Abstract
Improved understanding of the pattern of white matter changes in early and prodromal Alzheimer's disease (AD) states such as Mild Cognitive Impairment (MCI) is necessary to support earlier preclinical detection of AD, and debate remains whether white matter changes in MCI are secondary to gray matter changes. We applied neuropsychologically-based MCI criteria to a sample of normally aging older adults; 32 participants met criteria for MCI and 81 participants were classified as normal control (NC) subjects. Whole-head high resolution T1 and DTI scans were completed. Tract-Based Spatial Statistics was applied and a priori selected ROIs were extracted. Hippocampal volume and cortical thickness averaged across regions with known vulnerability to AD were derived. Controlling for cortical thickness, the MCI group showed decreased average FA and decreased FA in parietal white matter and in white matter underlying the entorhinal and posterior cingulate cortices relative to the NC group. Statistically controlling for cortical thickness, medial temporal FA was related to memory and parietal FA was related to executive functioning. These results provide further support for the potential role of white matter integrity as an early biomarker for individuals at risk for AD and highlight that changes in white matter may be independent of gray matter changes.
Keywords: Diffusion Tensor Imaging, Aging, Neuropsychological Tests, Magnetic Resonance Imaging, Memory, Executive Function
Introduction
The development of pharmacological treatments that may slow or halt the Alzheimer's disease (AD) process has served as an impetus for the early detection of dementia and the accurate identification of prodromal dementia states, including mild cognitive impairment (MCI). Recent work has focused on the identification of biomarkers for early detection, and structural magnetic resonance imaging (MRI) has proven to be a reliable biomarker for predicting future decline (Jack et al., 2010). Hippocampal volume and other indices of medial temporal lobe (MTL) atrophy are championed as promising MR biomarkers (Craig-Schapiro et al., 2009), but hippocampal atrophy may have multiple etiologies (Dhikav and Anand, 2012) and early gray matter (GM) changes can be detected beyond the MTL in prodromal AD and MCI (Dickerson et al., 2009; Wang et al., 2009). Dickerson and colleagues (2009) identified several cortical regions in which thickness is a potential biomarker for AD, referred to as “AD-signature” cortical thickness. This measure has predicted symptom severity, amyloid binding in asymptomatic older adults, conversion from questionable AD to mild AD (Bakkour et al., 2009), and progression to AD ten years after initially identifying cortical thinning in “AD-signature” areas among cognitively normal individuals (Dickerson et al., 2011).
Despite strong neuropathological evidence of white matter (WM) changes in AD (Brun and Englund, 1986), the role of WM in the development of AD and its utility as a structural MR biomarker has been largely overlooked. With the advent of diffusion tensor imaging (DTI), numerous studies have demonstrated changes in regional WM integrity in AD (Salat et al., 2010; Stricker et al., 2009), and decreases in WM integrity are also reliably found prior to the diagnosis of clinical dementia (Delano-Wood et al., 2010; Fellgiebel et al., 2005; Parente et al., 2008; Wang et al., 2009). WM changes may complement GM changes, explaining unique variance in the disease process. For example, the combination of DTI and cortical thickness measures has higher sensitivity and specificity to differentiate amnestic MCI (aMCI) from healthy control participants than either measure in isolation (Wang et al., 2009), highlighting the need to examine both imaging modalities in MCI. DTI studies of MCI have shown changes in WM integrity, most reliably within medial temporal and parietal regions (Bosch et al., 2012; Huang et al., 2012; Huang and Auchus, 2007; Jacobs et al., 2012; Medina et al., 2006; Rose et al., 2006; Scola et al., 2010; Zhuang et al., 2010) believed to underlie episodic memory (Buckner et al., 2008; Walhovd et al., 2009).
The relative importance of GM versus WM changes in MCI remains unclear. Although GM changes have traditionally been viewed as most important to the AD pathological process and clinical expression, recent research has challenged this assumption and there is increasing evidence of the potential utility of WM integrity as an early biomarker, with longitudinal evidence that it uniquely predicts cognitive decline relative to cerebrospinal fluid (CSF) biomarkers and GM volume (Scola et al., 2010; Selnes et al., 2013; Zhuang et al., 2012). In addition, there is evidence that changes in WM integrity may have unique effects that independently or synergistically contribute to effects of GM atrophy; for example, we have previously shown evidence that WM changes persist when statistically controlling for GM measures in AD (Salat et al., 2010; Stricker et al., 2009). To our knowledge, only three studies have tested whether WM changes persist when controlling for GM in MCI (Bosch et al., 2012; Delano-Wood et al., 2012; Selnes et al., 2013), with varying methods and results. Bosch and colleagues controlled for total GM volume, which may be insensitive to early AD-related changes, and argued that a majority of their WM findings were explained by GM atrophy. Delano-Wood and colleagues demonstrated that decreased WM integrity of the posterior cingulum persisted after controlling for hippocampal volume or whole-brain volume. Using methods similar to the present study, Selnes and colleagues demonstrated multiple regions with decreased WM integrity in MCI, the majority of which remained significant after controlling for local cortical thickness. The current study will determine whether WM changes persist after controlling for widespread cortical thinning in regions empirically validated as particularly sensitive to the AD disease process. Demonstrating unique effects of WM integrity in MCI, independent from AD-signature cortical thinning, would add to the growing support for an independent contribution of WM integrity to AD by demonstrating this effect in an at-risk MCI sample.
Similarly, preliminary evidence suggests that the relationship between WM integrity and cognition is independent of GM changes (Bosch et al., 2012; Delano-Wood et al., 2012; Grambaite et al., 2011), although most studies investigating the relationship between cognition and WM integrity have not controlled for GM. Studies investigating specific cognitive domains have provided initial evidence that decreased WM integrity correlates with memory (Bosch et al., 2012; Goldstein et al., 2009; Walhovd et al., 2009) and executive functioning in MCI (Chen et al., 2009; Grambaite et al.). WM integrity within posterior brain regions, most notably the posterior cingulate, is also related to memory (Delano-Wood et al., 2012; Fellgiebel et al., 2005; Rose et al., 2006; Walhovd et al., 2009). The importance of parietal WM to executive functioning in MCI has also been suggested (Chen et al., 2009; Jacobs et al., 2012; Kim et al., 2011). Because multiple studies show strong relationships between cortical thickness and cognition (Chang et al., 2010; Fjell et al., 2008; Walhovd et al., 2009), it is important to determine whether relationships between cognition and WM integrity are independent of cortical thinning.
The current study applied retrospective, neuropsychologically-based MCI criteria to a sample of normally aging older adults using the “comprehensive” criteria described by Jak et al. (2009b) to examine morphometric group differences in GM and WM integrity. The primary aim of this paper was to examine whether decreased WM integrity in MCI would persist when controlling for AD-signature cortical thickness. A secondary aim was to determine whether WM integrity contributes to variance in cognitive performance over and above that explained by AD-signature cortical thickness. We hypothesized that, relative to a normal control (NC) group, participants with neuropsychologically-defined MCI would exhibit decreased: 1) hippocampal volume, 2) AD-signature cortical thickness, and 3) WM integrity (i.e., lower fractional anisotropy) in a priori selected ROIs, particularly within medial temporal and parietal regions. Importantly, we predicted that differences in WM integrity across groups would be independent of cortical thinning. We also hypothesized that WM integrity of the medial temporal and parietal regions would be significantly related to poorer memory performance, and that WM integrity in parietal regions would be significantly related to poorer executive functioning, over and above AD-signature cortical thinning.
