Abstract
Background
Nitric oxide (NO) deficiency contributes to chronic kidney disease progression. NO deficiency could occur for many reasons, one of which is decreased NO synthase (NOS) abundance and/or activity.
Methods
In these experiments, we studied two groups of male Sprague Dawley rats given sham or surgical excision of both poles of the left kidney (at 2 days of age) followed by sham or surgical removal of the right kidney at 10 days. Rats were sacrificed at 9 weeks of age and the kidneys examined for abundance of neuronal NOS (nNOS)-α and -β, endothelial NOS, arginase II, argininosuccinate synthase and lysate, protein arginine methyltransferase 1, dimethylarginine dimethylamino-hydrolase 1 and 2, as well as renal pathology.
Results
The 5/6 nephrectomy (NX) group showed renal dysfunction, severe rapidly progressing glomerulosclerosis, and hypertension. Renal cortical nNOSα abundance was significantly reduced, whereas nNOSβ abundance was increased in the 5/6 NX group versus sham. Renal endothelial NOS was unchanged. Next, renal protein arginine methyltransferase 1 abundance was higher, whereas dimethylarginine dimethylamino-hydrolase 2 expression was lower in the 5/6 NX group versus sham. Renal arginase II, argininosuccinate synthase, and argininosuccinate lysate abundances were significantly decreased in 5/6 NX rats than those in sham.
Conclusion
The neonatal kidney is very susceptible to 5/6 NX-induced injury, and, as in adults, reciprocal changes in the nNOSα and nNOSβ in renal cortex occur during progression of chronic kidney disease and may contribute to the injury.
Keywords: chronic kidney disease, neuronal nitric oxide synthase, remnant kidney
1. Introduction
Nitric oxide (NO) deficiency occurs in humans and animals with chronic kidney disease (CKD) and may contribute to injury progression.1 In rats, development of CKD is associated with progressive loss of the α isoform of neuronal NO synthase (nNOS) from the kidney cortex.1,2 Furthermore, as nNOSα declines, the abundance of the nNOSβ isoform increases in adult rats subjected to 5/6 renal ablation/infarction.3 These changes in the renal cortical nNOS proteins may be causally linked to the progression of CKD.1,2
In addition to NOS isoform abundance, renal NOS activity can be regulated by asymmetric dimethylarginine (ADMA), which is mainly regulated by protein arginine methyl-transferase 1 (PRMT1, ADMA-synthesizing enzyme) and dimethylarginine dimethylamino-hydrolase (DDAH) 1 and 2 (ADMA-metabolizing enzymes). Because we had shown that increased renal PRMT1 expression and decreased renal DDAH activity contributed to the increase of ADMA in adult 5/6 nephrectomy (NX) rats,2 we also intended to study the ADMA-related enzymes in this neonatal 5/6 NX model.
Another possible mechanism for NO deficiency in CKD is substrate l-arginine deficiency.1 Because decreased endogenous l-arginine synthesis [by argininosuccinate synthase (ASS) and lysate (ASL)] and increased l-arginine consumption by means of other metabolic pathways (e.g., arginase II) could cause l-arginine deficiency. We also detected renal arginase II, ASS, and ASL expression in neonatal 5/6 NX rats.
Children are more susceptible to kidney injury and development of subsequent renal dysfunction than adults.4 In the normal adult, uninephrectomy (UNX) has a very mild phenotype that develops slowly, whereas in neonatal rats, UNX leads to rapidly evolving CKD and hypertension.5,6
In the present study, we investigated whether severe reduction of renal mass by 5/6 NX in neonatal rats is also associated with accelerated injury progression and/or reciprocal changes in density of the renal cortical nNOS isoforms, nNOSα and β. We also examined the l-arginine and ADMA-related enzymes in this neonatal 5/6 NX model.
