Abstract
Human amnion epithelial cells (hAECs) derived from term or pre-term amnion membranes have attracted attention from researchers and clinicians as a potential source of cells for regenerative medicine. The reason for this interest is evidence that these cells have highly multipotent differentiation ability, low immunogenicity, and anti-inflammatory functions. These properties have prompted researchers to investigate the potential of hAECs to be used to treat a variety of diseases and disorders in pre-clinical animal studies with much success.
hAECs have found widespread application for the treatment of a range of diseases and disorders. Potential clinical applications of hAECs include the treatment of stroke, multiple sclerosis, liver disease, diabetes and chronic and acute lung diseases. Progressing from pre-clinical animal studies into clinical trials requires a higher standard of quality control and safety for cell therapy products. For safety and quality control considerations, it is preferred that cell isolation protocols use animal product-free reagents.
We have developed protocols to allow researchers to isolate, cryopreserve and culture hAECs using animal product-free reagents. The advantage of this method is that these cells can be isolated, characterized, cryopreserved and cultured without the risk of delivering potentially harmful animal pathogens to humans, while maintaining suitable cell yields, viabilities and growth potential. For researchers moving from pre-clinical animal studies to clinical trials, these methodologies will greatly accelerate regulatory approval, decrease risks and improve the quality of their therapeutic cell population.
Keywords: Medicine, Issue 94, Amnion Membrane, Amniotic, Stem Cells, Epithelial, Cell Therapy, Perinatal, Placenta
Introduction
Cells derived from perinatal sources, such as the placenta, placental membranes, umbilical cord and amniotic fluid have attracted attention from researchers and clinicians as a potential source of cells for regenerative medicine1,2. The reason for this interest is that these cell types all possess some degree of plasticity and immunomodulatory capability3, properties that are fundamental to their potential therapeutic applications.
hAECs are a heterogeneous epithelial population that can be derived from term or pre-term amnion membrane4, providing an abundant potential source of regenerative cellular material. The properties that make hAECs appealing as a cellular therapy include their multipotency, low immunogenicity, and anti-inflammatory properties. hAECs have been found to be highly multipotent both in vitro and in vivo, capable of differentiating into mesodermal lineages (cardiomyocytes, myocytes, osteocytes, adipocytes), endodermal lineages (pancreatic cells, hepatic cells, lung cells) and ectodermal lineages (hair, skin, neural cells and astrocytes)5-10.
Reassuringly, despite their multipotency hAECs do not appear to either form tumors or promote tumour development in vivo. Furthermore, hAECs are also immune privileged, expressing low levels of class II human leukocyte antigens (HLAs)8. This property likely underlies their ability to evade immune rejection after allogeneic and xenogenic transplantation, as demonstrated in studies using immune competent monkeys, rabbits, guinea pigs, rats, and pigs11-13. hAECs display potent immunomodulatory and immunosuppressive properties and thus offer significant practical advantages for potential clinical applications in autoimmune disease therapy. hAECs are believed to exert immunomodulatory functions on both the innate and adaptive immune systems. One of the mechanisms suggested, is through the secretion of immunomodulatory factors14.
Current applications of hAECs in pre-clinical animal disease models include the treatment of stroke, multiple sclerosis, liver disease, diabetes and chronic and acute lung diseases. Researchers have shown interest in using hAECs to treat post-stroke brain inflammation due to their unique properties. There is evidence that hAECs can cross the blood brain barrier where they can engraft, survive for up to 60 days, differentiate into neurons, decrease inflammation and promote regeneration of damaged central nervous system tissue in animal models of neurological diseases15.
hAECs offer the ability to target and reverse multiple pathological pathways that contribute to the development and progression of multiple sclerosis. For example, results from pre-clinical animal studies suggest that hAECs are strongly immunosuppressive and can potentially induce peripheral immune tolerance and reverse ongoing inflammatory responses. hAECs have also been shown to have the capacity to differentiate into neural cells in vivo and enhance endogenous neuroregeneration through the secretion of a vast array of neurotrophic factors16.