Method
Participants
One hundred-thirteen participants underwent neuropsychological testing and structural MRI. Participants were recruited from two overlapping studies conducted at VA Boston Healthcare System. Twenty-nine participants represent a subset (based on their agreement to undergo structural MRI) of a larger sample recruited from the community through the Harvard Cooperative Program on Aging (HCPA) Claude Pepper Older American Independence Center (OAIC) in response to an advertisement appearing in the HCPA newsletter asking for healthy community-dwelling older African Americans. Eighty-four participants were part of the Understanding Cerebrovascular and Alzheimer's Risk in the Elderly (UCARE) program, recruited through the Boston University Alzheimer's Disease Center (BUADC) based on the criteria of being neurologically healthy and having a first-degree family relative with AD. Participants were excluded for the following: history of head trauma of “mild” severity or greater (Holm et al., 2005; e.g., within our sample loss of consciousness did not exceed 15 minutes), more than one head injury, any neurological disorder including dementia (i.e., Parkinson's disease, AD, vascular dementia), severe psychiatric illness, or brain surgery. All participants were literate with at least a 9th grade education. The VA Boston Healthcare System's institutional review board approved the study according to the Helsinki Declaration, and informed consent was obtained from each participant.
MCI Criteria
We applied the “comprehensive” criteria (Jak et al., 2009b) for retrospective diagnosis of MCI based on neuropsychological test scores. This required that at least two performances within a cognitive domain fell one standard deviation (SD) or more below normative expectations for that domain to contribute to MCI classification. A cutoff for impairment of 1 SD below normative data was adopted to strike a balance between reliability and sensitivity to detect mild impairment (Heaton et al., 2004; Jak et al., 2009b). Participants were excluded if they had MMSE < 24 (Caucasians) or MMSE < 23 (African Americans; Pedraza et al., 2012). Participants were classified as aMCI if memory was impaired (either alone or with additional domains impaired) and as nonamnestic MCI (naMCI) if domain(s) other than memory were impaired. Participants were classified as normal controls (NC) if no cognitive domains were impaired (performance on no more than one measure within a cognitive domain fell more than 1 SD below normative data). Using these criteria, 32 participants met criteria for MCI (14 aMCI, 18 naMCI), and 81 participants were classified as NC.
APOE ε4 genotyping
Genomic DNA was prepared directly from peripheral blood samples using the Pure Gene (Gentra Systems, Minneapolis, MN) DNA extraction kit, with minor modifications. The DNA was stored and an aliquot removed for APOE genotyping. PCR analysis was carried out essentially as described by (Wenham et al., 1991).
Neuropsychological Measures
All participants completed a neuropsychological battery assessing cognitive functioning in four domains: memory, attention/processing speed, language and executive functions. Publically available standard scores were used for group classification (see Table 1). Although the full range of neuropsychological information was necessary for determining MCI diagnosis through use of standard scores, after group classification was completed all analyses used raw scores. Only measures of memory and executive functioning were considered when examining relationships with morphometric measures. Self-report measures included the Geriatric Depression Scale (GDS; Yesavage et al., 1982) and the Lawton and Brody instrumental activities of daily living (IADL) questionnaire (Lawton and Brody, 1969).
Table 1.
Neuropsychological Measures and Normative Sources used for MCI Classification.
| Domain | Test | Normative Source |
|---|---|---|
| Memory | CVLT-II Trials 1-5 (T) | Delis et al. (2000) |
| CVLT-II LDFR (z) | Delis et al. (2000) | |
| *WMS-III VR Immediate (SS) | Wechsler (1997b) | |
| *WMS-III VR Delay (SS) | Wechsler (1997b) | |
|
| ||
| Executive | WCST Perseverative Errors (T) | Heaton et al. (1993) |
| Functioning | Stroop Color-Word (%ile) | Trenerry et al. (1989) |
| Trails B Time (T) | Heaton et al. (2004) | |
|
| ||
| Attention/ | WAIS-III Digit Span Total (SS) | Wechsler (1997a) |
| Processing | Ruff 2&7 Total Speed (T) | Ruff & Allen (1996) |
| Speed | Trails A Time (T) | Heaton et al. (2004) |
|
| ||
| Language | COWAT - FAS (T) | Heaton et al. (2004) |
| Animal Fluency (T) | Heaton et al. (2004) | |
| BNT Total (T) | Heaton et al. (2004) | |
Note. “CVLT-II” = California Verbal Learning Test - 2nd Edition, “WMS-III” = Wechsler Memory Scale - Third Edition, “VR” = Visual Reproduction, “SS” = scaled score, “WAIS-III” = Wechsler Adult Intelligence Scale - Third Edition, “Ruff 2&7” = Ruff 2&7 Selective Attention Test, “COWAT” = Controlled Oral Word Association Test, “BNT” = Boston Naming Test, “WCST” = Wisconsin Card Sorting Test.
Participants from the OAIC sample completed Logical Memory but did not complete Visual Reproduction (both were completed in the UCARE sample). Performance on Visual Reproduction has been shown to be more sensitive than Logical Memory for prediction of stable memory deficits in MCI (Teng et al., 2009) and to predict subsequent AD (Albert et al., 2001; Griffith et al., 2006; Salmon et al., 2002). Therefore we used Visual Reproduction whenever available for group classification, and substituted Logical Memory when it was unavailable (n = 29).
Mean Arterial Blood Pressure
Blood pressure (BP) was measured in a seated position with the arm at rest at heart level by a laboratory technician using a standard sphygmomanometer after five minutes of rest, and five minutes later. Average systolic and diastolic pressures were computed across the two measurements. Systolic and diastolic blood pressure were considered together to create a mean arterial blood pressure (MABP) using the following formula: MABP: 1/3 (Systolic − diastolic) + diastolic. MABP is a metric commonly used in clinical settings to obtain an accurate metric of overall blood pressure. MABP is believed to indicate perfusion pressure, particularly in body organs, and therefore may be more directly related to brain structure. In addition, prior studies have found significant relationships between MABP and cognition and brain structure in older adults (Brown et al., 2008; Guo et al., 2009; Leritz et al., 2010a). We controlled for this variable in all analyses.
Neuroimaging Protocol
Two participants were scanned using a Siemens 1.5 Tesla Sonata system, with the following parameters: MPRAGE; T1=1000 ms, TR=2.73 sec, TE=3.39 ms, flip angle=7°, slice thickness=1.33 mm, 128 slices, FOV=256×256 mm; DTI: repetition time (TR)=9000 ms echo time (TE)=68 ms, 60 slices total, acquisition matrix=128 × 128 (field of view; FOV=256 × 256 mm), slice thickness=2 mm (for 2 mm3 isotropic voxels) with 0 mm gap, with a b value=700 s/mm2, 10 T2 and 60 diffusion weighted images, and one image, the T2-weighted “low b” image with a b-value=0 s/mm2 as an anatomical reference volume. The remaining one hundred-eleven participants were scanned on the upgraded Siemens 1.5 Avanto System, with slightly different parameters; MPRAGE: T1=1000 ms, TR=2.73 sec, TE=3.31 ms, flip angle=7°, slice thickness=1.3 mm, 128 slices, FOV=256×256 mm; DTI: repetition time (TR)=7200 ms echo time (TE)=77 ms, 60 slices total, acquisition matrix=128 × 128 (field of view; FOV=256 × 256 mm), slice thickness=2 mm (for 2 mm3 isotropic voxels) with 0 mm gap, with a b value=700 s/mm2, 10 T2 and 60 diffusion weighted images, and one image, the T2-weighted “low b” image with a b-value=0 s/mm2 as an anatomical reference volume.