2. Methods
Studies were conducted on 14 male Sprague Dawley rat pups (10-day old) subjected to either sham surgery (N = 7) or 5/6 NX (N = 7), as described previously.7 Blood pressure was measured by tail cuff at 8 weeks of age, and rats were sacrificed at 9 weeks of age when an aortic blood sample was collected for measurement of plasma creatinine and blood urea nitrogen. The left kidney remnant was perfused blood-free with cold phosphate-buffered saline, removed, and weighed and a section placed in 10% buffered formalin for pathology. The remainder was separated into cortex, flash frozen in liquid nitrogen, and stored at −80°C for analysis. All studies were approved by the Institutional Animal Care & Use Committee of Virginia Commonwealth University and conducted in accordance with the standards described in the National Institutes of Health Guide to the Care and Use of Laboratory Animals.
Western blot analysis was conducted on kidney cortex (100–200 µg total protein) using a mouse monoclonal antibody (Santa Cruz, Santa Cruz, CA, USA) for nNOSα detection, and for nNOSβ, we used a C-terminal rabbit polyclonal antibody (Affinity BioReagents, Golden, CO, USA). Membranes were stripped and reprobed for endothelial NOS (eNOS) using a mouse monoclonal antibody (Transduction Laboratories, Lexington, KY, USA). For PRMT1, DDAH, arginase II, ASS, and ASL, we used 12% gels with an otherwise identical protocol to that used for the NOS. For PRMT1, we used a rabbit anti-PRMT1 antibody (Upstate; Billerica, MA, USA, 1:2000 dilution, overnight incubation) and a goat antirabbit antibody. For DDAH, we used a goat anti-rat DDAH1 antibody (Santa Cruz, 1:500 dilution, overnight incubation) and a goat anti-rat DDAH2 antibody (Santa Cruz, 1:100 dilution, overnight incubation), followed by a secondary donkey anti-goat antibody (Santa Cruz, 1:2000 dilution, 1 hour incubation). For arginase II, we used a rabbit antibody (Santa Cruz, 1:1000 dilution, overnight incubation), followed by a goat anti-rabbit IgG-horseradish peroxidase secondary antibody. For ASS and ASL, we used a rabbit ASS polyclonal antibody and a rabbit ASL polyclonal antibody (gifts from Dr. Gotoh), followed by a goat anti-rabbit IgG-horseradish peroxidase secondary antibody. Bands of interest were visualized using SuperSignal West Pico reagent (Pierce, Rockford, IL, USA) and quantified by densitometry as integrated optical density factored for Ponceau red staining to correct for any variations in total protein loading and for an internal standard (rat cerebellum for nNOS, endothelial cell lysate for eNOS). The protein abundance was represented as integrated optical density/Ponceau Red/Std. All details of these measurements were reported previously.2,3
Pathology was performed on 5-µm sections of formalin-fixed kidney, blocked in paraffin wax, and stained with periodic acid-Schiff. The level of renal injury was assessed on a blinded basis by determining the sclerotic damage to glomeruli using the 0 to 4+ scale, and were graded as follows: 0, no changes; 1+ injury involved <25% damage to the glomerulus; 2+ injury, 25%–50%; 3+ injury, 50%–75%; and 4+ injury, 75%–100% damage. Data were represented as percentage of damaged glomeruli (n = 100) showing any level of injury (scale 1+ to 4+) as published previously.2
Results are presented as means ± standard error. Parametric data were analyzed by unpaired t test and histologically by Mann-Whitney U test. A p < 0.05 was considered statistically significant.
3. Results
At 9 weeks of age, the 5/6 NX rats had lower body weight (270 ± 13 vs. 382 ± 9, p < 0.01) and much higher kidney weights (7.4 ± 0.4 g vs. 1.5 ± 0.1 g, p < 0.001) and blood pressure than shams (193 ± 11 vs. 125 ± 5 mmHg, p < 0.001). Both plasma creatinine and blood urea nitrogen levels were markedly increased in 5/6 NX rats (1.45 ± 0.20 vs. 0.36 ± 0.04 mg/dL, p < 0.005 and 170 ± 22 vs. 16 ± 5 mg/dL, respectively) compared with shams. These data have been published previously.7 The total number of damaged glomeruli (all levels of injury) was 3 ± 1% in shams and was much greater in the 5/6 NX group (63 ± 5%, p < 0.01). Robust induction of glomerulosclerosis was present in 5/6 NX rats as early as 8 weeks after surgery (Figure 1A). Tubulointerstitial fibrosis developed in parallel with glomerulosclerosis. As shown in Figure 1B, there were more damaged glomeruli at the 3+ (15.5 ± 3.0 vs. 0%) and 4+ levels (22.5 ± 5.0 vs. 0%) of severity in the 5/6 NX rats 8 weeks after surgery versus sham. This contrasted to the milder injury seen in adult male rats 15 weeks after 5/6 NX versus shams (40 ± 3% vs. 10 ± 2%, p < 0.01; data published previously2) as adult 5/6 NX rats had only ~5% and ~10% damaged glomeruli at the 3+ and 4+ levels of severity, respectively.2
Figure 1.
