Abstract
Excessive generation of wear particles after total joint replacement may lead to local inflammation and periprosthetic osteolysis. Modulation of the key transcription factor NF-κB in immune cells could potentially mitigate the osteolytic process. We previously showed that local delivery of ultrahigh-molecular-weight polyethylene (UHMWPE) particles recruited osteoprogenitor cells and reduced osteolysis. However, the biological effects of modulating the NF-κB signaling pathway on osteoprogenitor/mesenchymal stem cells (MSCs) remain unclear. Here we showed that decoy oligodeoxynucleotide (ODN) increased cell viability when primary murine MSCs were exposed to UHMWPE particles, but had no effects on cellular apoptosis. Decoy ODN increased transforming growth factor-beta 1 (TGF-β1) and osteoprotegerin (OPG) in MSCs exposed to UHMWPE particles. Mechanistic studies showed that decoy ODN upregulated OPG expression through a TGF-β1-dependent pathway. By measuring the alkaline phosphatase activity, osteocalcin levels, Runx2 and osteopontin expression, and performing a bone mineralization assay, we found that decoy ODN increased MSC osteogenic ability when the cells were exposed to UHMWPE particles. Furthermore, the cellular response to decoy ODN and UHMWPE particles with regard to cell phenotype, cell viability, and osteogenic ability was confirmed using primary human MSCs. Our results suggest that modulation of wear particle-induced inflammation by NF-κB decoy ODN had no adverse effects on MSCs and may potentially further mitigate periprosthetic osteolysis by protecting MSC viability and osteogenic ability.
Introduction
Total joint replacement is a highly successful surgical treatment for patients with end-stage arthritis. However, excessive generation of wear particles can cause chronic inflammation, which may result in periprosthetic osteolysis.1,2 Particle-induced osteolysis is a systemic response through secretion of chemokines from the cells in the local environment.3 These chemokine gradients mediate migration of multiple cell types, including macrophages4 and mesenchymal stem cells (MSCs).5 Infiltrated MSCs have an immunomodulatory function and osteogenic ability.6 A previous study showed that blocking MIP1α using a receptor antagonist mitigated osteolysis induced by ultrahigh-molecular-weight polyethylene (UHMWPE) particles in a murine model, suggesting that MSCs can protect particle-induced osteolysis in a confined region.5
NF-κB is a key transcriptional factor in inflammatory reactions. Recently, we demonstrated that suppression of NF-κB activity using decoy oligodeoxynucleotide (ODN) in primary mouse macrophages or the human THP1 macrophage cell line decreased the expression of multiple cytokines and chemokines in the presence of wear particles and endotoxin.7 The tissue microenvironment surrounding a joint replacement is composed of multiple cell types, including macrophages and MSCs. Thus, when evaluating possible treatments for periprosthetic osteolysis, the potential adverse effects of these interventions on other neighboring cells must be considered. Given the fundamental role of MSCs in protecting wear particle-induced osteolysis,5 the biological effects of NF-κB on MSCs are highly relevant.
In the current study, we used primary mouse and human bone marrow-derived MSCs to investigate the cellular response to NF-κB decoy ODN in the presence of clinically relevant UHMWPE particles. Cell viability, secretion of immune-modulating cytokines, and osteogenic differentiation ability of MSCs in response to these stimuli are characterized.
Materials and Methods
MSC isolation and culture
The method of isolating mouse bone marrow-derived MSCs was modified from published protocols.8,9 In brief, bone marrow was collected from the femurs and tibias of C57BL male mice aged 6–8 weeks old. Institutional guidelines for the care and use of laboratory animals were observed in all aspects of this project. The cells were carefully suspended and passed through a 70-μm strainer, spun down, and resuspended in α-minimal essential media (MEM) supplied with 10% heat inactivated MSC-certified fetal bovine serum (FBS; Invitrogen) and antibiotic–antimycotic solution (100 units of penicillin, 100 μg of streptomycin, and 0.25 μg of amphotericin B per mL; Hyclone, Thermo Scientific). The fresh media were replaced the next day to remove the unattached cells (passage 1). MSC surface marker expression was analyzed at passage 4. Cells between passages 4–7 were used for the experiments. Human MSCs at passage 2 were purchased from Lonza. Cells between passages 3–5 were used for the experiments as suggested by the company. Donors were selected with the following criteria: healthy male, age 20–40, Caucasian, or African American. The cells were grown in MSCGM™ (Lonza), and the instructions for cell culture were followed carefully.