Image Processing
T1 Image Processing
Cortical thickness measurements were obtained by first conducting cortical reconstruction using the FreeSurfer image analysis suite, which is freely available for download online (http://surfer.nmr.mgh.harvard.edu/). Methods have been previously described in detail (Dale et al., 1999; Fischl et al., 1999); see our previous work for a summary of this process (Leritz et al., 2010b).
Dickerson et al. (2009) used an exploratory map of cortical thinning in mild AD to define a priori ROIs for use in other analyses, termed “AD-signature” cortical thickness. We applied the AD-signature cortical thickness map to our subjects and derived a mean AD-signature cortical thickness value. We used average AD-signature cortical thickness because Bakkour and colleagues (Bakkour et al., 2009) previously demonstrated that this measure best predicted conversion from questionable AD dementia to mild AD dementia relative to hippocampal volume and the MTL thickness ROI.
DTI Image Processing
Diffusion data were processed using a multistep procedure involving the FreeSurfer image analysis suite and FSL (http://www.fmrib.ox.ac.uk.fsl/) processing streams, specifically Dtifit from FMRIB's Diffusion Toolbox (Behrens et al., 2003) and TBSS (Tract-Based Spatial Statistics, Smith et al., 2006), part of FSL (Smith et al., 2004). See our previous work (Leritz et al., 2010a; Salat et al., 2010) for additional details.
Regions of interest (ROIs) limited to the TBSS skeleton were created using T1-based WM parcellations automatically created during the FreeSurfer processing stream (Salat et al., 2009). These regional measures were based on gyral folding patterns (Desikan et al., 2006), which were subsequently diffused from the cortex into the subjacent WM, resulting in a WM parcellation for each gyral label, unique to each individual's anatomy (see Salat et al., 2012). Registration of the T1 image to the low b volume was performed using the FreeSurfer bbregister tool (Greve and Fischl, 2009), a procedure that utilizes tissue contrast (gray/white matter) as the basis of the registration cost function. Use of the FreeSurfer derived segmentations provided definition of WM regions directly beneath the cortex (Fischl et al., 2002; Salat et al., 2009). ROIs were selected to include those within medial temporal and parietal regions known to be affected early in the AD process, with additional preference given to WM regions underlying those regions that contributed to the AD-signature cortical thickness ROIs (Dickerson et al., 2009). ROIs included WM underlying the following cortical regions: entorhinal, parahippocampal (combined for some analyses into average medial temporal); posterior cingulate, precuneus, supramarginal, superior parietal (combined for some analyses into average parietal); transverse temporal, superior temporal, middle temporal, inferior temporal, temporal pole and superior frontal. Fractional anisotropy (FA), a representation of the degree of intravoxel coherence, is a commonly used metric of WM integrity, particularly in studies of MCI and AD. Lower FA indicates lower white matter integrity. Average FA was derived for each WM ROI. An average FA of these ROIs was also created to provide an average measure of FA comparable to the AD-signature cortical thickness summary measure (no empirically-derived FA-signature ROI has yet been created). White matter ROI values were extracted from voxels limited to the TBSS skeleton to reduce the influence of partial volume contamination. The ROI-segmented mean skeleton was deprojected from TBSS standard space to each participant's native diffusion volume using the inverse of the participant's transform to standard space to extract native values. Statistics were then extracted from each participant's native diffusion maps, with each segmentation comprised of voxels representing the center of WM tracts within each region. Right and left hemisphere ROIs were combined.
Statistical Analyses
Group comparisons were performed with ANCOVA to test for differences (NC versus MCI) in morphometric variables, controlling for age, education and MABP. In addition, similar ANCOVAs were performed for morphometric variables showing significant group differences, also controlling for AD-signature cortical thickness. To examine the relationship between ROIs and cognition, factor scores were created to reduce the number of neuropsychological variables. Raw scores for each variable contributing to the Memory and Executive domains for the MCI classification criteria were submitted into a principal components analysis using varimax rotation with two fixed factors; the minimum eigenvalue for extraction was set at 1. The two factors explained a total of 65% of the variance. The first factor extracted was called “Memory,” and explained 50% of the variance. Because CVLT-II variables showed comparable loadings on both factors, they were assigned to the Memory factor based on our conceptual understanding of these measures. See Table 2. The second factor was called “Executive,” explaining 15% of the variance. The demonstrated factor solution corresponded with the domains used for MCI classification. Factor scores were saved. Hierarchical multiple regressions were completed to determine the relative contribution of WM integrity and AD-signature cortical thickness to the Memory and Executive factors scores. Age, education and MABP were entered into the first step, AD-signature cortical-thickness was entered into the second step, and WM integrity was entered in the third step. Six hierarchical regressions were run in total, three for each cognitive factor score, with average FA, medial temporal FA, and parietal FA entered in the last step of each model. A significance level of p < .05 was used for all analyses and effect sizes (partial eta squared) are reported for all primary analyses. All analyses were conducted in SPSS (Version 19.0).
Table 2. Pattern Matrix from Factor Analysis of Memory and Executive Function Variables.
| Factor | ||
|---|---|---|
|
|
||
| Memory | Executive | |
| WMS-III LM I | .909 | .137 |
| WMS-III LM II | .906 | .162 |
| CVLT-II 1-5 Total* | .595 | .595 |
| CVLT-II LDFR* | .581 | .584 |
| Stroop | .114 | .805 |
| Trails B | -.123 | -.788 |
| WCST PE | -.142 | -.406 |
Note.
Factor loadings for both CVLT-II variables were similar for the Memory and Executive Factors. They were considered to be part of the Memory factor because of its conceptual relationship to other variables on this factor. “LM” = Logical Memory, “LDFR”=long delay free recall, “PE”=perseverative errors.
Results
The MCI and NC groups were comparable on age (t=-0.39, p=0.70), education (t=-0.29, p=0.77), sex (χ2=0.13, p=0.72), MABP (t=1.54, p=0.13), GDS (t=-1.06, p=0.29), Lawton and Brody IADL score (t=1.68, p=0.10) and MMSE (t=1.61, p=0.11). There was a greater proportion of African Americans in the MCI group, although this difference only approached significance (χ2=4.16, p=0.05). Due to targeted recruitment, a large percent (88%) had a self-reported positive family history of AD, and this percentage was comparable across MCI and NC groups (χ2=0.06, p=.81). Twenty-six percent of the sample was apolipoprotein ε4 positive. There were a higher percentage of APOE ε4 positive participants in the MCI group (31.25%) than in the NC group (23.5%), although this difference was not significant (χ2=1.60, p=0.21). These values exceed what has been documented in the general population (15%; Strittmatter and Roses, 1995), which may be attributable to the greater proportion of individuals with a family history of AD. See Table 3. See Table 4 for performance on neuropsychological measures across groups.
Table 3. Sample characteristics by MCI status.
| NC | MCI | |
|---|---|---|
| N | 81 | 32 |
| Sex: male/female | 30/51 | 13/19 |
| APOE ε4 +/− | 19/62 | 10/18 |
| Family history of AD +/− | 71/6 | 29/2 |
| Ethnicity: Caucasian/AA | 57/24 | 16/16 |
| MMSE | 28.05 (1.68) | 27.45 (1.95) |
| Age | 67.72 (9.12) | 68.50 (10.75) |
| Education | 14.98 (2.80) | 14.81 (2.43) |
| GDS | 3.93 (5.16) | 5.55 (5.55) |
| MABP | 95.28 (9.98) | 91.96 (11.28) |
| Lawton-Brody IADL | 26.57 (0.96) | 26.19 (1.36) |
Note. No significant differences between groups for variables represented above.