Human and rodent amnion epithelial cells have already demonstrated their therapeutic efficacy for the treatment of liver disease in animal models. In a carbon tetrachloride damage induction model of liver disease, hAEC transplantation lead to engraftment of viable hAECs in the liver, accompanied with reduced hepatocyte apoptosis, and decreased hepatic inflammation and fibrosis17.
hAECs can be stimulated to expressed pancreatic factors including insulin and glucose transporters. Several studies have investigated the potential for hAECs to restore blood glucose levels in diabetic mice18. In mice receiving hAECs, both animal body weight and blood glucose levels decreased to normal levels following injection of cells. These studies present a strong case for the use of hAECs for the treatment of diabetes mellitus.
hAECs have a proven role in the prevention and repair of experimental acute and chronic lung injury in both adult and neonatal models19. These studies found that hAECs differentiate in vitro into functional lung epithelial cells expressing multiple lung-associated proteins, including Cystic Fibrosis Transmembrane Conductance Regulator (CFTR), the ion channel that is mutated in patients with cystic fibrosis20. Additionally, when hAECs are delivered to the injured adult and neonatal lung, they exert their reparative effects via the modulation of host immune cells, reducing pulmonary leucocyte recruitment, including neutrophils, macrophages and lymphocytes21-23.
Given their abundance, safety record, and proven clinical applications for multiple diseases, clinical trials using hAECs is inevitable. With the goal of accelerating the translation of hAEC therapies into clinical-trials, we developed methods to isolate, cryopreserve and culture hAECs in a manner suitable for clinical trials, using animal product-free reagents in accordance with current good manufacturing practices (cGMP) guidelines.
We based this protocol a previously published protocol that we were using successfully to isolate hAECs using animal-derived reagents6. We altered the original protocol to replace animal-derived products with animal product-free reagents, and subsequent optimization was performed to optimize cell yield, viability and purity. Our goal was to develop a protocol that would comply with regulatory standards for cell manufacturing for human clinical trials.
Protocol
NOTE: Placentae should be collected from singleton healthy pregnancies, with a preference for term elective caesarean sections. Written, informed consent should be given for the collection of their placenta. Your relevant human research ethics committee should approval all collection and use of human tissues.
1. Isolation of Amnion Epithelial Cells
Place the placenta onto a sterile surface within a class II biological safety cabinet.
Using sterile hanks balanced salt solution (HBSS), wash as much blood as possible from the placenta surface and membranes.
With the placenta placed with the umbilical cord facing up, mechanically separate the outer edge of the amnion and chorion membranes.
Once separated, manually strip the amnion membrane from the chorion membrane of the placenta, working around the edge of the membrane, towards the umbilical cord. NOTE: Be careful to remove any pieces of contaminating chorion membrane from the amnion.
Using sterile scissors, cut the amnion membrane approximately 2 cm from the base of the umbilical cord and place in a 500 ml sealed container containing 250 ml HBSS.
Wash thoroughly by shaking the sealed bottle containing the amnion membrane.
Remove the amnion membrane from the collection container using sterile forceps, and place into a 15 cm Petri dish.
Cut the amnion membrane into pieces approximately 5 cm long (when held vertically with forceps), discarding bloody or torn pieces.
Place in a 500 ml sealed container and wash with 250 ml HBSS approximately 3-5 times or until the amnion membrane becomes translucent with no contaminating blood. NOTE: Any blood present may reduce the efficiency of the enzymatic digest solution.
Transfer all pieces of amnion membrane into a container containing 50 ml of the enzymatic digest solution, taking care to minimize carryover of HBSS.
Incubate the amnion pieces in enzymatic digest solution in a water bath or warm room at 37 °C for 15 min with gentle agitation to remove any remaining blood.