Decoy ODN
The NF-κB decoy sequences used for the studies were 5′-CCTTGAAGGGATTTCCCTCC-3′ and 3′-GGAACTTCCC TAA-AGGGAGG-5′. Scrambled decoy ODN had the following sequences 5′-TTGCCGTA-CCTGACTTAGCC-3′ and 3′-AACGGCATGGACTGAATCGG-3′.10 The synthetic ODNs were a gift from Dr. Kensuke Egashira of Kyushu University, Japan. The ODN was washed with 70% ethanol, dried, and dissolved in sterile Tris-EDTA (10 mM Tris, 1 mM EDTA), and the supernatant was purified over a NAP 10 column. The ODN concentration (0.5 μM) in the current study using MSCs was the same as our previous experiments using macrophages; this dose was sufficient to inhibit the NF-κB activity without significant cytotoxic effects.7
UHMWPE particles
Conventional UHMWPE particles were a gift from Dr. Timothy Wright (Hospital for Special Surgery, New York); the particles were obtained from joint simulator tests and isolated according to an established protocol.11 Frozen aliquots of particles containing serum were lyophilized for 4–7 days. The dried material was digested in 5 M sodium hydroxide at 60°C for 1 h and ultrasonicated for 10 min. The digested particle suspension was centrifuged through a 5% sucrose gradient at 40K rpm at 10°C for 3 h. The collected particles at the surface of the sucrose solution were incubated at 80°C for 1 h and centrifuged again through an isopropanol gradient (0.96 and 0.90 g/cm3) at 40K rpm at 10°C for 1 h. The purified particles at the interface between the two layers of isopropanol were harvested, and the isopropanol was evaporated from the particle mixture and then lyophilized until dry. Particles were resuspended in 95% ethanol, which was then evaporated completely. The particles tested negative for endotoxin using a Limulus Amebocyte Lysate Kit (BioWhittaker). The mean diameter of the particles was 0.43±0.104 μm (mean±standard error, range 0.26–0.81 μm) measured by electron microscopy.
Cell surface marker analysis by flow cytometry
Mouse MSCs exposed to 0.5 μM of decoy or scrambled ODN were maintained in the MSC culture medium for 3 days. The cells were then detached and washed with Dulbecco's phosphate-buffered saline (DPBS) and resuspended in DPBS containing 2% FBS and 1 mM EDTA. The cells were stained with Sca1,12 CD44, CD105, CD11b, CD45, and CD3413 (eBioscience) and analysis was performed by LSRII (BD Bioscience). Data were analyzed by FlowJo 7.6 (Tree Star, Inc.). The experiments were confirmed twice independently.
Cell viability assay
Mouse and human MSCs were exposed to 0.5 μM decoy or scrambled ODN, with or without UHMWPE particles or lipopolysaccharide (LPS; Sigma-Aldrich). Particles were coated on the bottom of the 24-well culture plate as previously described.7 Cell viability studies (repeated four times) were quantified by measuring the lactate dehydrogenase (LDH) activity using CytoTox 96® nonradioactive cytotoxicity assay (Promega). The experiments were confirmed twice independently.
Cell apoptosis assay
Mouse MSCs exposed to the ODN, particles, and LPS were stained with the propidium iodide staining solution and APC-conjugated Annexin V (eBioscience) and analyzed by the LSR II flow cytometer (BD Bioscience). Data were analyzed by FlowJo 7.6 (Tree Star, Inc.), and the Annexin V-positive cells were defined as apoptotic cells. The experiments were confirmed twice independently.
Osteogenic differentiation assay
Mouse MSCs exposed to decoy ODN or scrambled ODN, with or without UHMWPE particles, were grown in the osteogenic medium (α-MEM supplemented with 10% FBS, 100 nM dexamethasone, 10 mM β-glycerol phosphate, and 50 μM ascorbate-2-phosphate) or control medium. The human MSC osteogenic differentiation medium was purchased from Lonza, and the manufacturer's protocol was carefully followed. Cell lysate at week 2 was used for the alkaline phosphatase (ALP) activity assay (QuantiChrome™ Alkaline phosphatase assay kit, Cat.No.DALP-250; Bioassay Systems). Culture supernatant at week 3 was used to detect osteocalcin levels (osteocalcin EIA ELISA Kit; mouse, Cat.No.BT-470; human, Cat.No.BT-480; Biomedical Technologies). Cellular mineralization in mouse MSCs was determined using the Alizarin red stain. The results were photographed and the staining was eluted by 10% cetylpyridinium chloride and quantified by measuring the absorbance at 562 nm. The time points for the different osteogenic assays were chosen based on a previous study.14 All the assays were done with six repetitions in each group, and the experiments were confirmed twice independently.
Adipogenesis and chondrogenesis assay
The differentiation assay protocols were modified slightly from the published methods.15 For the adipogenesis assay, cells were incubated in α-MEM supplemented with 10% FBS, 1 μM dexamethasone, 0.5 μM IBMX, 2 μg/mL insulin, and 2 μM rosiglitazone for 2 weeks. The results were analyzed by Oil Red O staining at day 14. The assay was repeated six times. For the chondrogenesis assay, cells were centrifuged to form a pellet and incubated at high-glucose DMEM supplemented with 100 nM dexamethasone, 1% ITS, 50 μM ascorbate-2-phosphate, 1 mM sodium pyruvate, 50 μg/mL proline, and 20 ng/mL transforming growth factor-beta 3 (TGF-β3). The pellets were resuspended and air-dried on glass slides and stained with toluidine blue at day 21.
RNA extraction and quantitative polymerase chain reaction
Cellular RNAs were extracted using the RNeasy RNA purification kit (Qiagen). RNAs were reverse transcribed into complementary DNA (cDNA) using a high-capacity cDNA archive kit (Applied Biosystems). Probes for 18s rRNA, TGF-β1, RANKL, osteoprotegerin (OPG), Runx2, osteopontin, Smurf2, and TAZ were purchased from Applied Biosystems. Reverse-transcriptase polymerase chain reaction (PCR) was performed in triplicate in an ABI 7900HT Sequencing Detection System (Applied Biosystems), using 18s rRNA as the internal control. The −ΔΔCt relative quantization method was used to evaluate the gene expression level. The experiments were confirmed twice independently.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) kits for TGF-β1 and IL-10 were purchased from eBioscience. Assay kits for OPG and RANKL were purchased from R&D. Manufacturers' protocols were followed carefully. The optical densities were determined using a Bio-Rad 3550-UV microplate reader (Bio-Rad) set at 450 nm. The assays were performed in triplicate and confirmed twice independently.