Table 4. Mean (SD) for raw score performance on neuropsychological measures by MCI status*.
| NC (N=81) | MCI (N=32) | ||
|---|---|---|---|
| Memory | CVLT-II trials 1-5 | 51.40 (10.22) | 42.09 (12.56) |
| CVLT-II LDFR | 10.90 (2.86) | 8.66 (3.93) | |
| WMS-III LM I | 44.69 (9.55) | 37.59 (9.20) | |
| WMS-III LM II | 28.33 (8.08) | 23.25 (6.74) | |
| a WMS-III VR I | 80.51 (12.83) | 67.35 (17.85) | |
| a WMS-III VR II | 59.69 (21.23) | 36.38 (23.67) | |
| Attention/Processing Speed | WAIS-III DS total | 18.40 (3.92) | 14.97 (3.57) |
| b Ruff 2&7 total speed | 255.25 (52.59) | 219.09 (46.18) | |
| Trails A | 36.77 (13.17) | 44.54 (13.03) | |
| Language | Letter fluency (FAS) | 44.48 (11.34) | 33.47 (11.10) |
| Animal fluency | 19.35 (4.60) | 15.50 (4.00) | |
| BNT | 56.28 (3.82) | 53.09 (5.43) | |
| Executive Functions | WCST PE | 15.31 (11.68) | 26.81 (15.80) |
| Stroop interference | 97.69 (22.78) | 71.88 (21.15) | |
| Trails B | 81.11 (37.52) | 123.26 (47.75) |
Note. “DS” = digit span, “LDFR”= long delay free recall, “LM”= logical memory, “PE”= perseverative errors
Independent sample t-tests showed that all comparisons were significant at p < .05
Scores for these measures were not collected from the whole sample, rather they represent performance of 61 NC and 23 MCI participants.
Sum of Automatic Detection Speed and Controlled Search Speed raw scores.
Group comparisons
Group comparisons of AD-signature cortical thickness, hippocampal volume and FA, controlling for age, education and MABP
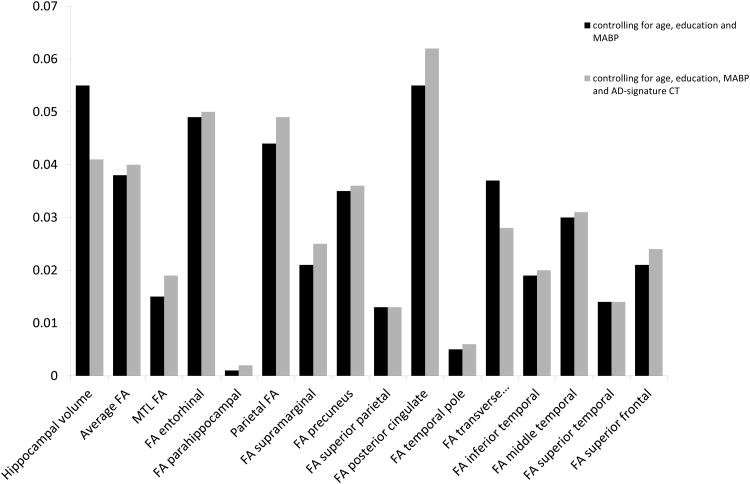
Several measures demonstrated lower values in the MCI group relative to the NC group, including hippocampal volume (F4,108=6.270, p=0.014), average FA (F4,108=4.223, p=0.042) and average parietal FA (F4,108=5.018, p=0.027), and this difference approached significance for AD-signature cortical thickness (F4,108=3.336, p=0.071, η2=0.030). Average medial temporal FA was not significantly different across groups (p=0.198). Within individual ROIs, lower FA was demonstrated in the MCI group relative to the NC group in WM underlying entorhinal (F4,108=5.572, p=0.02), posterior cingulate (F4,108=6.234, p=0.014), and transverse temporal (F4,108=4.184, p=0.043) cortical regions, and this difference approached significance in WM underlying middle temporal (p=0.069) and precuneus (p=0.051) cortical regions. No significant differences in FA were found in WM underlying parahippocampal, supramarginal, temporal pole, inferior temporal, superior temporal, superior frontal or superior parietal cortical regions (all p's>0.05). See Table 5 and Figure 1.
Table 5. Means (SD) for morphometric MRI data by MCI status.
| NC | MCI | |
|---|---|---|
| AD-signature cortical thickness (mm)* | 2. 489 (0.131) | 2. 448 (0.159) |
| Hippocampal volume (mm3)* | 7018.532 (798.870) | 6658.820 (811.937) |
| Average FA* | 0.340 (0.019) | 0.333 (0.020) |
| MTL FA | 0.310 (0.023) | 0.303 (0.027) |
| FA entorhinal* | 0.293 (0.024) | 0.281 (0.024) |
| FA parahippocampal | 0.328 (0.029) | 0.325 (0.036) |
| Parietal FA* | 0.369 (0.024) | 0.360 (0.022) |
| FA supramarginal | 0.333 (0.024) | 0.327 (0.022) |
| FA precuneus | 0.357 (0.028) | 0.349 (0.028) |
| FA superior parietal | 0.380 (0.024) | 0.375 (0.026) |
| FA posterior cingulate* | 0.405 (0.032) | 0.390 (0.030) |
| FA temporal pole | 0.299 (0.021) | 0.295 (0.032) |
| FA transverse temporal* | 0.308 (0.028) | 0.296 (0.029) |
| FA inferior temporal | 0.343 (0.022) | 0.336 (0.023) |
| FA middle temporal | 0.331 (0.019) | 0.324 (0.024) |
| FA superior temporal | 0.345 (0.019) | 0.339 (0.021) |
| FA superior frontal | 0.365 (0.020) | 0.359 (0.024) |
p<0.05
Figure 1.
Partial eta squared is provided for all group comparison analyses, shown with and without controlling for AD-signature cortical thickness.
Group Comparisons controlling for AD-signature cortical thickness
Group comparisons controlling for age, education, MABP and AD-signature cortical thickness
The MCI group had lower hippocampal volume (F5,107=4.585, p=0.035), parietal FA (F5,107=5.457, p=0.021) and average FA (F5,107=4.417, p=0.038) than the NC group when controlling for AD-signature cortical thickness. Within individual ROIs, the MCI group had lower FA than the NC group in WM underlying entorhinal (F5,107=5.586, p=0.020) and posterior cingulate (F5,107=7.050, p=0.009) cortical regions when controlling for AD-signature cortical thickness, and this difference only approached significance in WM underlying transverse temporal (F5,107=3. 072, p=0.082) cortical region. See Figure 1.
Association of morphometric and neuropsychological variables
Hierarchical multiple regression analyses demonstrated that AD-signature cortical thickness explained a significant amount of variance (p=.001) in the Memory factor over and above age, education and MABP. Medial temporal FA explained a significant amount of variance (p=.008) in the Memory factor over and above age, education, MABP and AD-signature cortical thickness. Average FA and parietal FA did not explain additional unique variance (beyond AD-signature cortical thickness) in the Memory Factor (p>.05). AD-signature cortical thickness explained a significant amount of variance (p=.005) in the Executive factor over and above age, education and MABP. Parietal FA explained a significant amount of variance (p=.032) in the Executive factor, over and above age, education, MABP and AD-signature cortical thickness. Average FA and parietal FA did not explain additional unique variance (beyond AD-signature cortical thickness) in the Memory Factor (p>.05). See Table 6.
