Abstract
Background
Concurrent chemoradiotherapy is the current standard treatment for inoperable stage III non-small cell lung cancer (NSCLC). In this study we aimed to investigate the efficacy and toxicity of CCRT with split dose of cisplatin (30 mg/m2) and vinorelbine (20 mg/m2) in patients with inoperable stage III NSCLC followed in our oncology clinic.
Material/Methods
Medical records of 97 patients with inoperable stage III NSCLC treated with concurrent chemoradiotherapy with cisplatin-vinorelbine were retrospectively analyzed. Cisplatin (30 mg/m2) and vinorelbine (20 mg/m2) were administered on days 1, 8, 22, and 29 during radiotherapy. Two cycles of consolidation chemotherapy were given. All patient data, including pathological, clinical, radiological, biochemical, and hematological data, were assessed retrospectively using our database system.
Results
Our study included 97 unresectable stage III NSCLC patients who were treated with CCRT. Median age was 58 years old (range 39–75) and 87 (89.7%) of the patients were men. ECOG performance score was 0–1 in 93 patients (95.9%). Squamous histology, the most common histology, was diagnosed in 46 patients (47.4%). Median follow-up time was 23.8 months. Median progression-free survival (PFS) and median overall survival time (OS) were 10.3 months and 17.8 months, respectively. Objective response rate and clinical benefit rate were 75.3% and 83.5%, respectively. Distant and local relapse rate were 57.1% and 42.9%, respectively. Hematological and non-hematological grade 3–4 toxicities were seen in 13 (13.4%) and 16 (16.5%) patients, respectively. Six (6.1%) patients died due to toxicity.
Conclusions
The results of this study suggest that split-dose cisplatin may offer fewer grade III–IV toxicities without sacrificing efficacy and could be an option in patients with inoperable stage III NSCLC during CCRT. Similar to past studies, despite high response rate during CCRT, distant relapse is the major parameter that influences patient survival in long-term in NSCLC.
MeSH Keywords: Chemoradiotherapy, Cisplatin, Lung Neoplasms, Vinca Alkaloids
Background
Lung cancer is the leading cause of cancer-related death among all types of cancers [1]. Non-small cell lung cancer (NSCLC) accounts for the majority of cases and one-third of them presented at locally advanced stage [2]. Concurrent chemoradiotherapy (CCRT) has been accepted as a standard treatment modality for patients with unresectable stage III NSCLC disease. CCRT provides better overall survival (OS) compared to sequential chemotherapy followed by radiotherapy or thoracic radiotherapy only [3–5]. Despite the high response rate with CCRT, median overall survival time for these patients is 15–25 months. The optimal chemotherapy regimen in CCRT has not been clearly defined. The most commonly used regimens are mitomycin-vindesine and cisplatin, etoposide and cisplatin, paclitaxel and carboplatin, and vinorelbine and cisplatin [3–6]. There is no phase III randomized trial comparing cisplatin-based regimens during CCRT and studies in the literature report similar response and survival rates.
With this study, we aimed to investigate the efficacy and toxicity of CCRT with split low-dose cisplatin (30 mg/m2) and vinorelbine (20 mg/m2) during radiotherapy and 2 additional cycles of consolidation therapy after CCRT with the same drugs in patients with inoperable stage III NSCLC followed in our oncology clinic.
Material and Methods
Patient population
We retrospectively analyzed medical records from 2006 to 2012 of 97 patients with inoperable stage IIIA and IIIB NSCLC treated with concurrent chemoradiotherapy with cisplatin and vinorelbine. All data of the patients, including pathological, clinical, radiological, biochemical, and hematological data, were assessed retrospectively using our database system.
Staging was determined according to the TNM classification seventh edition [7]. This study was approved by the Baskent University Institutional Review Board and supported by the Baskent University Research Fund.
All patients had histologically confirmed of non-small cell lung carcinoma. PET-CT (18F-FDG) scans and cranial MRI were used for staging and radiotherapy planning for all patients. The decision regarding mediastinal lymph node sampling was made by the thoracic tumor board.
Chemotherapy and radiotherapy
Cisplatin (30 mg/m2) and vinorelbine (20 mg/m2) from peripheral route were administered on days 1, 8, 22, and 29 during radiotherapy. Two cycles of consolidation chemotherapy (total 4 cycles) with the same drugs were given as cisplatin (80 mg/m2) and vinorelbine (25 mg/m2).