(A) Histologic changes in 5/6 NX rat and sham control. Representative photographs from a 5/6 NX rat 8 weeks after surgery (right panel) and control (left panel) by periodic acid-Schiff stain. Bar = 500 µm. (B) Percent damaged glomeruli in shams (black bars) and 5/6 nephrectomized rats (gray bars) according to severity of glomerular injury (1+ <25%; 2+ 25–50%; 3+ 51–75%; 4+ >75%). *p < 0.05 versus sham. NX = nephrectomy.
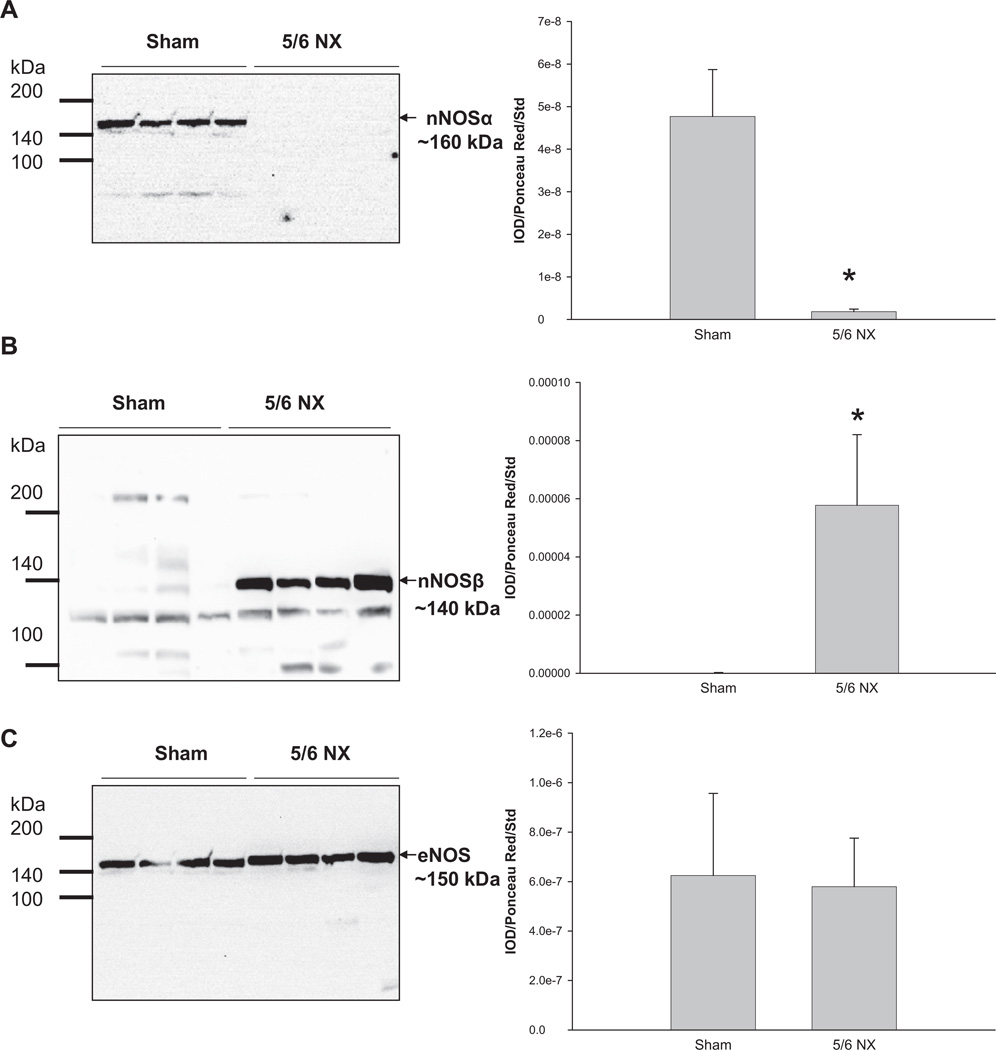
The renal nNOSα abundance was reduced almost to zero in the 5/6 NX group (Figure 2A), whereas nNOSβ abundance was increased (Figure 2B). In contrast, renal cortical eNOS protein abundance was more variable and was not different between the two groups (Figure 2C).