Table 6. Hierarchical regression models.
| Step and predictors | Statistics for step | Statistics for predictors | ||
|---|---|---|---|---|
|
|
||||
| R2 | ΔR2 | β | T | |
| DV: Memory Factor | ||||
| Step 1 | 0.165** | |||
| Age | -0.142 | -1.616 | ||
| Education | 0.254 | 2.998** | ||
| MABP | 0.068 | 0.772 | ||
| Step 2 | 0.243** | 0.078** | ||
| AD-signature CT | 0.322 | 3.595** | ||
| Step 3 | 0.291** | 0.048** | ||
| MTL FA | 0.224 | 2.682** | ||
| DV: Executive Factor | ||||
| Step 1 | 0.197** | |||
| Age | 0.184 | 1.942 | ||
| Education | -0.244 | -2.817** | ||
| MABP | -0.147 | -1.629 | ||
| Step 2 | 0.253** | 0.056** | ||
| AD-signature CT | -0.266 | -2.960** | ||
| Step 3 | 0.284** | 0.032* | ||
| Parietal FA | -0.209 | -2.174* | ||
Note. Statistics for predictors represent values for full model. “DV” = dependent variable, “MABP” = mean arterial blood pressure, AD = Alzheimer's disease, CT = cortical thickness, MTL FA = average medial temporal lobe fractional anisotropy
p < .05
p < .01
Discussion
In this sample of healthy older adults with a high rate of family history of Alzheimer's disease, an MCI group characterized using comprehensive neuropsychological criteria (Jak et al., 2009b) exhibited decreased hippocampal volume and WM integrity relative to normal controls. Nearly all differences in WM integrity were statistically independent of AD-signature cortical thinning. Further, WM integrity was related to cognition, independent of cortical thinning.
Consistent with our hypotheses, the current results demonstrated decreased hippocampal volume in the MCI group. Despite the well-known finding of hippocampal volume loss in MCI (Shi et al., 2009), this is only the second study to demonstrate this when defining MCI through a neuropsychological approach (Jak et al., 2009a). We also demonstrated a trend toward widespread cortical thinning (i.e., decreased AD-signature cortical thickness) in MCI.
As hypothesized, the present study demonstrated decreased WM microstructural integrity as manifested in lower average FA (of our selected ROIs) and lower parietal FA among the MCI group. Parietal WM degeneration has been previously documented in MCI (Bosch et al., 2012; Chua et al., 2008; Delano-Wood et al., 2012; Medina et al., 2006; Rose et al., 2006; Scola et al., 2010; Shim et al., 2008; Shu et al., 2011; Zhuang et al., 2010). Within ROIs comprising our measure of posterior WM, FA in the posterior cingulate was significantly decreased. Decreased WM integrity in the posterior cingulate has emerged as a highly replicable finding across DTI studies of MCI and AD (Catheline et al., 2010; Chua et al., 2009; Chua et al., 2008; Delano-Wood et al., 2012). These results suggest that alterations within posterior WM regions, particularly the posterior cingulate, may serve as a potential imaging biomarker of early AD-related brain changes. The results of the present study extend the existing literature by demonstrating that parietal WM changes remained significant even when controlling for AD-signature cortical thickness, suggesting that posterior WM changes are independent of grey matter atrophy within this susceptible population.
The lack of decreased WM integrity in our average medial temporal WM ROI is unexpected and inconsistent with our hypotheses. Within individual ROIs, the MCI group showed decreased FA in WM underlying the entorhinal, but not parahippocampal, cortex. Other studies have identified decreased WM integrity in parahippocampal WM in MCI (Liu et al., 2009; O'Dwyer et al., 2011; Rose et al., 2006; Zhang et al., 2007). One possible explanation for this discrepancy is that our sample may represent early MCI, as these are individuals who were neurologically normal per history, identified only by neuropsychological status. Another possible explanation for the lack of parahippocampal WM differences in our study may be due to collapsing across aMCI and naMCI groups. Zhuang and colleagues (2010) demonstrated a differing pattern of results in a large sample of aMCI, naMCI and healthy control subjects; although widespread differences relative to control subjects were found in both MCI groups, significant differences in temporal WM were only found in the aMCI group. Of note, however, is that they found no significant differences when directly comparing aMCI and naMCI groups. Our results are consistent with Grambaite and colleagues (2011); their investigation also demonstrated decreased integrity in the WM underlying the entorhinal, but not parahippocampal, cortex in aMCI relative to a “less-advanced” MCI sample. AD neuropathology is present in the entorhinal cortex in the earliest stages of the disease process, even before the hippocampus and parahippocampal gyrus (Braak and Braak, 1996; Gomez-Isla et al., 1996), and a similar pattern of findings has been documented in morphometry studies focused on GM alterations (Desikan et al., 2009; Devanand et al., 2012; Fennema-Notestine et al., 2009). Similarly, the more anterior (entorhinal) findings would be expected given that in AD the degenerative changes in the parahippocampal WM seem to follow an anterior to posterior gradient (Salat et al., 2010). WM underlying the entorhinal cortex has not been frequently included as an ROI in past DTI studies. Our methodological approach that applies T1-based cortical parcellations to the underlying WM facilitated inclusion of this ROI. Potential advantages of this method include correspondence between WM and cortical thickness ROIs and a clearly defined method by which to replicate analyses. Given our finding of decreased WM integrity in the WM underlying the entorhinal cortex in this mixed aMCI and naMCI sample, which persisted even when controlling for AD-signature cortical thickness, this region deserves further investigation in future MCI studies.
The current results provide support for the relative independence of decreased WM integrity in MCI from GM atrophy as measured by widespread cortical thinning in regions known to be sensitive to AD. These results are consistent with those of Selnes et al. (2012) who also demonstrated that most of the MCI group differences in WM integrity persisted despite controlling for localized cortical thinning. Collectively, our findings also suggest that changes in WM integrity explain unique variance and may precede, or have additive or synergistic effects on, GM atrophy in individuals at risk for AD. This interpretation is consistent with Brun and Englund's (1986) neuropathological findings that indicate WM changes in AD are not likely to be solely due to Wallerian degeneration. Further support for the early importance of white matter changes was recently demonstrated by Selnes and colleagues (2013) in a longitudinal study. They showed that measures of WM integrity better predicted decline in cognition and MTL atrophy over 2-3 years than did CSF biomarkers in a sample of patients with subjective cognitive complaints and MCI and proposed that changes in WM may precede GM atrophy in the Alzheimer's pathological cascade model (Jack et al., 2010). The current study adds to a growing body of literature that suggests that WM integrity may serve as an independent MR biomarker in studies examining individuals at risk for AD. Further, including measures of WM integrity in future biomarker studies may have significant practical importance. For example, some investigators have proposed that vascular processes may precede or accelerate AD neuropathology (de la Torre, 2002; Leszek et al., 2012). Vascular risk factors are easily modifiable, thus, could possibly prevent development of dementia later in life through early preventative efforts or could potentially slow cognitive decline (Richard and Pasquier, 2012). Further determining white matter's contribution to early detection by regularly including DTI in conversion studies and in clinical trials may significantly impact future treatment and prevention efforts.