For thoracic radiotherapy (TRT), 3-dimensional conformal radiation therapy (3D-CRT) utilizing co-registered FDG-PET-CT-based treatment planning was delivered to all patients. Gross tumor volume (GTV) included all primary tumor(s) and abnormally enlarged hilar or mediastinal lymph nodes greater than 1 cm in diameter seen on CT or metabolically active areas on PET-CT images. Clinical target volumes (CTVs) were defined by adding 1-cm margins to GTVs, and elective nodal stations were not included in the CTV. Planning target volume-1 (PTV1) was created by adding an additional 1.5-cm margin to CTVs, and PTV2 (boost field) was defined as the GTVs plus a 1.5-cm margin. For all patients, 3D-CRT plans were aimed to minimize the volume of normal lung and surrounding normal tissues irradiated while providing coverage of PTVs by isodose surfaces between 95% and 107%. TRT was delivered through the anteroposterior-posteroanterior portals with customized multi-leaf collimators for PTV 1–46 Gy/23 fractions, followed by off-spinal cord oblique boost dose up to 66 Gy/33 fractions for PTV 2.
Toxicity
Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria Adverse Events (CTCAE) version 3.0. Patients were evaluated weekly during concurrent CRT and consolidation chemotherapy.
Response evaluation
Response to treatment was defined by using RECIST criteria [8]. Clinical examination at every 3 months and radiological examination at every 6 months imaging were used for patient surveillance. Types of first relapses (distant and local) were recorded.
Statistics
All results are presented as rate for categorical values or mean and median for continuous variables. Overall survival (OS) was determined as time between histological diagnosis and death/last control. Progression-free survival (PFS) was determined as time between diagnosis and progression or death. Survival curves were estimated according to the Kaplan-Meier method and log-rank test was used for univariate comparisons. Adjusted hazard ratios (HRs) and 95% confidence intervals (95% CIs) were used for estimation. All data were analyzed using SPSS version 17.0 (SPSS Inc, Chicago, IL) and a p value of <0.05 was considered statistically significant.
Results
After analyzing the medical records, 97 consecutive unresectable stage IIIA-B NSCLC patients treated with CCRT in our centre were included into this study. Patients’ baseline characteristics are shown in Table 1. Median age was 58 years old (range 39–75), and 87 (89.7%) of the patients were men. ECOG performance score was 0–1 in 93 patients (95.9%). Squamous histology, the most common histology, was diagnosed in 46 patients (47.4%). There were 41 (42.3%) stage IIIA patients and 56 (57.7%) stage IIIB patients.
Table 1.
Patient characteristics.
| No. of patients | % | |
|---|---|---|
| Age, years | ||
| <65 | 78 | 80.4 |
| 65+ | 19 | 19.6 |
| Sex | ||
| Male | 87 | 89.7 |
| Female | 10 | 10.3 |
| ECOG PS | ||
| 0 | 26 | 26.8 |
| 1 | 67 | 69.1 |
| 2 | 4 | 4.1 |
| Smoking | ||
| Yes | 93 | 95.9 |
| No | 4 | 4.1 |
| Histology | ||
| Squamous | 46 | 47.4 |
| Adenocarcinoma | 41 | 42.3 |
| Others | 10 | 10.3 |
| Comorbidities | ||
| Yes | 22 | 22.7 |
| No | 75 | 77.3 |
| AJCC stage | ||
| IIIA | 41 | 42.2 |
| IIIB | 56 | 57.8 |
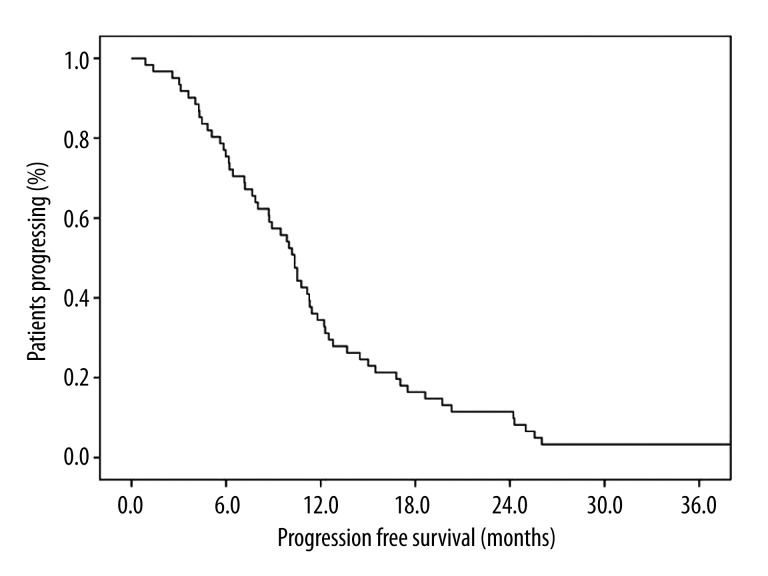
Objective response rate (CR+PR) and clinical benefit rate (CR+PR+SD) were 75.3% and 83.5%, respectively (Table 2). Median follow-up time was 23.8 months (range 0.9–60). Median PFS and OS were 10.3 ([(95% CIs), 9.2–11.5) and 17.8 months ([(95% CIs), 11.4–24.4) (Figures 1 and 2). When we evaluated the 61 (62.8%) patients who relapsed, distant and local relapse rates were 57.1 and 42.9%, respectively.
