that culturing primary melanocytes by the method described in this report does not interfere with normal Mc1r signaling and does not salvage defective Mc1r signaling caused by the extension loss-of-function truncating mutation.
Figure 3.
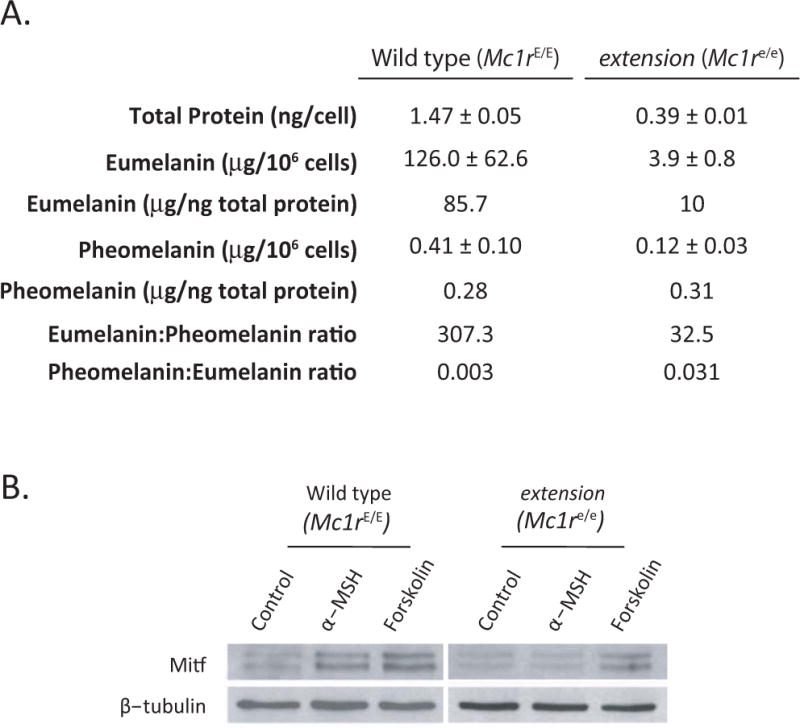
Determination of melanin levels and MSH responsiveness of primary melanocytes. A) Total protein content of purified primary melanocytes grown for 4–5 weeks in culture was determined by the BCA Protein Assay (Thermo Fisher Scientific). Similarly, eumelanin and pheomelanin were quantitatively analyzed by HPLC based on the formation of pyrrole-2,3,5-tricarboxylic acid (PTCA) by permanganate oxidation of eumelanin and 4-amino-3-hydroxyphenylalanine (4-AHP) by hydriodic acid reductive hydrolysis of pheomelanin, respectively as described (Ito and Wakamatsu 2003). B) Western analysis of Mitf expression in response to 100 nM α-MSH or 100 μM forskolin with β-tubulin loading control.
In summary, we describe a novel method for the isolation and purification of primary murine melanocytes based on inclusion of conditioned media from stem cell factor-secreting keratinocytes. Since this protocol lacks exogenous pharmacologic cAMP stimulants, melanocytes purified by this method displayed similar melanocytic pigmentation patterns as those found in skin from which they were derived. This method, useful for melanocytes isolated from either Mc1r-intact and Mc1r-defective strains, will allow experimental clarification of the role of pheomelanin and MC1R signaling in melanocytic physiology and UV responses.