Analyses investigating associations with cognition highlighted that the clinical significance of WM changes may also be independent from gray matter changes. Overall, these results show that across a sample of healthy older adults and individuals with MCI, WM integrity explains variance in cognition over and above cortical thickness in regions shown to be sensitive to early AD-related changes. Analyses within specific cognitive domains partially supported our hypotheses. As predicted, medial temporal FA was significantly related to memory performance. Further, consistent with results of Grambaite and colleagues (2011), we demonstrated that this relationship was independent of cortical thinning. Unlike their study wherein they locally controlled for cortical thinning, we demonstrate that this relationship persists when accounting for widespread cortical thickness in a pattern known to be sensitive to early AD neuropathological changes (Dickerson et al., 2011). Also consistent with Grambaite and colleagues, we did not find a relationship between parietal WM integrity and memory performance. Other studies have demonstrated a relationship between parietal WM regions such as the posterior cingulate (Fellgiebel et al., 2005; Rose et al., 2006), but these studies did not control for gray matter changes. Results also revealed a relationship between parietal WM integrity and executive functioning, consistent with findings from other investigators (Chen et al., 2009; Jacobs et al., 2012; Kim et al., 2011). The current results extend prior findings by demonstrating that this relationship is independent of cortical thinning. Additional work applying a voxelwise approach is needed to more extensively examine which WM regions correlate with memory and executive functioning.
This study has a number of limitations. Investigation of the relationship between WM and cognition collapsed across groups to avoid arbitrarily restricting range and to maximize power. Further, because the same measures used for the Memory and Executive domains in MCI diagnosis were used for the Memory and Executive Factors for these analyses, inclusion of group status in the regression model or limiting analyses to subgroups may be viewed as partially dependent. Therefore, results from our regression analyses must be interpreted with caution, as results suggest that there is a relationship between these cognitive domains and FA, supporting potential clinical implications of variation in white matter integrity over and above cortical thinning, but do not suggest specificity of these relationships to MCI. Because a subjective or objective change in cognition over time was not required for MCI diagnosis in the current study, and follow-up data is not available, it remains possible that the current results could reflect normal variability in morphology that is also related to cognitive strengths and weaknesses, rather than an early Alzheimer's process. The elevated prevalence of family history and APOE ε4 rates offer some argument against this, but future work is needed with prospectively diagnosed MCI to selectively include MCI presumed to be due to AD (Albert et al., 2011). Longitudinal studies are needed to examine the relative ability of WM integrity versus measures of GM to predict conversion from MCI to Alzheimer's dementia and to replicate the current results in a sample destined to develop AD. The current sample, while at risk for AD, may reflect a variety of etiologies for MCI. While the elevated rate of family history of AD and the APOE ε4 allele relative to the general population increases the likelihood of preclinical AD within this sample, it may limit the generalizability of our results. In addition, the difference in rate of MCI in African American and Caucasian participants approached significance. Although we used normative data with corrections for ethnicity whenever possible (Heaton et al., 2004), not all measures included in this study had this option. Our group is currently working on a separate manuscript to examine potential differences across our African American and Caucasian participants. Due to small sample sizes and an effort to limit the number of analyses, we chose not to analyze aMCI and naMCI subgroups separately given evidence that naMCI frequently represents early AD (Fischer et al., 2007; Rountree et al., 2007; Schneider et al., 2009). However, this may have affected our ability to detect medial temporal lobe effects (Zhuang et al., 2010). Ongoing studies will expand our MCI sample, allowing for us to more specifically investigate morphometric differences in amnestic and non-amnestic MCI.
In summary, we found evidence that neuropsychologically-defined MCI is associated with reduced gray and white matter integrity, and group differences in WM integrity persisted even when controlling for cortical thickness in regions sensitive to the AD process. This is the first study to demonstrate that decreased integrity in entorhinal and parietal WM in MCI may be independent of widespread gray matter changes. Moreover, results suggest that changes in WM integrity are clinically significant and cannot be fully attributed to gray matter atrophy. Future work will explore whether similar results can be demonstrated in prospectively diagnosed MCI and further examination of potential differences by MCI subtype is needed. Improved understanding of the pattern of WM changes that develop in early AD and prodromal AD states such as MCI has important implications for early detection and monitoring treatment effects in clinical trials, and the current study suggests a prominent and preferential role of parietal and temporal lobe WM integrity and relationships to cognition during this critical stage.
Acknowledgments
This work was supported by the National Institute of Neurologic Disorders and Stroke (grant numbers K23NS062148,); the National Institute of Nursing Research (grant number R01NR010827), the National Institute on Aging (grant numbers P60AG08812, P01AG004390); and by Medical Research Service VA Merit Review Awards to William Milberg and Regina McGlinchey. This research was conducted while Nikki Stricker was a Gilbert Foundation/AFAR Research Grant recipient. The authors would like to thank Marge Ahlquist for her assistance with BP collection and phlebotomy on all participants.
Footnotes
The authors report no conflicts of interest.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers & Dementia. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. Journal of the International Neuropsychological Society. 2001;7(5):631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology. 2009;72(12):1048–1055. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance in Medicine. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Bosch B, Arenaza-Urquijo EM, Rami L, Sala-Llonch R, Junque C, Sole-Padulles C, Bartres-Faz D. Multiple DTI index analysis in normal aging, amnestic MCI and AD. Relationship with neuropsychological performance. Neurobiology of Aging. 2012;33(1):61–74. doi: 10.1016/j.neurobiolaging.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer's disease. Acta Neurologica Scandinavica Supplement. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Brown AD, McMorris CA, Longman RS, Leigh R, Hill MD, Friedenreich CM, Poulin MJ. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiology of Aging. 2008;31(12):2047–2057. doi: 10.1016/j.neurobiolaging.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Annals of Neurology. 1986;19(3):253–262. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Catheline G, Periot O, Amirault M, Braun M, Dartigues JF, Auriacombe S, Allard M. Distinctive alterations of the cingulum bundle during aging and Alzheimer's disease. Neurobiology of Aging. 2010;31(9):1582–1592. doi: 10.1016/j.neurobiolaging.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Chang YL, Bondi MW, Fennema-Notestine C, McEvoy LK, Hagler DJ, Jr, Jacobson MW, Dale AM. Brain substrates of learning and retention in mild cognitive impairment diagnosis and progression to Alzheimer's disease. Neuropsychologia. 2010;48(5):1237–1247. doi: 10.1016/j.neuropsychologia.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TF, Chen YF, Cheng TW, Hua MS, Liu HM, Chiu MJ. Executive dysfunction and periventricular diffusion tensor changes in amnesic mild cognitive impairment and early Alzheimer's disease. Human Brain Mapping. 2009;30(11):3826–3836. doi: 10.1002/hbm.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua TC, Wen W, Chen X, Kochan N, Slavin MJ, Trollor JN, Sachdev PS. Diffusion tensor imaging of the posterior cingulate is a useful biomarker of mild cognitive impairment. American Journal of Geriatric Psychiatry. 2009;17(7):602–613. doi: 10.1097/JGP.0b013e3181a76e0b. [DOI] [PubMed] [Google Scholar]