Figure 2.
Relative abundance of renal cortex (A) nNOSα, (B) nNOSβ, and (C) eNOS. Representative whole membranes show the nNOSα (~160 kDa), nNOSβ (~140 kDa), and eNOS (~150 kDa). N = 7 per group. *p < 0.05 versus sham. nNOSα = neuronal nitric oxide synthase-α; nNOSβ = neuronal nitric oxide synthase-β; eNOS = endothelial nitric oxide synthase.
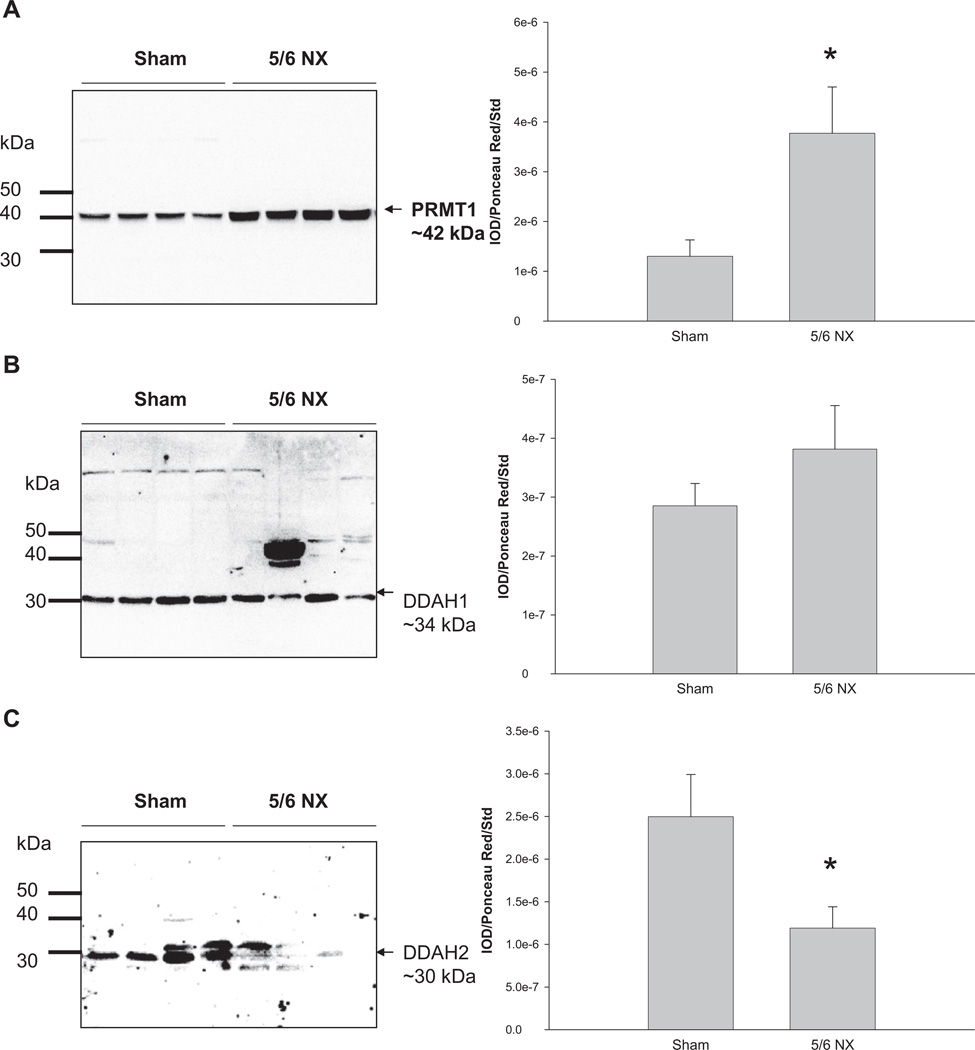