References
- Abdel-Malek Z, et al. Mitogenic, melanogenic, and cAMP responses of cultured neonatal human melanocytes to commonly used mitogens. J Cell Physiol. 1992;150(2):416–25. doi: 10.1002/jcp.1041500226. [DOI] [PubMed] [Google Scholar]
- Abdel-Malek Z, et al. Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proc Natl Acad Sci U S A. 1995;92(5):1789–93. doi: 10.1073/pnas.92.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Malek ZA, et al. The melanocortin 1 receptor and the UV response of human melanocytes–a shift in paradigm. Photochem Photobiol. 2008;84(2):501–8. doi: 10.1111/j.1751-1097.2008.00294.x. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Halaban R. Establishment of proliferative, pure cultures of pigmented chicken melanocytes from neural tubes. J Invest Dermatol. 1985;84(2):158–61. doi: 10.1111/1523-1747.ep12275408. [DOI] [PubMed] [Google Scholar]
- Busca R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000;13(2):60–9. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]
- Cui R, et al. Central Role of p53 in the Suntan Response and Pathologic Hyperpigmentation. Cell. 2007;128(5):853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- D’Orazio JA, et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443(7109):340–4. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- Eisinger M, et al. Stimulation of growth of human melanocytes by tumor promoters. Carcinogenesis. 1983;4(6):779–81. doi: 10.1093/carcin/4.6.779. [DOI] [PubMed] [Google Scholar]
- Fernandez L, et al. MC1R: three novel variants identified in a malignant melanoma association study in the Spanish population. Carcinogenesis. 2007;28(8):1659–64. doi: 10.1093/carcin/bgm084. [DOI] [PubMed] [Google Scholar]
- Fritsch P, et al. Keratinocyte substrate adhesion is magnesium-dependent and calcium-independent. Cell Biol Int Rep. 1979;3(7):593–8. doi: 10.1016/0309-1651(79)90057-2. [DOI] [PubMed] [Google Scholar]
- Furuya R, et al. The proliferation and differentiation of neonatal epidermal melanocytes in F1 hairless mice of HR-1 × HR/De in serum-free culture. J Dermatol. 1998;25(4):211–21. doi: 10.1111/j.1346-8138.1998.tb02384.x. [DOI] [PubMed] [Google Scholar]
- Gilchrest BA, et al. Attachment and growth of human keratinocytes in a serum-free environment. J Cell Physiol. 1982;112(2):197–206. doi: 10.1002/jcp.1041120207. [DOI] [PubMed] [Google Scholar]
- Grichnik JM, et al. The SCF/KIT pathway plays a critical role in the control of normal human melanocyte homeostasis. J Invest Dermatol. 1998;111(2):233–8. doi: 10.1046/j.1523-1747.1998.00272.x. [DOI] [PubMed] [Google Scholar]
- Halaban R, Alfano FD. Selective elimination of fibroblasts from cultures of normal human melanocytes. In Vitro. 1984;20(5):447–50. doi: 10.1007/BF02619590. [DOI] [PubMed] [Google Scholar]
- Halaban R, et al. Human melanocytes cultured from nevi and melanomas. J Invest Dermatol. 1986;87(1):95–101. doi: 10.1111/1523-1747.ep12523594. [DOI] [PubMed] [Google Scholar]
- Han J, et al. Melanocortin 1 receptor variants and skin cancer risk. Int J Cancer. 2006;119(8):1976–84. doi: 10.1002/ijc.22074. [DOI] [PubMed] [Google Scholar]
- Hauser JE, et al. Melanin content and MC1R function independently affect UVR-induced DNA damage in cultured human melanocytes. Pigment Cell Res. 2006;19(4):303–14. doi: 10.1111/j.1600-0749.2006.00315.x. [DOI] [PubMed] [Google Scholar]
- Hearing VJ. The melanosome: the perfect model for cellular responses to the environment. Pigment Cell Res. 2000;13(Suppl 8):23–34. doi: 10.1034/j.1600-0749.13.s8.7.x. [DOI] [PubMed] [Google Scholar]
- Hill HZ, Hill GJ. UVA, pheomelanin and the carcinogenesis of melanoma. Pigment Cell Res. 2000;13(Suppl 8):140–4. doi: 10.1034/j.1600-0749.13.s8.25.x. [DOI] [PubMed] [Google Scholar]
- Hirobe T, et al. Excess tyrosine rescues the reduced activity of proliferation and differentiation of cultured recessive yellow melanocytes derived from neonatal mouse epidermis. Eur J Cell Biol. 2007;86(6):315–30. doi: 10.1016/j.ejcb.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Hirobe T, et al. Steel factor controls the proliferation and differentiation of neonatal mouse epidermal melanocytes in culture. Pigment Cell Res. 2003;16(6):644–55. doi: 10.1046/j.1600-0749.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- Ito S, Wakamatsu K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review. Pigment Cell Res. 2003;16(5):523–31. doi: 10.1034/j.1600-0749.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- Ito S, et al. Chemical analysis of melanins and its application to the study of the regulation of melanogenesis. Pigment Cell Res. 2000;13(Suppl 8):103–9. doi: 10.1034/j.1600-0749.13.s8.19.x. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, et al. Significance of the melanocortin 1 receptor in regulating human melanocyte pigmentation, proliferation, and survival. Ann N Y Acad Sci. 2003;994:359–65. doi: 10.1111/j.1749-6632.2003.tb03200.x. [DOI] [PubMed] [Google Scholar]