Statistical analysis
One-way ANOVA with Tukey's post hoc testing was conducted using Prism 5 (GraphPad Software). Data are reported as mean±standard error of the mean. A p value of <0.05 was chosen as the threshold of significance.
Results
NF-κB decoy ODN had no significant toxic effects on mouse MSCs
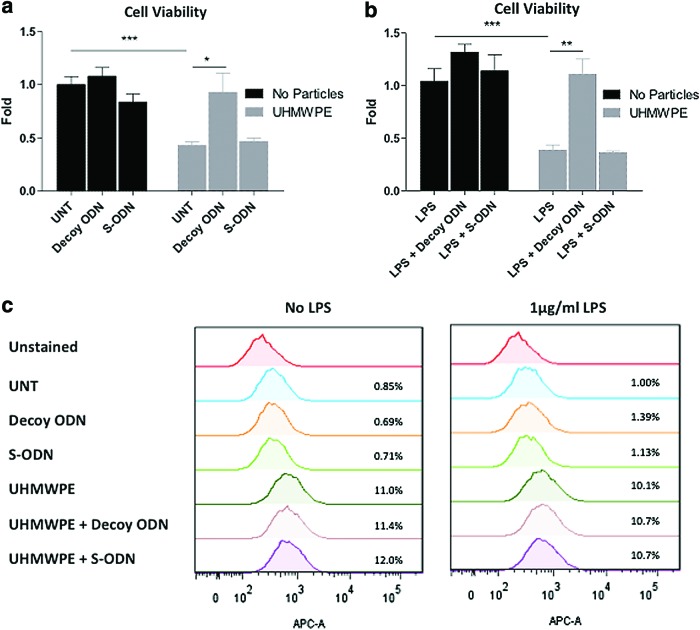
The differentiation capabilities of harvested murine MSCs into osteoblast, adipocyte, and chondrocyte phenotypes were confirmed (Supplementary Fig. S1a; Supplementary Data are available online at www.liebertpub.com/tea). MSCs were plated and exposed to 0.5 μM decoy ODN or scrambled ODN for 72 h, and the expression of surface markers was analyzed by flow cytometry. Surface marker expression patterns (CD44+/CD105+/Sca1+/CD11b−/CD34−/CD45−) in the MSCs were consistent with previous reports,12,13 while decoy ODN and scrambled ODN had no significant effects on surface marker expression (Supplementary Fig. S1b). Cellular viability of mouse MSCs exposed to ODN and UHMWPE particles for 48 h was determined by the LDH assay. The viable cell amount was reduced to 42.9%±3.4% (without LPS) or 38.5%±4.8% (with 1 μg/mL LPS) in the cells exposed to UHMWPE particles compared to the untreated control, and the reduction was reversed by decoy ODN treatment (Fig. 1a, b). In the apoptosis assay, MSCs exposed to the particles caused 11.0% cell apoptosis. The apoptotic cells were slightly decreased to 10.1% in the presence of LPS, but this did not reach statistical significance (Fig. 1c). Notably, decoy ODN had no protective effects on particle-induced cell apoptosis, suggesting that the decoy ODN-mediated protective effect was not correlated with the apoptosis pathway.
FIG. 1.
NF-κB decoy ODN treatment increased mouse MSC viability but not cell apoptosis in response to UHMWPE particles. Mouse MSCs were exposed to (a) control or (b) 1 μg/mL LPS, together with 0.5 μM decoy ODN or scrambled ODN, with or without UHMWPE particles. Cell viability was quantified at 48 h using the LDH assay. (c) Mouse MSCs exposed to ODN, UHMWPE particles, and LPS for 24 h were stained with Annexin V to evaluate the cellular apoptotic response. The apoptotic cells were analyzed by flow cytometry. The error bars represent the SEM. *p<0.05, **p<0.01, and ***p<0.005. LDH, lactate dehydrogenase; LPS, lipopolysaccharide; MSCs, mesenchymal stem cells; ODN, oligodeoxynucleotide; SEM, standard error of mean; S-ODN, scrambled ODN; UHMWPE, ultrahigh-molecular-weight polyethylene; UNT, untreated control. Color images available online at www.liebertpub.com/tea
NF-κB decoy ODN induced TGF-β1 and OPG expression in mouse MSCs exposed to UHMWPE particles
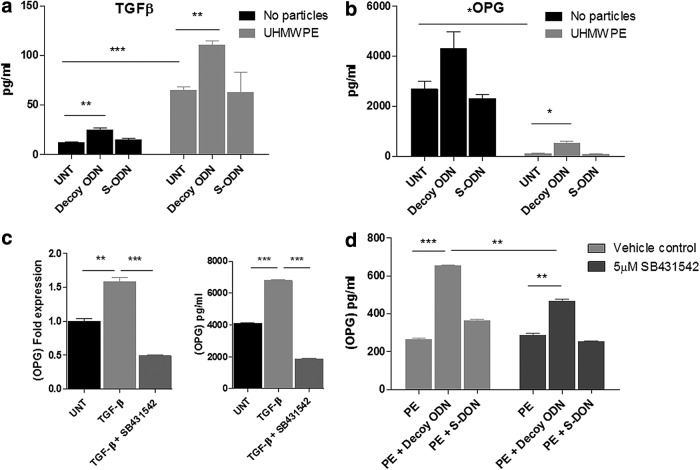
Cultured supernatants were collected from mouse MSCs exposed to ODN and UHMWPE particles for 24 h. TGF-β1 and IL-10 secretion was chosen for evaluation because of the immune modulation and osteogenic regulatory functions, as outlined in previous reports on MSC and bone formation.16–19 TGF-β secretion was increased by NF-κB decoy ODN treatment in MSCs (24.79±4.07 pg/mL) compared to the untreated controls (11.96±1.77 pg/mL). The induction persisted when the cells were exposed to UHMWPE particles, but at a significantly higher level (decoy ODN: 64.66±3.79 pg/mL; UHMWPE only: 110.39±7.17 pg/mL; p<0.01) (Fig. 2a). IL-10 secretion was undetectable by ELISA (below 30 pg/mL) in the collected supernatant. Cells exposed to decoy ODN and/or UHMWPE particles did not induce IL-10 expression.