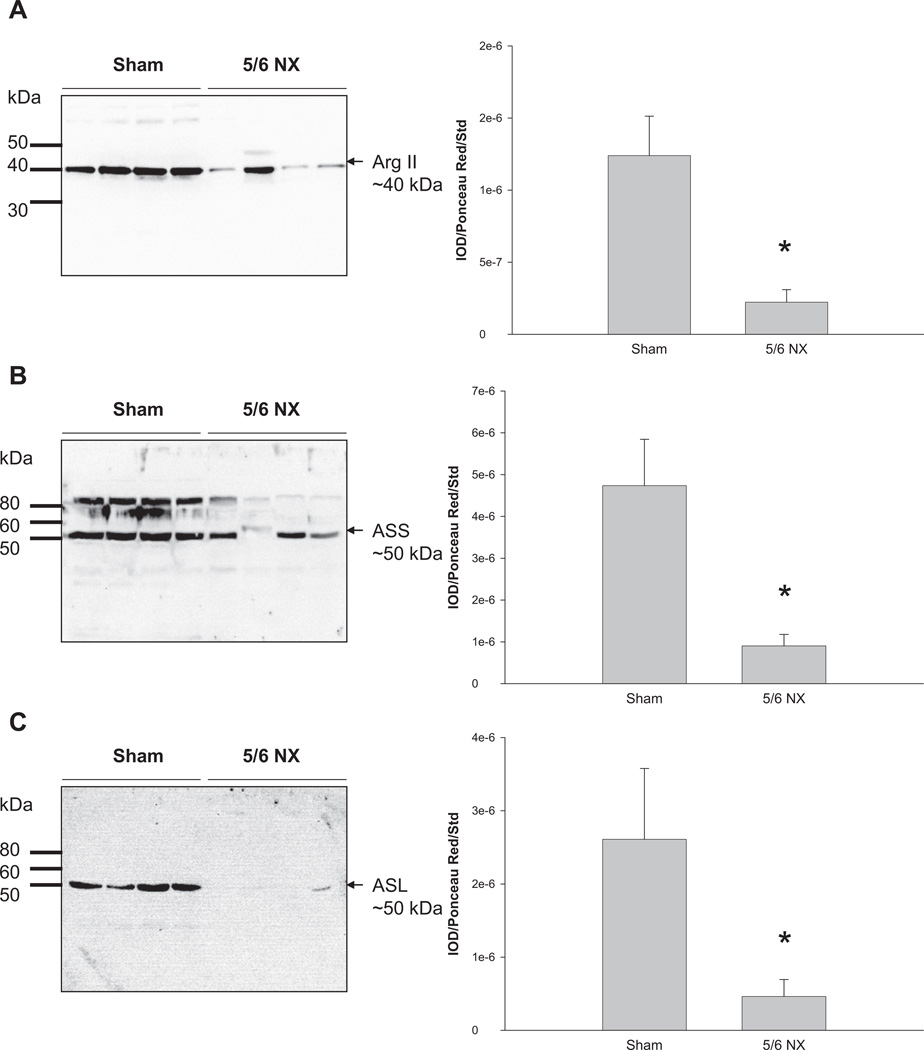
Renal PRMT1 abundance was higher in the 5/6 NX group versus sham (Figure 3A). We found no difference in renal DDAH1 abundance between the two groups (Figure 3B), whereas DDAH2 expression was lower in 5/6 NX group versus sham (Figure 3C). Renal arginase II, ASS, and ASL abundances were significantly decreased in the 5/6 NX group versus sham (Figure 4).