- Kitano Y. Effects of dibutyryl adenosine 3′,5′-cyclic monophosphate on human melanocytes in vitro. Acta Derm Venereol. 1976;56(3):223–8. [PubMed] [Google Scholar]
- Kowalczuk C, et al. Effect of increased intracellular melanin concentration on survival of human melanoma cells exposed to different wavelengths of UV radiation. Int J Radiat Biol. 2001;77(8):883–9. doi: 10.1080/09553000110062521. [DOI] [PubMed] [Google Scholar]
- Kunisada T, et al. Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. J Exp Med. 1998;187(10):1565–73. doi: 10.1084/jem.187.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisada T, et al. Review: ligands for receptor tyrosine kinases expressed in the skin as environmental factors for melanocyte development. J Investig Dermatol Symp Proc. 2001;6(1):6–9. doi: 10.1046/j.0022-202x.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- Levy C, et al. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med. 2006;12(9):406–14. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Mackenzie MA, et al. Activation of the receptor tyrosine kinase Kit is required for the proliferation of melanoblasts in the mouse embryo. Dev Biol. 1997;192(1):99–107. doi: 10.1006/dbio.1997.8738. [DOI] [PubMed] [Google Scholar]
- Marcelo CL, Tomich J. Cyclic AMP, glucocorticoid, and retinoid modulation of in vitro keratinocyte growth. J Invest Dermatol. 1983;81(1 Suppl):64s–8s. doi: 10.1111/1523-1747.ep12540609. [DOI] [PubMed] [Google Scholar]
- McGill GG, et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 2002;109(6):707–18. doi: 10.1016/s0092-8674(02)00762-6. [DOI] [PubMed] [Google Scholar]
- Murphy M, et al. Steel factor is required for maintenance, but not differentiation, of melanocyte precursors in the neural crest. Dev Biol. 1992;153(2):396–401. doi: 10.1016/0012-1606(92)90124-y. [DOI] [PubMed] [Google Scholar]
- Newton RA, et al. Human melanocytes expressing MC1R variant alleles show impaired activation of multiple signaling pathways. Peptides. 2007;28(12):2387–96. doi: 10.1016/j.peptides.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Pittelkow MR, Shipley GD. Serum-free culture of normal human melanocytes: growth kinetics and growth factor requirements. J Cell Physiol. 1989;140(3):565–76. doi: 10.1002/jcp.1041400323. [DOI] [PubMed] [Google Scholar]
- Price ER, et al. alpha-Melanocyte-stimulating hormone signaling regulates expression of microphthalmia, a gene deficient in Waardenburg syndrome. J Biol Chem. 1998;273(49):33042–7. doi: 10.1074/jbc.273.49.33042. [DOI] [PubMed] [Google Scholar]
- Rees JL. The genetics of sun sensitivity in humans. Am J Hum Genet. 2004;75(5):739–51. doi: 10.1086/425285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins LS, et al. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993;72(6):827–34. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- Stratigos AJ, et al. Melanocortin receptor-1 gene polymorphisms and the risk of cutaneous melanoma in a low-risk southern European population. J Invest Dermatol. 2006;126(8):1842–9. doi: 10.1038/sj.jid.5700292. [DOI] [PubMed] [Google Scholar]
- Suzuki I, et al. Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinology. 1996;137(5):1627–33. doi: 10.1210/endo.137.5.8612494. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, et al. Melanin acts as a potent UVB photosensitizer to cause an atypical mode of cell death in murine skin. Proc Natl Acad Sci U S A. 2004;101(42):15076–81. doi: 10.1073/pnas.0403994101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A, et al. Normal murine melanocytes in culture. In Vitro Cell Dev Biol. 1987;23(7):519–22. doi: 10.1007/BF02628423. [DOI] [PubMed] [Google Scholar]
- Valverde P, et al. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11(3):328–30. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- Valverde P, et al. The Asp84Glu variant of the melanocortin 1 receptor (MC1R) is associated with melanoma. Hum Mol Genet. 1996;5(10):1663–6. doi: 10.1093/hmg/5.10.1663. [DOI] [PubMed] [Google Scholar]
- Wenczl E, et al. (Pheo)melanin photosensitizes UVA-induced DNA damage in cultured human melanocytes. J Invest Dermatol. 1998;111(4):678–82. doi: 10.1046/j.1523-1747.1998.00357.x. [DOI] [PubMed] [Google Scholar]
- Westerhof W. The discovery of the human melanocyte. Pigment Cell Res. 2006;19(3):183–93. doi: 10.1111/j.1600-0749.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- Widlund HR, Fisher DE. Microphthalamia-associated transcription factor: a critical regulator of pigment cell development and survival. Oncogene. 2003;22(20):3035–41. doi: 10.1038/sj.onc.1206443. [DOI] [PubMed] [Google Scholar]
- Yoon TJ, Hearing VJ. Co-culture of mouse epidermal cells for studies of pigmentation. Pigment Cell Res. 2003;16(2):159–63. doi: 10.1034/j.1600-0749.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- Yuspa SH, et al. Clonal growth of mouse epidermal cells in medium with reduced calcium concentration. J Invest Dermatol. 1981;76(2):144–6. doi: 10.1111/1523-1747.ep12525490. [DOI] [PubMed] [Google Scholar]