Table 2.
Response rates.
| No. of patients | % | |
|---|---|---|
| Progressive disease | 7 | 7.2 |
| Stable disease | 8 | 8.2 |
| Partial response | 55 | 56.7 |
| Complete response | 18 | 18.6 |
Figure 1.
Kaplan-Meier progression-free survival of patients treated with concurrent chemoradiotherapy with split low-dose cisplatin-vinorelbine followed by 2 additional courses of consolidation chemotherapy, 10.3 months ([(95% CIs), 9.2–11.5).
Figure 2.
Kaplan-Meier overall survival of patients treated with concurrent chemoradiotherapy with split low-dose cisplatin-vinorelbine followed by 2 additional courses of consolidation chemotherapy, 17.8 months ([(95% CIs), 11.4–24.4).
In the whole group, 92.8% of patients had received the planned therapy of CCRT and consolidation chemotherapy. The reason for not completing full course chemotherapy and CCRT was treatment related to death in 4 patients. During the study period, 43 patients died. Response evaluation could not be done in 9 patients: 2 were lost during CCRT, 4 died during consolidation chemotherapy, and 3 were lost to follow-up. Furthermore, hematological and non-hematological grade 3–4 toxicities were seen in 13 (13.4%) and 16 (16.5%) patients, respectively (Table 3). Additionally, drug extravasation occurred in 6 (6.2%) patients during the chemotherapy regimen, and we did hot compression and close follow-up. All patients recovered without any sequel from extravasation.
Table 3.
Major toxicities during CCRT and consolidation chemotherapy.
| Grade 3 and 4 toxicities (CCRT) | ||
|---|---|---|
| Toxicities | No. of patients | % |
| Hematological toxicities | 13 | 13.4 |
| Anemia | 7 | 7.2 |
| Neutropenia | 8 | 8.2 |
| Febrile Neutropenia | 6 | 6.1 |
| Thrombocytopenia | 2 | 2.0 |
| Non-hematological toxicities | 16 | 16.5 |
| Esophagitis | 13 | 13.4 |
| Renal toxicity | 2 | 2.0 |
| Pulmonary toxicity | 6 | 6.1 |
| Rate of mortality | 4 | 4.1 |
| Pulmonary toxicity | 2 | 2.0 |
| Febrile neutropenia | 3* | 3.0 |
One patient also had pulmonary toxicity.
Discussion
NSCLC is still the leading cause of cancer-associated death in both women and men. Despite advances in monitoring and radiologic imaging techniques, most patients were diagnosis at advanced stage. CCRT is the standard treatment for unresectable stage III NSCLC patients. Data in the literature strongly suggest that CCRT significantly improves survival rates when compared to thoracic radiotherapy and sequential chemoradiotherapy [3–5]. Although CCRT is the standard, the best chemotherapy combination is not known. Randomized trials demonstrated that full-dose chemotherapy with cisplatin-based regimen improves long-term survival compared with sequential chemoradiotherapy, but using full-dose chemotherapy resulted in excessive toxicity [3,4,9,10]. There is no published phase III randomized trial that directly compares different combination regimens in CCRT, but cisplatin plus etoposide and weekly carboplatin plus paclitaxel have been most commonly used combination regimens with thoracic radiotherapy. These combinations have been demonstrated to be effective and safe in randomized studies [6,11–13], with reported median overall survival times between 15 and 23 months. Failure of loco-regional control was reported at around 20%, and failure at distant sites was far more commonly reported than local relapse and major cause of death [6,11].