Remove the amnion membrane pieces from the enzymatic digest solution and place in a new sterile container.
Add 100 ml of fresh enzymatic digest solution to the amnion membrane and incubate at 37 °C for 60 min in a water bath or warm room with gentle shaking (first digestion).
Remove the amnion membrane pieces and place into new container taking care to allow the enzymatic digest solution to drain from each piece of membrane. NOTE: This can be achieved by dragging amnion pieces along the inside rim of the container.
Add 100 ml of fresh enzymatic digest solution to the amnion membrane pieces and incubate 60 min in a water bath or warm room at 37 °C with gentle shaking (second digestion).
Pass the cell suspension from Step 1.14 through a 70 µm filter and centrifuge at 1,000 x g for 10 min.
Remove and discard the supernatant, and resuspend the cell pellet in 4 ml HBSS containing 1 mg/ml soybean-based enzyme inhibitor. Gently pipette repeatedly with a 1 ml pipette to obtain a single cell suspension. NOTE: At this stage cells can be left on ice until cells from the second digest are at the same stage of processing.
Remove the amnion membrane pieces and place into new container taking care to allow the enzymatic digest solution to drain from each piece of membrane. NOTE: This can be achieved by dragging amnion pieces along the inside rim of the container.
Pass the cell suspension from Step 1.18 through a 70 µm filter and centrifuge at 1,000 x g for 10 min.
Remove and discard the supernatant, and resuspend the cell pellet in 4 ml HBSS containing 1 mg/ml soybean-based enzyme inhibitor. Gently pipette repeatedly with a 1 ml pipette to obtain a single cell suspension.
Pool cells from the two digests or alternatively keep cell digests separated until after cell counts. NOTE: This prevents mixing of potentially low viability cells with high viability cells.
Add HBSS to a final volume of 10-20 ml and perform cell counts using trypan blue to measure viability.
Confirm the epithelial cell phenotype by flow cytometry. Make up appropriate antibody cocktails using anti-EpCAM-PE (1:2 dilution) anti-CD90-PeCy5 (1:250) and anti-CD105-APC (1:100) in HBSS.
Resuspend a subset of cells from one or both digests in the antibody cocktail at a concentration of 25 x 106 cells/ml.
For flow cytometry, stain 1 x 106 cells with antibody cocktails containing isotype controls (eg. IgG1 isotype control-PE + anti-CD90-PeCy5 + anti-CD105-APC). Keep ~1 x 106 cells unstained for flow cytometer set-up.
Incubate cells in antibody cocktails at 4 °C for 60 min.
Wash cells 3x in HBSS and resuspend at 5 to 10 million cells per ml for flow cytometry.
Perform flow cytometry and note results. The purity of the cell isolate (the percentage of EpCAM positive and CD90/CD105 negative cells) is expected to be 90-95% and <1% respectively.
Set aside a suitable number of cells for testing for appropriate quality control or release criteria as determined by final application. NOTE: This can include disease/viral screening, or other safety or biological criteria.
For the remaining cells, follow the cryopreservation protocol, or alternatively place directly into culture (direct to culture step 3.6).
2. Cryopreservation of hAECs
Determine cell number and viability using flow cytometry data or manual counting with trypan blue exclusion.
Centrifuge at 700 x g for 5 min.
Resuspend cells between 1-10 million cells per ml in animal product-free cryopreservation media.
Pipette a suitable volume of cells into O-ringed cryopreservation vials and place into a controlled rate freezing apparatus where cooling occurs at approximately 1°C/min until -80°C is achieved.
Transfer vials into liquid nitrogen for long-term storage.
3. Thawing and Culture of Cyropreserved hAECs
Remove cryopreservation vials from liquid nitrogen and place on ice. NOTE: For transfer only - proceed rapidly to next step.