FIG. 2.
NF-κB decoy ODN and UHMWPE particles enhanced the secretion of TGF-β1 and OPG in mouse MSCs. Mouse MSCs were exposed to 0.5 μM decoy ODN or scrambled ODN, with or without UHMWPE particles. The culture supernatants were collected 24 h later. Secretion of TGF-β1 (a) and OPG (b) was quantified by ELISA. (c) Mouse MSCs were treated with 1 ng/mL TGF-β1, with or without 5 μM SB431542 for 24 h. Cellular RNA and culture supernatants were collected for analysis of OPG expression by quantitative PCR (left) and ELISA (right). (d) Mouse MSCs exposed to UHMWPE particles were treated with 0.5 μM decoy ODN or scrambled ODN, with or without 5 μM SB431542. Supernatants were collected 24 h later and OPG expression was quantified by ELISA. The error bars represent SEM. *p<0.05, **p<0.01, and ***p<0.005. ELISA, enzyme-linked immunosorbent assay; OPG, osteoprotegerin; PCR, polymerase chain reaction; PE, UHMWPE particles; S-ODN, scrambled ODN; TGF-β, transforming growth factor-beta; UNT, untreated control.
Secretion of RANKL and OPG by MSCs was used to evaluate the effects of NF-κB decoy ODN on MSC osteogenic differentiation potential. OPG secretion was increased by treatment with NF-κB decoy ODN in MSCs (4302.96±681.6 pg/mL), compared to the untreated controls (2685.36±309.23 pg/mL), but this comparison did not reach statistical significance. MSCs exposed to UHMWPE particles had a significantly lower OPG expression (124.26±2.03 pg/mL); NF-κB ODN treatment enhanced the expression level of OPG (543.77±58.36 pg/mL) (Fig. 2b). The RANKL expression level was out of the detectable range using ELISA, and there was no significant change in response to ODN and particles (data not shown).
NF-κB decoy ODN induced OPG expression through a TGF-β1-dependent pathway
Previous reports suggested that OPG expression was enhanced by TGF-β1 signaling.20 To investigate the potential correlation between TGF-β1 and OPG expression, mouse MSCs were treated with 1 ng/mL TGF-β1 alone or in combination with the TGF-β receptor kinase inhibitor SB431542. The culture supernatant and cellular RNA were collected 24 h later, and the expression of OPG was evaluated. TGF-β1 treatment increased OPG expression at both the mRNA (1.57±0.06-fold) and protein (6780±50 pg/mL) levels (Fig. 2c); the induction of OPG was suppressed by addition of SB431542. Furthermore, induction of OPG expression by decoy ODN using mouse MSCs exposed to UHMWPE particles was partially reduced by SB431542 treatment (from 653±3 pg/mL to 466±11 pg/mL, Fig. 2d).