Figure 3.
Relative abundance of renal cortex (A) PRMT1, (B) DDAH1, and (C) DDAH2. Representative Western blots of whole membranes show PRMT1 (~42 kDa), DDAH1 (~34 kDa), and DDAH2 bands (~30 kDa). N = 7 per group. *p < 0.05 versus sham. PRMT1 = protein arginine methyltransferase 1; DDAH1 = dimethylarginine dimethylamino-hydrolase 1; DDAH2 = dimethylarginine dimethylamino-hydrolase 2.
Figure 4.
Relative abundance of renal cortex (A) arginase II, (B) argininosuccinate synthase (ASS), and (C) argininosuccinate lysate (ASL). Representative Western blots of whole membranes show arginase II (~40 kDa), ASS (~50 kDa), and ASL bands (~50 kDa). N = 7 per group. *p < 0.05 versus sham. ASS = argininosuccinate synthase; ASL = argininosuccinate lysate.
4. Discussion
Reduction in renal mass is a commonly used model of CKD in the rat, with progression of injury depending on several factors including amount of renal mass removed, time after renal mass reduction, and sex- and strain-dependent vulnerability to CKD. Age is also important, and in the present study, we showed that after 5/6 NX, neonatal rats develop rapidly progressing hypertension and kidney damage over a course of 8 weeks. In the young adult Sprague Dawley male, there is little hypertension and more moderate damage, even 15 weeks after 5/6 NX.2 The findings are similar with UNX, which has a mild phenotype that develops slowly in adults, whereas in neonatal rats, UNX leads to rapidly evolving CKD and hypertension.5,6 There was a remarkable hypertrophic response of the remnant left kidney in the neonates with increases of ~400%, whereas 5/6 NX in young adults led to increases of ~60% in left kidney weight.2 This exaggerated hypertrophic response may contribute to the severity of the injury.
Recently, we reported the existence of two isoforms of nNOS (splice variants) in the normal rat kidney.3 The nNOSα decreases as severity of kidney injury progresses, irrespective of the model.1 In situations where renal cortical nNOS is preserved (because of sex- or strain-dependent resistance to progression of CKD), the cortical nNOSα abundance is also preserved, suggesting a causal relationship.1 The abundance of nNOSβ increased in adult rats after 5/6 ablation infarction of renal mass,3 and we also reported increased nNOSβ abundance in a rapamycin-treated transplant model.8 Because nNOSβ abundance increases in parallel with increasing glomerular damage, if this is intended as a compensatory effect, it is clearly inadequate to prevent progression of the injury. In accordance with our previous findings in adult rats, we found that nNOSα decreased, but nNOSβ increased in 5/6 NX rats when surgery was performed in neonates. These changes were very marked and paralleled the severity of the renal injury. On the other hand, renal eNOS expression was unchanged in 5/6 NX rats (present study) and in adults.2
Next, we found renal PRMT1 increased, but DDAH2 decreased, which might result in the accumulation of ADMA. Although ADMA level was not analyzed because of limited blood volume in this study, we have shown elevated ADMA level in adult 5/6 NX rats.2 Because kidney is a major site of ADMA catabolism by DDAH, reduced nephron mass could consequently influence renal metabolism of systemic ADMA. On the other hand, increased PRMT1 expression could also influence NO bioavailability by means of inducing ADMA synthesis.
Dietary intervention with l-arginine has resulted in amelioration of a number of experimental kidney diseases,9 including remnant kidney.10 However, whether l-arginine level is decreased and whether l-arginine supplementation is beneficial to children with CKD are inconclusive. ASS/ ASL, the two arginase isoenzymes, the three NOS isoenzymes, arginine decarboxylase, and cationic amino acid transporters have been recognized as key factors in regulating arginine metabolism.11 Nevertheless, their relative importance in regulating NO production in CKD remains unclear. Our study demonstrated that renal ASS/ASL and arginase II abundances were decreased in neonatal 5/6 NX rats, which was consistent with our recent findings showing that a fall in l-arginine production in adult remnant kidney is because of an early loss of ASS and ASL conversion of citrulline.12 As fetal rat kidney is prone to express low ASS expression compared with adult kidney,13 suggesting a substantial l-arginine deficiency might occur because of decreased l-arginine production in the neonatal remnant kidney. As arginase pathway accounts for the major consumption of l-arginine and arginase II is the major isoform expressed in the kidney, our data revealed NO deficiency attributed to l-arginine deficiency might not mainly be because of consumption by arginase in this model.