Thaw the cryopreservation vials rapidly at 37 °C until only a small piece (~5 mm x 5 mm) of ice remains. NOTE: It is important that the cell solution does not warm to reduce the toxic effects of DMSO in the cryopreservation media.
Dilute cryopreservation media 10-fold with cold (~4 °C) serum-free media, added drop-wise in a suitable sized tube or container.
Centrifuge at 700 x g for 5 min at RT.
Resuspend in serum-free medium, to an approximate concentration of 1 million cells per ml. Determine cell number and viability using automated or manual counting with trypan blue exclusion. Expected viability post-thaw will be 5-10% less than pre-cryopreservation viability.
Plate cells on standard cell culture-treated plastic ware, or alternatively use Type I collagen to coat culture surfaces.
- For Type I Collagen coating follow the protocol below
- Prepare acid soluble Type I Collagen from human placenta (15 mg collagen with 25 ml deionized water and 50 µl glacial acetic acid).
- Cover beaker with Parafilm and stir moderately at 37 °C until collagen strands are dissolved.
- Sterile filter collagen solution using a 0.22 µm filter.
- Dilute the filtered collagen stock 1:10 with deionized water. This diluted stock is the working concentration solution for coating plastic and membrane surfaces.
- Collagen coat plastic, glass and membrane surfaces for 2 hr at RT at 37°C, or O/N at 2-8 °C.
- Remove excess fluid from the coated surface, and allow it to dry O/N.
- Rinse with sterile tissue culture grade water or HBSS before introducing cells and medium.
Plate hAECs at a density of 25,000 cells/cm2 in serum-free medium, and allow attachment to occur for 72-96 hr.
Following attachment, change media by carefully aspirating and replacing with a suitable volume of serum-free medium every 2-3 days.
- Passage cells using the protocol below
- Aspirate cell culture media and wash with 1x volume of sterile HBSS
- Add 1x volume of enzymatic digest solution and incubate for 5-15 min, or until cells are observed detaching from the cell culture surface.
- Collect enzymatic digest solution containing cells, ensuring cells are detached from the cell culture surface by repeatedly pipetting the enzymatic digest solution against the cell culture surface
- Centrifuge at 700 x g for 5 min.
- Resuspend the cell pellet in a suitable volume of HBSS containing 1 mg/ml soybean-based enzyme inhibitor. Gently pipette repeatedly with a 1ml pipette to obtain a single cell suspension.
- Add serum-free medium to an approximate concentration of 1 million cells/ml. Determine cell number and viability using automated or manual counting with trypan blue exclusion.
- Plate hAECs at a density of 25,000 cells/cm2 in serum-free medium, and continue with standard culturing methods.
Representative Results
When this procedure is followed correctly, an average yield of 120 million hAECs should be expected, with a typical range of 80-160 million cells. From these yields, an average viability of 83 ± 4% can be expected. The increased average yield and slightly lower viability in the clinical method may be due to higher trypsin activity than the animal-derived product, and perhaps also due to the lack of serum proteins. Isolated hAECs have an average cell surface profile of 92% EpCAM positive cells with <1% CD90, CD105 mesenchymal marker positive cells. These markers were selected due to their specificity for epithelial24,25 and mesenchymal26 lineages respectively. This process can take approximately 4-5 hr to complete (Figure 1).
Cryopreservation requires 20-60 min depending on the number of vials and final cell yield. We have previously tested a range of cryopreservation media and found that many animal product-free media perform suitably, however the optimal DMSO concentration is approximately 5-10%. Typically one can expect the viability post-thaw to be 5-10% less than pre-cryopreservation viability, and this loss can be significantly greater for the inexperienced user (Figure 2).