NF-κB decoy ODN decreased the reduction of osteogenesis in mouse MSCs caused by UHMWPE particle exposure
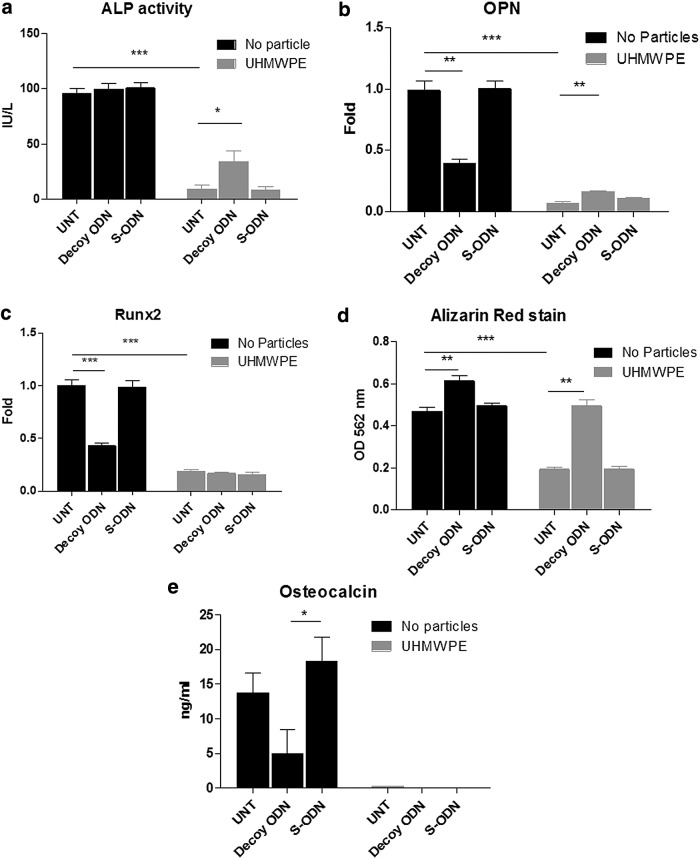
To investigate the roles of NF-κB signaling in the MSC osteogenic differentiation ability, mouse MSCs were cultured in the osteogenic medium containing 0.5 μM decoy ODN or scrambled ODN, with or without UHMWPE particles. The ALP activity by MSCs (day 14) was reduced from 95.97±4.09 IU/L to 9.13±3.35 IU/L when the cells were exposed to UHMWPE particles. Decoy ODN treatment enhanced the ALP activity to 34.08±9.24 IU/L in particle-exposed cells (Fig. 3a), but the induction level did not return to values seen without particle exposure. Osteopontin and Runx2 RNA expression at day 14 was reduced by either decoy ODN alone or particle exposure. Osteopontin but not Runx2 expression was increased by decoy ODN in the presence of particles (Fig. 3b, c). Bone mineralization (Alizarin red stain) of MSCs at day 21 was reduced to 58.7%±2.0% by particle exposure and decoy ODN was able to reverse the reduction. Decoy ODN also increased bone mineralization in MSCs with no particles by 32.6%±5.5% (Fig. 3d). Osteocalcin levels in MSCs at day 21 were reduced by NF-κB decoy ODN (13.74±2.853 ng/mL to 4.96±3.42 ng/mL, Fig. 3e), and the expression was below the detectable level (1 ng/mL) when cells were exposed to the particles.
FIG. 3.
NF-κB decoy ODN increased osteogenic ability of mouse MSCs exposed to UHMWPE particles. Mouse MSCs were cultured in the osteogenic differentiation medium exposed to 0.5 μM decoy ODN or scrambled ODN, with or without UHMWPE particles. Cell lysates were harvested at day 14 for the ALP kinetic assay (a) or the quantitative PCR gene expression assay for Runx2 and osteopontin (OPN) at day 14 (b, c). Cultured supernatants at day 21 were collected and osteocalcin levels were determined by ELISA (d), and cells at day 21 were fixed and assayed by Alizarin red stain (e). The cells were destained and quantified by reading at O.D. 562 nm. The error bars represent SEM. *p<0.05, **p<0.01, and ***p<0.005. ALP, alkaline phosphatase; S-ODN, scrambled ODN; UNT, untreated control.
NF-κB decoy ODN increased cell viability and osteogenic ability in human MSCs exposed to UHMWPE
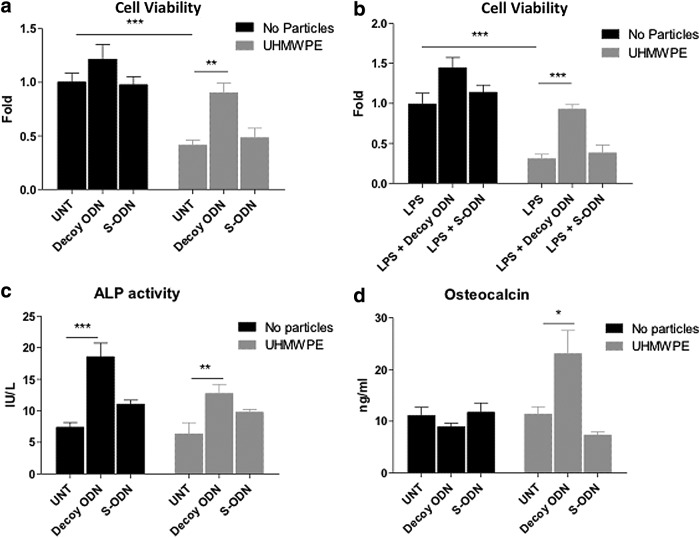
The viable cell amounts were reduced to 41.3%±4.7% when the cells were exposed to UHMWPE particles compared to untreated controls; the reduction was reversed by the addition of decoy ODN treatment to a value of 90.2%±9.1% (Fig. 4a). The viable cell number was reduced to 30.7%±5.6% when cells were exposed to UHMWPE particles and LPS, whereas decoy ODN treatment protected cells from this toxic effect (Fig. 4b).
FIG. 4.
NF-κB decoy ODN increased cell viability and osteogenic ability in human MSCs exposed to UHMWPE. Human MSCs were exposed to (a) control or (b) 1 μg/mL LPS, together with 0.5 μM decoy ODN or scrambled ODN, with or without UHMWPE particles. Cell viability was quantified at 48 h using the LDH assay. (c, d) Human MSCs were cultured in the osteogenic differentiation medium and exposed to 0.5 μM decoy ODN or scrambled ODN, with or without UHMWPE particles. Cell lysates were harvested at day 14 for the ALP kinetic assay (c). Cultured supernatants at day 21 were collected and osteocalcin levels were determined by ELISA (d). The error bars represent SEM. *p<0.05, **p<0.01, and ***p<0.005. S-ODN, scrambled ODN; UNT, untreated control.