Vinorelbine is a semi-synthetic vinca alkaloid and is the most active member of this group against NSCLC. The combination of cisplatin-vinorelbine was shown to be effective in advanced and adjuvant setting of NSCLC [14–16]. Phase II studies showed that cisplatin-vinorelbine is also an effective and safe radio sensitizer in CCRT, with median overall survival time between 21 and 23 months [17,18]. These studies used vinorelbine dose of 20 mg/m2, not the usual 25 mg/m2 dose used in metastatic or adjuvant settings.
With this study, we reported results of 97 unresectable stage III NSCLC patients treated with CCRT associated with split low dose of cisplatin-vinorelbine and followed by 2 cycles of the same drugs. Of the total, 9 patients did not receive protocol treatment. Median PFS and OS were 10.3 [(95% CIs), 9.2–11.5)] and 17.8 months [(95% CIs), 11.4–24.4)], respectively. These results were comparable with other studies in the literature [4–6,9–13].
PET-CT plays an important role in the evaluation of patients with stage III lung cancer and is recommended. When compared with CT imaging, PET-CT significantly increases sensitivity and specificity of radiological staging, especially in evaluation of mediastinal lymph nodes. However, in areas of endemic granulomatous infection, PET has been shown to increase the rates of false-positives in mediastinal lymph nodes. Therefore, positive PET-CT findings should be checked with tissue confirmation with invasive interventions [19,20]. PET-CT was used in 97 patients (100%) for clinical staging and 3D-radiotherapy planning. Treatment response was also evaluated by PET-CT in 57 patients (64.4%). In this study, we actively used PET-CT for staging. Diagnostic mediastinoscopy was used in 14 (14.4%) patients for staging purposes. We believe that mediastinoscopy should be done in every case with FDG positive mediastinal lymph nodes; this is a weakness of our study. All patients were discussed by the multi-disciplinary board, but rejection of procedure by patients, technical difficulties of reaching to FDG-positive lymph nodes, and having T4 tumors were major reasons for not doing mediastinoscopy routinely.
Median follow-up period was 23.8 months [(95%CIs 0.9–60)]. Median OS and PFS were 17.8 months [(95% CIs) 11.4–24.4] and 10.3 months [(95% CIs), 9.2–11.5], respectively. In the literature, CCRT studies reported median OS and PFS between 12–25 and 10–15 months, respectively. In our study, 4 (4.1%) patients had ECOG performance of 2, and all of these patients were dead in less than 6 months. Although our results showed that our OS and PFS was at the lower edge of that in the literature, this study included patients with low performance scale and we think that our study truly reflects the situation of real clinical practice.
Objective response rate (CR+PR) and disease control rate (CR+PR+SD) were determined as 75.3% and 85%, respectively. This response rate in this study is quite close the other studies in the literature. During the follow-up period 61 (63%) patients relapsed at systemic and local site in 57.1% and 42.9%, respectively. This result showed that most patients with unresectable stage III NSCLC have a high distant relapse rate, systemic disease at diagnosis, and poor long-term survival rates. Because of high relapse rate at distant sites, consolidation chemotherapy had been added to CCRT, either with the same regimen or switched to another chemotherapy regimen. The positive effect of consolidation chemotherapy after CCRT with either the same or switched regimen has not been shown in the literature. We used the same chemotherapy regimen (cisplatin-vinorelbine) for 2 more cycles, similar to other studies.
Completion of full planed treatment was achieved in 88 (92.8%) patients. The occurrence of severe hematological and non-hematological grade III and IV toxicities were detected in 13 (13.4%) and 16 (16.5%) patients, respectively. In the literature, no chemotherapy regimen incorporated to CCRT showed a clear advantage over other regimens in efficacy. A trial that compared cisplatin-vinorelbine, cisplatin-paclitaxel, and cisplatin-gemcitabine in CCRT showed similar efficacy but different toxicity profiles of these regimens. Their conclusion was cisplatin-gemcitabine may have unacceptable toxicity compared to other regimens [21]. Mariano et al. reported cisplatin-vinorelbine had less toxicity and had the highest completion rate of full treatment course in a review article [22]. We showed that split low-dose cisplatin-vinorelbine with CCRT seemed to be less toxic and that the completion rate of full treatment course was higher when compared to that in the literature. Therefore, we suggest that decreasing the cisplatin and vinorelbine dose and giving split dose of cisplatin may provide less toxicity without sacrificing efficacy.