- Chua TC, Wen W, Slavin MJ, Sachdev PS. Diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease: a review. Current Opinion in Neurology. 2008;21(1):83–92. doi: 10.1097/WCO.0b013e3282f4594b. [DOI] [PubMed] [Google Scholar]
- Craig-Schapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer's disease. Neurobiology of Disease. 2009;35(2):128–140. doi: 10.1016/j.nbd.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Vascular basis of Alzheimer's pathogenesis. Annals of the New York Academy of Sciences. 2002;977:196–215. doi: 10.1111/j.1749-6632.2002.tb04817.x. [DOI] [PubMed] [Google Scholar]
- Delano-Wood L, Bondi MW, Jak AJ, Horne NR, Schweinsburg BC, Frank LR, Salmon DP. Stroke risk modifies regional white matter differences in mild cognitive impairment. Neurobiology of Aging. 2010;31(10):1721–1731. doi: 10.1016/j.neurobiolaging.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano-Wood L, Stricker NH, Sorg SF, Nation DA, Jak AJ, Woods SP, Bondi MW. Posterior cingulum white matter disruption and its associations with verbal memory and stroke risk in mild cognitive impairment. Journal of Alzheimers Disease. 2012;29(3):589–603. doi: 10.3233/JAD-2012-102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. Second. San Antonio: The Psychological Corporation; 2000. [Google Scholar]
- Desikan RS, Cabral HJ, Hess CP, Dillon WP, Glastonbury CM, Weiner MW, Fischl B. Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer's disease. Brain. 2009;132(Pt 8):2048–2057. doi: 10.1093/brain/awp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Bansal R, Liu J, Hao X, Pradhaban G, Peterson BS. MRI hippocampal and entorhinal cortex mapping in predicting conversion to Alzheimer's disease. Neuroimage. 2012;60(3):1622–1629. doi: 10.1016/j.neuroimage.2012.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhikav V, Anand KS. Are vascular factors linked to the development of hippocampal atrophy in Alzheimer's disease? Journal of Alzheimers Disease. 2012;32(3):711–718. doi: 10.3233/JAD-2012-120928. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Buckner RL. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral Cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Stoub TR, Shah RC, Sperling RA, Killiany RJ, Albert MS, Detoledo-Morrell L. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76(16):1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellgiebel A, Muller MJ, Wille P, Dellani PR, Scheurich A, Schmidt LG, Stoeter P. Color-coded diffusion-tensor-imaging of posterior cingulate fiber tracts in mild cognitive impairment. Neurobiology of Aging. 2005;26(8):1193–1198. doi: 10.1016/j.neurobiolaging.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, Hagler DJ, Jr, McEvoy LK, Fleisher AS, Wu EH, Karow DS, Dale AM. Structural MRI biomarkers for preclinical and mild Alzheimer's disease. Human Brain Mapping. 2009;30(10):3238–3253. doi: 10.1002/hbm.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer P, Jungwirth S, Zehetmayer S, Weissgram S, Hoenigschnabl S, Gelpi E, Tragl KH. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007;68(4):288–291. doi: 10.1212/01.wnl.0000252358.03285.9d. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Amlien I, Bjornerud A, Reinvang I, Gjerstad L, Fladby T. Morphometric changes in the episodic memory network and tau pathologic features correlate with memory performance in patients with mild cognitive impairment. American Journal of Neuroradiology. 2008;29(6):1183–1189. doi: 10.3174/ajnr.A1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein FC, Mao H, Wang L, Ni C, Lah JJ, Levey AI. White Matter Integrity and Episodic Memory Performance in Mild Cognitive Impairment: A Diffusion Tensor Imaging Study. Brain Imaging and Behavior. 2009;3(2):132–141. doi: 10.1007/s11682-008-9055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. Journal of Neuroscience. 1996;16(14):4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grambaite R, Reinvang I, Selnes P, Fjell AM, Walhovd KB, Stenset V, Fladby T. Pre-dementia memory impairment is associated with white matter tract affection. Journal of the International Neuropsychological Society. 2011;17(1):143–153. doi: 10.1017/S1355617710001360. [DOI] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith HR, Netson KL, Harrell LE, Zamrini EY, Brockington JC, Marson DC. Amnestic mild cognitive impairment: diagnostic outcomes and clinical prediction over a two-year time period. Journal of the International Neuropsychological Society. 2006;12(2):166–175. doi: 10.1017/S1355617706060267. [DOI] [PubMed] [Google Scholar]
- Guo X, Pantoni L, Simoni M, Bengtsson C, Bjorkelund C, Lissner L, Skoog I. Blood pressure components and changes in relation to white matter lesions: a 32-year prospective population study. Hypertension. 2009;54(1):57–62. doi: 10.1161/HYPERTENSIONAHA.109.129700. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtis G. Wisconsin Card Sorting Test (WCST) manual, revised and expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: demographically adjusted neuropsychological norms for African American and Caucasian Adults. Lutz, FL: Psychological Assessment Resources, Inc; 2004. [Google Scholar]
- Holm L, Cassidy JD, Carroll LJ, Borg J. Summary of the WHO Collaborating Centre for Neurotrauma Task Force on Mild Traumatic Brain Injury. Journal of Rehabilitation Medicine. 2005;37(3):137–141. doi: 10.1080/16501970510027321. [DOI] [PubMed] [Google Scholar]
- Huang H, Fan X, Weiner M, Martin-Cook K, Xiao G, Davis J, Diaz-Arrastia R. Distinctive disruption patterns of white matter tracts in Alzheimer's disease with full diffusion tensor characterization. Neurobiology of Aging. 2012;33(9):2029–2045. doi: 10.1016/j.neurobiolaging.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Auchus AP. Diffusion tensor imaging of normal appearing white matter and its correlation with cognitive functioning in mild cognitive impairment and Alzheimer's disease. Annals of the New York Academy of Sciences. 2007;1097:259–264. doi: 10.1196/annals.1379.021. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurology. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HI, Van Boxtel MP, Jolles J, Verhey FR, Uylings HB. Parietal cortex matters in Alzheimer's disease: an overview of structural, functional and metabolic findings. Neuroscience and Biobehavioral Reviews. 2012;36(1):297–309. doi: 10.1016/j.neubiorev.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Jak AJ, Bangen KJ, Wierenga CE, Delano-Wood L, Corey-Bloom J, Bondi MW. Contributions of neuropsychology and neuroimaging to understanding clinical subtypes of mild cognitive impairment. International Review of Neurobiology. 2009a;84:81–103. doi: 10.1016/S0074-7742(09)00405-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC. Quantification of five neuropsychological approaches to defining mild cognitive impairment. American Journal of Geriatric Psychiatry. 2009b;17(5):368–375. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Park JS, Ahn HJ, Seo SW, Lee JM, Kim ST, Na DL. Voxel-based analysis of diffusion tensor imaging in patients with subcortical vascular cognitive impairment: correlates with cognitive and motor deficits. Journal of Neuroimaging. 2011;21(4):317–324. doi: 10.1111/j.1552-6569.2010.00527.x. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- Leritz EC, Salat DH, Milberg WP, Williams VJ, Chapman CE, Grande LJ, McGlinchey RE. Variation in blood pressure is associated with white matter microstructure but not cognition in African Americans. Neuropsychology. 2010a;24(2):199–208. doi: 10.1037/a0018108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leritz EC, Salat DH, Williams VJ, Schnyer DM, Rudolph JL, Lipsitz L, Milberg WP. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage. 2010b;54(4):2659–2671. doi: 10.1016/j.neuroimage.