The ALP activity in human MSCs (day 14) demonstrated no significant change when the cells were exposed to UHMWPE particles. Decoy ODN treatment enhanced ALP activity 2–2.5-fold regardless of particle exposure (Fig. 4c). Consistent with mouse MSCs, osteocalcin expression in human MSCs was partially reduced by decoy ODN (from 11.11±1.63 ng/mL to 8.94±0.65 ng/mL). However, the decoy ODN increased osteocalcin expression (11.39±1.42 ng/mL to 23.09±4.46 ng/mL) in the presence of UHMWPE particles (Fig. 4d).
Discussion
The dual roles of NF-κB in apoptosis and antiapoptosis are determined, in part, by the type of apoptotic stimulus.21 Our data showed that NF-κB decoy ODN increased cell viability in MSCs exposed to UHMWPE particles but had no effect on cell apoptosis, indicating that the decoy ODN effects are more likely due to changes in cell proliferation. The results suggest that excessive NF-κB activation could be one of the mechanisms by which UHMWPE particles reduce MSC viability. Previous reports showed that MSCs treated with LPS increase cell proliferation.22 This phenomenon was not observed in our current studies, which may due to a lower dose of LPS (100 ng/mL) used in our experiments. Interestingly, the cell viability was significantly reduced in the presence of UHMWPE and LPS, which could be reversed by addition of NF-κB decoy ODN. The results suggest that suppression of NF-κB activity could be important in protecting infiltrating MSCs in inflammatory states.23
MSCs can modulate immune responses through secretion of anti-inflammatory cytokines. Cross talk between TGF-β1 and NF-κB is important in preventing an overwhelming inflammatory response.24,25 In human intestinal lamina propria mononuclear cells, treatment with TGF-β1 can suppress NF-κB activation through induction of IκB expression. On the other hand, one study using mouse fibroblast cell lines showed that NF-κB activation can also suppress TGF-β1 signaling.25 Our data showed that inhibition of NF-κB activity in MSCs by decoy ODN induced TGF-β1 expression in the presence of UHMWPE particles. The induced TGF-β1 expression in MSCs exposed to UHMWPE particles may therefore suppress the particle-induced inflammatory response.
TGF-β1 can also play an important role in bone remodeling. Activation of TGF-β1 signaling can increase cell proliferation and early differentiation of osteoprogenitor cells.26 Transgenic animal studies in conditional knockout mice showed that deficiency in TGF-β1 signaling causes bone developmental defects at various locations.26 A previous study showed that a single dose of TGF-β1 reversed the inhibition of bone ingrowth due to the presence of polyethylene particles in a rabbit bone harvest chamber model.17 However, continuous infusion of TGF-β1 in a similar model (without the perturbating influence of particles) showed no significant effect on bone growth.27 This is similar to our observation that NF-κB decoy ODN only reversed osteogenesis when in the presence of UHMWPE particles.
Takai et al. showed that TGF-β1 increased OPG expression in the mouse bone marrow stromal cell line ST2.20 Direct treatment with TGF-β1 on ST2 cells induced transient transcription of OPG mRNA after 3–6 h of treatment. In this study, we showed that OPG induced by the decoy ODN was partially regulated by TGF-β1 in mouse bone marrow-derived MSCs exposed to UHMWPE particles (Fig. 2d).
The RANK/RANKL/OPG system is a dominant regulator of osteoclastogenesis in bone.28 RANKL is mainly expressed by preosteoblasts and stromal cells, while RANK is expressed on the cell surface of osteoclast precursors. Interaction of RANKL with RANK can activate NF-κB signaling in osteoclast precursors and induce cell differentiation so that the cells become mature osteoclasts. Similar to RANKL, OPG is also expressed in preosteoblasts and stromal cells. Secretion of OPG inhibits RANK/RANKL interaction through competitive binding to RANK.28 Therefore, the ratio of RANKL/OPG is a critical factor for osteoclast maturation and activation.28 Expression and activation of RANK/RANKL are tightly correlated with NF-κB signaling. Inhibition of NF-κB activity with decoy ODN may suppress the stimulatory effects of RANK/RANKL signaling in osteoblasts/osteoclasts29 and enhance OPG expression in MSCs/preosteoblasts by an independent pathway. The consequent reduction of RANKL/OPG ratio may result in suppression of osteoclastogenesis. The potential cross talk between MSCs/osteoprogenitors and osteoclasts in the presence of wear particles and NF-κB decoy ODN would be important to evaluate in future coculture studies.
In the absence of particles, we showed that the decoy ODN reduced Runx2, osteopontin, and osteocalcin expression but increased bone mineralization. A similar phenomenon was observed when considering tumor necrosis factor-alpha (TNF-α) effects on bone healing.30 Previous reports suggested that TNF-α enhances osteoclastogenesis and inhibits osteogenesis at an early stage of inflammation, but induces osteogenesis at the later tissue repair stages of inflammation.30 The role of NF-κB in osteogenesis in previous reports31–35 and our current findings may be due to different time points of evaluation, different proinflammatory stimuli, and the specific osteogenic markers tested.