As with other models of experimentally induced CKD, reduction of renal nNOSα and compensatory increase of nNOSβ are seen after 5/6 NX in neonates. Specific therapy targeting NO deficiency might be a promising approach to delay CKD progression. Additionally, elucidation of the differences and similarities in the mechanisms involved in the progression of CKD in children and adults might provide insights into prevention of childhood CKD.
References
- 1.Baylis C. Arginine, arginine analogs and nitric oxide production in chronic kidney disease. Nat Clin Pract Nephrol. 2006;2:209–220. doi: 10.1038/ncpneph0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tain YL, Freshour G, Dikalova A, Griendling K, Baylis C. Vitamin E reduces glomerulosclerosis, restores renal neuronal NOS, and suppresses oxidative stress in the 5/6 nephrectomized rat. Am J Physiol Renal Physiol. 2007;292:F1404–F1410. doi: 10.1152/ajprenal.00260.2006. [DOI] [PubMed] [Google Scholar]
- 3.Smith C, Merchant M, Fekete A, et al. Splice variants of neuronal nitric oxide synthase are present in the rat kidney. Nephrol Dial Transplant. 2009;24:1422–1428. doi: 10.1093/ndt/gfn676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. 2006;69:184–189. doi: 10.1038/sj.ki.5000032. [DOI] [PubMed] [Google Scholar]
- 5.Nagata M, Schärer K, Kriz W. Glomerular damage after uninephrectomy in young rats. I. Hypertrophy and distortion of capillary architecture. Kidney Int. 1992;42:136–147. doi: 10.1038/ki.1992.271. [DOI] [PubMed] [Google Scholar]
- 6.Woods LL, Weeks DA, Rasch R. Hypertension after neonatal uninephrectomy in rats precedes glomerular damage. Hypertension. 2001;38:337–342. doi: 10.1161/01.hyp.38.3.337. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh SS, Krieg RJ, Sica DA, Wang R, Fakhry I, Gehr T. Cardiac hypertrophy in neonatal nephrectomized rats: the role of the sympathetic nervous system. Pediatr Nephrol. 2009;24:367–377. doi: 10.1007/s00467-008-0978-8. [DOI] [PubMed] [Google Scholar]
- 8.Tain YL, Muller V, Szabo AJ, Erdely A, Smith C, Baylis C. Sex differences in response to rapamycin in kidney transplantation: impact on constitutive nitric oxide synthase. Nitric Oxide. 2008;18:80–86. doi: 10.1016/j.niox.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reyes AA, Karl IE, Klahr S. Role of arginine in health and in renal disease. Am J Physiol. 1994;267:F331–F346. doi: 10.1152/ajprenal.1994.267.3.F331. [DOI] [PubMed] [Google Scholar]
- 10.Reyes AA, Purkerson ML, Karl I, Klahr S. Dietary supplementation with L-arginine ameliorates the progression of renal disease in rats with subtotal nephrectomy. Am J Kidney Dis. 1992;20:168–176. doi: 10.1016/s0272-6386(12)80546-4. [DOI] [PubMed] [Google Scholar]
- 11.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen GF, Baylis C. In vivo renal arginine release is impaired throughout development of chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F95–F102. doi: 10.1152/ajprenal.00487.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husson A, Brasse-Lagnel C, Fairand A, Renouf S, Lavoinne A. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur J Biochem. 2003;270:1887–1899. doi: 10.1046/j.1432-1033.2003.03559.x. [DOI] [PubMed] [Google Scholar]