Cell culture can be performed with hAECs to enable characterization, differentiation, or other specific in vitro procedures. These cells initially attach to cell surfaces with an efficiency of 50-80% (personal observation). This increases to 70-90% with subsequent passages. Attachment may be increased with the use of surface coating materials such as collagen type I, or similar products. hAECs maintain their epithelial phenotype in serum-free culture media (Figure 3). We have found significant changes in cell surface marker profiles following repeated passage. Additionally, after approximately 5 passages hAECs can either reach senescence, or go through morphological changes consistent with epithelial to mesenchymal transition. After repeated passage, hAECs maintain a normal karyotype, long telomere lengths and cell cycle distribution. These properties indicate a low risk of transformation or tumorigenicity. In addition to possessing immune regulatory and anti-inflammatory properties, hAECs have been shown to be highly multipotent in vitro and in vivo, differentiating into cell lineages representative of the three primary germ layers (Figure 4).
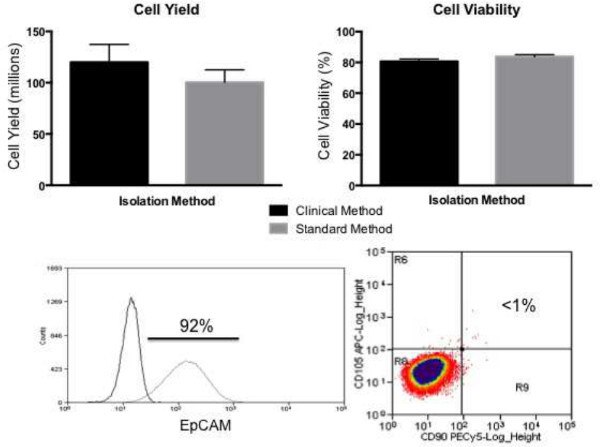 Figure 1: Expected cell yield, viability and purity for clinical isolation
protocol. Similar cell yields and viability can be achieved using animal-product
free reagents compared to standard animal product-containing methods. A typical isolation
should be >90% EpCAM and <1% CD90/CD105 positive cells. Please click
here to view a larger version of this figure.
Figure 1: Expected cell yield, viability and purity for clinical isolation
protocol. Similar cell yields and viability can be achieved using animal-product
free reagents compared to standard animal product-containing methods. A typical isolation
should be >90% EpCAM and <1% CD90/CD105 positive cells. Please click
here to view a larger version of this figure.
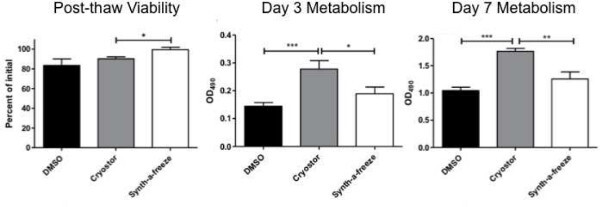 Figure 2: Post thaw viability and metabolism for serum-containing and serum-free
cryopreservation media. Compared to FBS containing 10% DMSO, commercially available
animal product-free cryopreservation media showed similar or increased post-thaw viability
and metabolism. Please click
here to view a larger version of this figure.
Figure 2: Post thaw viability and metabolism for serum-containing and serum-free
cryopreservation media. Compared to FBS containing 10% DMSO, commercially available
animal product-free cryopreservation media showed similar or increased post-thaw viability
and metabolism. Please click
here to view a larger version of this figure.
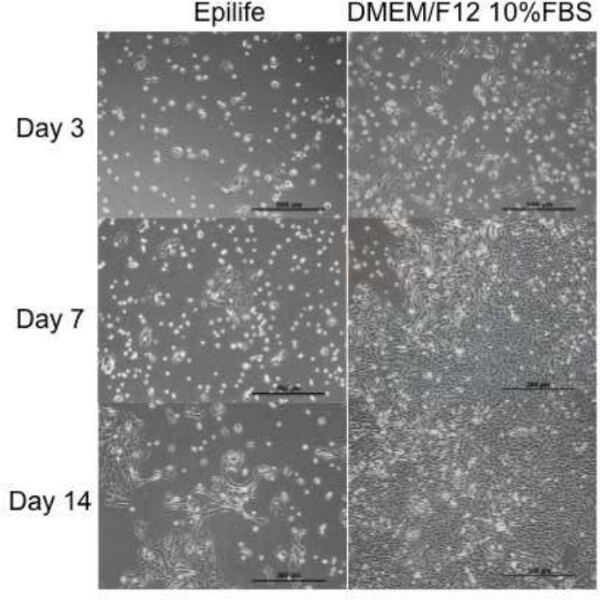 Figure 3: Typical attachment and growth profile of hAECs in serum-free and
serum-containing cell culture medium. Animal product-free culture media showed
suitable cell attachment, proliferation, and maintenance of epithelial phenotype. However
further development of serum-free media is required to achieve equal results to
serum-containing media. Scale bars represent 500 µm.