In the presence of particles, we found that several osteogenic markers were enhanced by decoy ODN, except for Runx2. It is likely that NF-κB regulates osteogenesis in a Runx2-independent pathway, such as by β-catenin signaling.36 In other studies, the suppression of NF-κB promoted osteogenesis in primary bone marrow-derived MSCs and conditional NF-κB-deficient mice.31 The suppressive effects of NF-κB on osteogenesis are mediated by direct upregulation of Smurf1/2 expression to enhance β-catenin degradation.31 On the contrary, Cho et al. demonstrated that NF-κB activated by TNF-α promoted osteogenesis through upregulation of a transcriptional coactivator with PDZ-binding motif (TAZ) in adipose-derived MSCs.33 In our experimental model, we confirmed that the genes involved in osteogenic regulation were suppressed by NF-κB decoy ODN (Supplementary Fig. S2). However, the expression of Smurf2 and TAZ was reduced, but the RANKL/OPG ratio was increased in the presence of UHMWPE particles. Both positive and negative regulatory effects on bone formation by NF-κB signaling may occur in MSCs, however, the osteogenic signaling was enhanced by decoy ODN in the presence of UHMWPE particles.
Conclusion
Suppression of NF-κB activity protects cell viability and osteogenic ability of MSCs exposed to UHMWPE particles. Modulation of the inflammatory response induced by UHMWPE wear particles using an NF-κB decoy ODN does not cause significant adverse effects on MSCs and may potentially enhance their protective function (summarized in Fig. 5). The strategic use of NF-κB ODN could potentially mitigate wear particle-induced periprosthetic osteolysis.
FIG. 5.
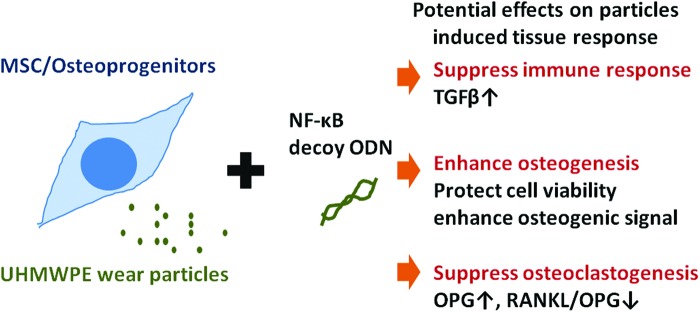
Summary of NF-κB decoy ODN effects on MSCs in the presence of UHMWPE particles. Suppression of NF-κB activity by decoy ODN may protect MSCs from wear particle-induced bone loss using three distinct pathways, including (1) suppressing the immune response, (2) enhancing osteogenesis, and (3) suppressing osteoclastogenesis. Color images available online at www.liebertpub.com/tea
Supplementary Material
Acknowledgments
This work was supported by NIH grants 2R01AR055650 and 1R01AR063717 and the Ellenburg Chair in Surgery at Stanford University.
Disclosure Statement
No competing financial interests exist.
References
- 1.Lin T.H., Tamaki Y., Pajarinen J., Waters H.A., Woo D.K., Yao Z., and Goodman S.B.Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-kappaB as a therapeutic target. Acta Biomater 10,1, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Purdue P.E., Koulouvaris P., Nestor B.J., and Sculco T.P.The central role of wear debris in periprosthetic osteolysis. HSS J 2,102, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodman S.B., and Ma T.Cellular chemotaxis induced by wear particles from joint replacements. Biomaterials 31,5045, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren P.G., Lee S.W., Biswal S., and Goodman S.B.Systemic trafficking of macrophages induced by bone cement particles in nude mice. Biomaterials 29,4760, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibon E., Yao Z., Rao A.J., Zwingenberger S., Batke B., Valladares R., Smith R.L., Biswal S., Gambhir S.S., and Goodman S.B.Effect of a CCR1 receptor antagonist on systemic trafficking of MSCs and polyethylene particle-associated bone loss. Biomaterials 33,3632, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Blanc K., and Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol 12,383, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Lin T.H., Yao Z., Sato T., Keeney M., Li C., Pajarinen J., Yang F., Egashira K., and Goodman S.B.Suppression of wear-particle-induced pro-inflammatory cytokine and chemokine production in macrophages via NF-kappaB decoy oligodeoxynucleotide: a preliminary report. Acta Biomater 10,3747, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peister A., Mellad J.A., Larson B.L., Hall B.M., Gibson L.F., and Prockop D.J.Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood 103,1662, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Huang C.K., Lee S.O., Lai K.P., Ma W.L., Lin T.H., Tsai M.Y., Luo J., and Chang C.Targeting androgen receptor in bone marrow mesenchymal stem cells leads to better transplantation therapy efficacy in liver cirrhosis. Hepatology 57,1550, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Shimizu H., Nakagami H., Morita S., Tsukamoto I., Osako M.K., Nakagami F., Shimosato T., Minobe N., and Morishita R.New treatment of periodontal diseases by using NF-kappaB decoy oligodeoxynucleotides via prevention of bone resorption and promotion of wound healing. Antioxid Redox Signal 11,2065, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Campbell P., Ma S., Yeom B., McKellop H., Schmalzried T.P., and Amstutz H.C.Isolation of predominantly submicron-sized UHMWPE wear particles from periprosthetic tissues. J Biomed Mater Res 29,127, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Houlihan D.D., Mabuchi Y., Morikawa S., Niibe K., Araki D., Suzuki S., Okano H., and Matsuzaki Y.Isolation of mouse mesenchymal stem cells on the basis of expression of Sca-1 and PDGFR-alpha. Nat Protoc 7,2103, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Mafi R., Hindocha S., Mafi P., Griffin M., and Khan W.S.Sources of adult mesenchymal stem cells applicable for musculoskeletal applications—a systematic review of the literature. Open Orthop J 5Suppl 2,242, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Z., Nelson