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszek J, Sochocka M, Gasiorowski K. Vascular factors and epigenetic modifications in the pathogenesis of Alzheimer's disease. Journal of the Neurological Sciences. 2012;323(1-2):25–32. doi: 10.1016/j.jns.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Liu Y, Spulber G, Lehtimaki KK, Kononen M, Hallikainen I, Grohn H, Soininen H. Diffusion tensor imaging and Tract-Based Spatial Statistics in Alzheimer's disease and mild cognitive impairment. Neurobiology of Aging. 2011;32(9):1558–1571. doi: 10.1016/j.neurobiolaging.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Medina D, DeToledo-Morrell L, Urresta F, Gabrieli JD, Moseley M, Fleischman D, Stebbins GT. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiology of Aging. 2006;27(5):663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- O'Dwyer L, Lamberton F, Bokde AL, Ewers M, Faluyi YO, Tanner C, Hampel H. Multiple indices of diffusion identifies white matter damage in mild cognitive impairment and Alzheimer's disease. PLoS One. 2011;6(6):e21745. doi: 10.1371/journal.pone.0021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente DB, Gasparetto EL, da Cruz LC, Jr, Domingues RC, Baptista AC, Carvalho AC, Domingues RC. Potential role of diffusion tensor MRI in the differential diagnosis of mild cognitive impairment and Alzheimer's disease. American Journal of Roentgenology. 2008;190(5):1369–1374. doi: 10.2214/AJR.07.2617. [DOI] [PubMed] [Google Scholar]
- Pedraza O, Clark JH, O'Bryant SE, Smith GE, Ivnik RJ, Graff-Radford NR, Lucas JA. Diagnostic validity of age and education corrections for the Mini-Mental State Examination in older African Americans. Journal of the American Geriatric Society. 2012;60(2):328–331. doi: 10.1111/j.1532-5415.2011.03766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard F, Pasquier F. Can the treatment of vascular risk factors slow cognitive decline in Alzheimer's disease patients? Journal of Alzheimer's Disease. 2012;32(3):765–772. doi: 10.3233/JAD-2012-121012. [DOI] [PubMed] [Google Scholar]
- Rose SE, McMahon KL, Janke AL, O'Dowd B, de Zubicaray G, Strudwick MW, Chalk JB. MRI diffusion indices and neuropsychological performance in amnestic mild cogntive impairment. Journal of Neurology, Neurosurgery and Psychiatry. 2006;77(10):1122–1128. doi: 10.1136/jnnp.2005.074336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rountree SD, Waring SC, Chan WC, Lupo PJ, Darby EJ, Doody RS. Importance of subtle amnestic and nonamnestic deficits in mild cognitive impairment: prognosis and conversion to dementia. Dementia and Geriatric Cognitive Disorders. 2007;24(6):476–482. doi: 10.1159/000110800. [DOI] [PubMed] [Google Scholar]
- Ruff RM, Allen CC. Ruff 2 & 7 Selective Attention Test: Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc; 1996. [Google Scholar]
- Salat DH, Lee SY, van der Kouwe AJ, Greve DN, Fischl B, Rosas HD. Age-associated alterations in cortical gray and white matter signal intensity and gray to white matter contrast. Neuroimage. 2009;48(1):21–28. doi: 10.1016/j.neuroimage.2009.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, van der Kouwe AJ, Greve DN, Pappu V, Lee SY, Rosas HD. White matter pathology isolates the hippocampal formation in Alzheimer's disease. Neurobiology of Aging. 2010;31(2):244–256. doi: 10.1016/j.neurobiolaging.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Williams VJ, Leritz EC, Schnyer DM, Rudolph JL, Lipsitz LA, Milberg WP. Inter-individual variation in blood pressure is associated with regional white matter integrity in generally healthy older adults. Neuroimage. 2012;59(1):181–192. doi: 10.1016/j.neuroimage.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon DP, Thomas RG, Pay MM, Booth A, Hofstetter CR, Thal LJ, Katzman R. Alzheimer's disease can be accurately diagnosed in very mildly impaired individuals. Neurology. 2002;59(7):1022–1028. doi: 10.1212/wnl.59.7.1022. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Annals of Neurology. 2009;66(2):200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scola E, Bozzali M, Agosta F, Magnani G, Franceschi M, Sormani MP, Falini A. A diffusion tensor MRI study of patients with MCI and AD with a 2-year clinical follow-up. Journal of Neurology Neurosurgery and Psychiatry. 2010;81(7):798–805. doi: 10.1136/jnnp.2009.189639. [DOI] [PubMed] [Google Scholar]
- Selnes P, Aarsland D, Bjornerud A, Gjerstad L, Wallin A, Hessen E, Fladby T. Diffusion tensor imaging surpasses cerebrospinal fluid as predictor of cognitive decline and medial temporal lobe atrophy in subjective cognitive impairment and mild cognitive impairment. Journal of Alzheimer's Disease. 2013;33(3):723–736. doi: 10.3233/JAD-2012-121603. [DOI] [PubMed] [Google Scholar]
- Selnes P, Fjell AM, Gjerstad L, Bjornerud A, Wallin A, Due-Tonnessen P, Fladby T. White matter imaging changes in subjective and mild cognitive impairment. Alzheimer's & Dementia. 2012;8(5 Suppl):S112–121. doi: 10.1016/j.jalz.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer's disease: Meta-analyses of MRI studies. Hippocampus. 2009;19(11):1055–1064. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- Shim YS, Yoon B, Shon YM, Ahn KJ, Yang DW. Difference of the hippocampal and white matter microalterations in MCI patients according to the severity of subcortical vascular changes: neuropsychological correlates of diffusion tensor imaging. Clinical Neurology and Neurosurgery. 2008;110(6):552–561. doi: 10.1016/j.clineuro.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Shu N, Wang Z, Qi Z, Li K, He Y. Multiple diffusion indices reveals white matter degeneration in Alzheimer's disease and mild cognitive impairment: a tract-based spatial statistics study. Journal of Alzheimer's Disease. 2011;26(Suppl 3):275–285. doi: 10.3233/JAD-2011-0024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stricker NH, Schweinsburg BC, Delano-Wood L, Wierenga CE, Bangen KJ, Haaland KY, Bondi MW. Decreased white matter integrity in late-myelinating fiber pathways in Alzheimer's disease supports retrogenesis. Neuroimage. 2009;45(1):10–16. doi: 10.1016/j.neuroimage.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(11):4725–4727. doi: 10.1073/pnas.92.11.4725. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng E, Tingus KD, Lu PH, Cummings JL. Persistence of neuropsychological testing deficits in mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2009;28(2):168–178. doi: 10.1159/000235732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenerry MR, Crosson B, DeBoe J, Leber WR. Stroop Neuropsychological Screening Test: Manual. Odessa, FL: Psychological Assessment Resources; 1989. [Google Scholar]
- Walhovd KB, Fjell AM, Amlien I, Grambaite R, Stenset V, Bjornerud A, Fladby T. Multimodal imaging in mild cognitive impairment: Metabolism, morphometry and diffusion of the temporal-parietal memory network. Neuroimage. 2009;45(1):215–223. doi: 10.1016/j.neuroimage.2008.10.053. [DOI] [PubMed] [Google Scholar]
- Wang L, Goldstein FC, Veledar E, Levey AI, Lah JJ, Meltzer CC, Mao H. Alterations in cortical thickness and white matter integrity in mild cognitive impairment measured by whole-brain cortical thickness mapping and diffusion tensor imaging. American Journal of Neuroradiology. 2009;30(5):893–899. doi: 10.3174/ajnr.A1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Inteligence Scale - third edition manual 1997a [Google Scholar]
- Wechsler D. Wechsler Memory Scale - third edition manual 1997b [Google Scholar]
- Wenham PR, Price WH, Blandell G. Apolipoprotein E genotyping by one-stage PCR. Lancet. 1991;337(8750):1158–1159. doi: 10.1016/0140-6736(91)92823-k. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Jahng GH, Bayne W, Mori S, Schad L, Weiner MW. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68(1):13–19. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L, Sachdev PS, Trollor JN, Kochan NA, Reppermund S, Brodaty H, Wen W. Microstructural white matter changes in cognitively normal individuals at risk of amnestic MCI. Neurology. 2012;79(8):748–754. doi: 10.1212/WNL.0b013e3182661f4d. [DOI] [PubMed] [Google Scholar]
- Zhuang L, Wen W, Zhu W, Trollor J, Kochan N, Crawford J, Sachdev P. White matter integrity in mild cognitive impairment: a tract-based spatial statistics study. Neuroimage. 2010;53(1):16–25. doi: 10.1016/j.neuroimage.2010.05.068. [DOI] [PubMed] [Google Scholar]