Figure 3: Typical attachment and growth profile of hAECs in serum-free and
serum-containing cell culture medium. Animal product-free culture media showed
suitable cell attachment, proliferation, and maintenance of epithelial phenotype. However
further development of serum-free media is required to achieve equal results to
serum-containing media. Scale bars represent 500 µm.
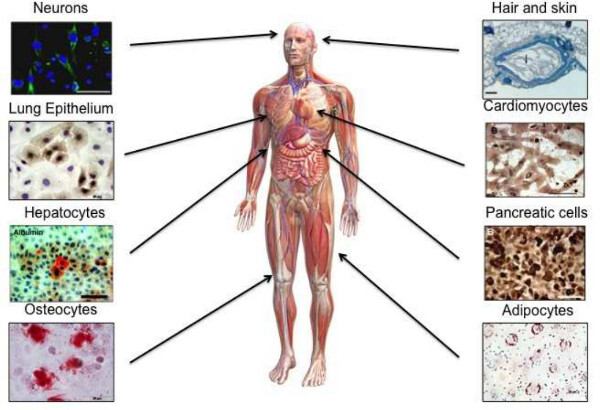 Figure 4: Multipotent differentiation of hAECs. At early passage, hAECs
have been shown to differentiate into multiple cell lineages representative of the three
primary germ layers. These lineages include, but are not limited to; neurons, hair and skin
cells, lung epithelium, cardiomyocytes, hepatocytes, pancreatic cells, osteocytes and
adipocytes. (Figure adapted from 27)
Figure 4: Multipotent differentiation of hAECs. At early passage, hAECs
have been shown to differentiate into multiple cell lineages representative of the three
primary germ layers. These lineages include, but are not limited to; neurons, hair and skin
cells, lung epithelium, cardiomyocytes, hepatocytes, pancreatic cells, osteocytes and
adipocytes. (Figure adapted from 27)
Discussion
There are several critical parameters that can have a significant impact in the success of this methodology. Storage of the placenta or amnion for up to 3 hr before isolation of hAECs may be desirable for logistic or scheduling purposes, however it is recommended that the tissue is processed as soon as possible. If tissue is to be stored, it is recommended that storage be performed following dissection and washing of the amnion membrane. Amnion can be stored in sterile HBSS containing antibiotics at 4 °C, however cell viability can decrease with extended storage time. We, and others have found variability in cell yield and viability, and to minimize this variability it is recommended that placenta are collected from healthy term births, preferably delivered by cesarean section.
We found that contamination of the amnion membrane with blood cells will result in inhibition of enzyme activity and a reduction in cell yield. Therefore a critical step in the protocol is thorough washing of the placenta and the amnion membrane is recommended. It is recommended that the amnion tissue be washed until it appears white/clear to avoid downstream enzyme inhibition. Isolation of a relatively homogeneous population of epithelial cells is important for characterization and quality control testing.