E.R., Smith R.L., and Goodman S.B.The sequential expression profiles of growth factors from osteoprogenitors [correction of osteroprogenitors] to osteoblasts in vitro. Tissue Eng 13,2311, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Zhu H., Guo Z.K., Jiang X.X., Li H., Wang X.Y., Yao H.Y., Zhang Y., and Mao N.A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc 5,550, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Ma S., Xie N., Li W., Yuan B., Shi Y., and Wang Y.Immunobiology of mesenchymal stem cells. Cell Death Differ 21,216, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman S.B., Song Y., Chun L., Regula D., and Aspenberg P. Effects of TGFbeta on bone ingrowth in the presence of polyethylene particles. J Bone Joint Surg Br Vol 81,1069, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Trindade M.C., Lind M., Nakashima Y., Sun D., Goodman S.B., Schurman D.J., and Smith R.L.Interleukin-10 inhibits polymethylmethacrylate particle induced interleukin-6 and tumor necrosis factor-alpha release by human monocyte/macrophages in vitro. Biomaterials 22,2067, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Wong N., Trindade M.C., Patel R., Yaszay B., Goodman S.B., and Smith R.L.Effects of interleukin-10 on titanium particle-induced macrophage transcription factor activation and cytokine expression in vitro. J Biomed Mater Res Part A 69,40, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Takai H., Kanematsu M., Yano K., Tsuda E., Higashio K., Ikeda K., Watanabe K., and Yamada Y.Transforming growth factor-beta stimulates the production of osteoprotegerin/osteoclastogenesis inhibitory factor by bone marrow stromal cells. J Biol Chem 273,27091, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Kaltschmidt B., Kaltschmidt C., Hofmann T.G., Hehner S.P., Droge W., and Schmitz M.L.The pro- or anti-apoptotic function of NF-kappaB is determined by the nature of the apoptotic stimulus. Eur J Biochem 267,3828, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Wang Z.J., Zhang F.M., Wang L.S., Yao Y.W., Zhao Q., and Gao X.Lipopolysaccharides can protect mesenchymal stem cells (MSCs) from oxidative stress-induced apoptosis and enhance proliferation of MSCs via Toll-like receptor(TLR)-4 and PI3K/Akt. Cell Biol Int 33,665, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Zimmerli W., Trampuz A., and Ochsner P.E.Prosthetic-joint infections. N Engl J Med 351,1645, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Monteleone G., Mann J., Monteleone I., Vavassori P., Bremner R., Fantini M., Del Vecchio Blanco G., Tersigni R., Alessandroni L., Mann D., Pallone F., and MacDonald T.T.A failure of transforming growth factor-beta1 negative regulation maintains sustained NF-kappaB activation in gut inflammation. J Biol Chem 279,3925, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Bitzer M., von Gersdorff G., Liang D., Dominguez-Rosales A., Beg A.A., Rojkind M., and Bottinger E.P.A mechanism of suppression of TGF-beta/SMAD signaling by NF-kappa B/RelA. Genes Dev 14,187, 2000 [PMC free article] [PubMed] [Google Scholar]
- 26.Chen G., Deng C., and Li Y.P.TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 8,272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman S., Song Y., Chun L., Aspenberg P., Plouhar P., Glancy T., Regula D., and Smith R.L.Effects of local infusion of TGFbeta on bone ingrowth in rabbit chambers. J Biomed Mater Res 53,475, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Khosla S.Minireview: the OPG/RANKL/RANK system. Endocrinology 142,5050, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Wada T., Nakashima T., Hiroshi N., and Penninger J.M.RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med 12,17, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Osta B., Benedetti G., and Miossec P.Classical and paradoxical effects of TNF-α on bone homeostasis. Front Immunol 5,48, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang J., Liu F., Lee M., Wu B., Ting K., Zara J.N., Soo C., Al Hezaimi K., Zou W., Chen X., Mooney D.J., and Wang C.Y.NF-kappaB inhibits osteogenic differentiation of mesenchymal stem cells by promoting beta-catenin degradation. Proc Natl Acad Sci U S A 110,9469, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X., Hu C., Wang G., Li L., Kong X., Ding Y., and Jin Y.Nuclear factor-kappaB modulates osteogenesis of periodontal ligament stem cells through competition with beta-catenin signaling in inflammatory microenvironments. Cell Death Dis 4,e510, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho H.H., Shin K.K., Kim Y.J., Song J.S., Kim J.M., Bae Y.C., Kim C.D., and Jung J.S.NF-kappaB activation stimulates osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue by increasing TAZ expression. J Cell Physiol 223,168, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Nakamura H., Aoki K., Masuda W., Alles N., Nagano K., Fukushima H., Osawa K., Yasuda H., Nakamura I., Mikuni-Takagaki Y., Ohya K., Maki K., and Jimi E.Disruption of NF-kappaB1 prevents bone loss caused by mechanical unloading. J Bone Miner Res 28,1457, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Otero J.E., Chen T., Zhang K., and Abu-Amer Y.Constitutively active canonical NF-kappaB pathway induces severe bone loss in mice. PLoS One 7,e38694, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James A.W.Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica 2013,684736, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.