Conclusions
The current study showed that split low-dose cisplatin-vinorelbine provides results similar to that in the literature regarding response rate and survival parameters and it may be an option for CCRT in patients with unresectable stage III NSCLC. We reported a higher rate of treatment-related death (4 patients had performance score of 2 at the basal state) and lower rates of grade 3 and 4 hematological and non-hematological toxicities when compared with the literature. Furthermore, results of this study suggest that split low-dose cisplatin-vinorelbine may decrease grade III and IV toxicities during CCRT without decreasing the efficacy. Similar to past studies, despite a high response rate during CCRT, distant relapse is the major parameter that influences patient survival on long-term in NSCLC. Developing more effective systemic treatment is key for long-term success in NSCLC.
Footnotes
Source of support: Departmental sources
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Van Meerbeeck JP. Staging of non-small cell lung cancer: Consensus, controversies and challenges. Lung Cancer. 2001;34(S2):95–107. doi: 10.1016/s0169-5002(01)00356-7. [DOI] [PubMed] [Google Scholar]
- 3.Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non–small-cell lung cancer. J ClinOncol. 1999;17:2692–99. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 4.Curran WJ, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J. Natl Cancer Inst. 2011;103:1452–60. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vokes EE, Herndon JE, Kelley MJ, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III nonsmall cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol. 2007;25:1698–704. doi: 10.1200/JCO.2006.07.3569. [DOI] [PubMed] [Google Scholar]
- 6.Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23:5883–91. doi: 10.1200/JCO.2005.55.405. [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–74. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 8.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 9.Zatloukal P, Petruzelka L, Zemanova M, et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer. 2004;46:87–98. doi: 10.1016/j.lungcan.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95-01 Study. J Clin Oncol. 2005;23:5910–17. doi: 10.1200/JCO.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 11.Albain KS, Crowley JJ, Turrisi AT, et al. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: a Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol. 2002;20:3454–60. doi: 10.1200/JCO.2002.03.055. [DOI] [PubMed] [Google Scholar]
- 12.Gandara DR, Chansky K, Albain KS, et al. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol. 2003;21:2004–10. doi: 10.1200/JCO.2003.04.197. [DOI] [PubMed] [Google Scholar]
- 13.Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol. 2008;26:5755–60. doi: 10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 14.Furuse K, Ohta M, Fukuoka M, et al. [Early phase II clinical study of KW-2307 in patients with lung cancer. Lung Cancer Section in KW-2307 Study Group]. Gan To Kagaku Ryoho. 1994;21:785–93. [in Japanese] [PubMed] [Google Scholar]
- 15.Le Chevalier T, Brisgand D, Douillard JY, et al. Randomized study of vinorelbine and cisplatin versus vindesine and cisplatin versus vinorelbine alone in advanced non-small-cell lung cancer: results of a European multicenter trial including 612 patients. J Clin Oncol. 1994;12:360–67. doi: 10.1200/JCO.1994.12.2.360. [DOI] [PubMed] [Google Scholar]
- 16.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–27. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 17.Hirose T, Mizutani Y, Ohmori T, et al. The combination of cisplatin and vinorelbine with concurrent thoracic radiation therapy for locally advanced stage IIIA or IIIB non-small-cell lung cancer. Cancer Chemother Pharmacol. 2006;58:361–67. doi: 10.1007/s00280-005-0164-7. [DOI] [PubMed] [Google Scholar]
- 18.Naito Y, Kubota K, Nihei K, et al. Concurrent chemoradiotherapy with cisplatin and vinorelbine for stage III non-small cell lung cancer. J Thorac Oncol. 2008;3:617–22. doi: 10.1097/JTO.0b013e3181753b38. [DOI] [PubMed] [Google Scholar]
- 19.Terán MD, Brock MV. Staging lymph node metastases from lung cancer in the mediastinum. J Thorac Dis. 2014;6(3):230–36. doi: 10.3978/j.issn.2072-1439.2013.12.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sümbül AT, Sezer A, Abalı H, et al. An Old Enemy Not to be Forgotten during PET CT Scanning of Cancer Patients: Tuberculosis. Contemp Oncol (Pozn) 2014;18:1–4. doi: 10.5114/wo.2014.43985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vokes EE, Herndon JE, Crawford J, et al. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: cancer and leukemia group B study 9431. J Clin Oncol. 2002;20:4191–98. doi: 10.1200/JCO.2002.03.054. [DOI] [PubMed] [Google Scholar]
- 22.Provencio M, Isla D, Sánchez A, Cantos B. Inoperable stage III non-small cell lung cancer: Current treatment and role of vinorelbine. J Thorac Dis. 2011;3:197–204. doi: 10.3978/j.issn.2072-1439.2011.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]