If low yields, viability or contamination of the cell yield with mesenchymal cells occurs there are several modifications that can be performed for troubleshooting. If cell yields are low, it is likely that enzymatic activity was inhibited due to blood or serum contamination. This can be resolved with more thorough and additional washing steps and/or discarding bloody or contaminated amnion pieces. Enzyme incubation time can also be increased, but this can be detrimental to cell viability. Decreased viability or contamination with mesenchymal cells can be avoided by decreasing the digest time. However, this may also decrease the total yield of cells. Times ranging from 30 min to 2 hr can be used for this protocol. These times need to be optimized for desired cell purity and total yield/viability. One limitation of this technique is that increasing cell yield or viability can come at the expense of each other. Additionally, serum-free components are currently not as effective of maintaining cell viability following exposure to enzymatic activity.
Cells can be cryopreserved without any major decrease in cell viability or loss of metabolism by storage in a temperature controlled liquid nitrogen system. This can range from a simple isopropanol-filled cryopreservation device to a state-of-the-art controlled rate freezing system. To our knowledge, the optimal freezing rate for hAECs in this system has not been determined, however the standard rate of 1 °C/min results in suitable maintenance of cell viability and post-thaw metabolism. During thawing of cryopreserved cells, is important to keep thawing time to a minimum to reduce cell exposure to DMSO. Cells may attach and proliferate at a lower rate following cryopreservation and thawing compared to culturing freshly isolated cells.
For clinical application it may be desirable to culture cells to increase cell number or for downstream characterization and/or in vitro manipulation. Readers should understand how their specific in vitro manipulation might alter the regulatory pathways involved in the clinical application of these cells. We investigated that an animal product–free culture medium such as EpiLife was suitable for the maintenance and expansion of hAECs. However, it is required to optimize the growth factor concentrations to achieve increased growth rates for such cell type. The issue of cell adherence during culture by using a human collagen-coating matrix, increases the plating efficiency. However, following prolonged exposure to enzymatic digestion the plating efficiency will be sub-optimal.
In summary, this protocol has been developed to facilitate the isolation and culture of human amnion epithelial cells (hAECs) using animal product-free reagents in accordance with current good manufacturing practices (cGMP) guidelines. The advantage of this method compared to alternative isolation methods, is that these cells can be isolated, characterized, cryopreserved and cultured without the risk of delivering potentially harmful animal pathogens to humans, while maintaining suitable cell yields, viabilities and growth potential. For researchers moving from pre-clinical animal studies to clinical trials, these methodologies will greatly accelerate regulatory approval, decrease risks and improve the quality of their therapeutic cell population.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors acknowledge financial support from the Victorian Government’s Operational Infrastructure Support Program.
References
- Murphy SV, Wallace EM, Jenkin G. In: Stem Cells and Regenerative Medicine. Appasani K, editor. Vol. 1. Springer Science and Business Media; 2011. pp. 243–264. [Google Scholar]
- Murphy SV, Atala A. Amniotic fluid and placental membranes: unexpected sources of highly multipotent cells. Semin. Reprod. Med. 2013;31(1):62–68. doi: 10.1055/s-0032-1331799. [DOI] [PubMed] [Google Scholar]
- Parolini O, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26(2):300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- Lim R, et al. Preterm human amnion epithelial cells have limited reparative potential. Placenta. 2013;34(6):486–492. doi: 10.1016/j.placenta.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Tamagawa T, Ishiwata I, Saito S. Establishment and characterization of a pluripotent stem cell line derived from human amniotic membranes and initiation of germ layers in vitro. Hum Cell. 2004;17(3):125–130. doi: 10.1111/j.1749-0774.2004.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Miki T, Marongiu F, Ellis E, Strom CS. Isolation of amniotic epithelial stem cells. Curr Protoc Stem Cell Biol. 2007;1:Unit 1E.3. doi: 10.1002/9780470151808.sc01e03s3. [DOI] [PubMed] [Google Scholar]
- Murphy S, et al. Amnion epithelial cell isolation and characterization for clinical use. Curr Protoc Stem Cell Biol. 2010;1:Unit 1E.6. doi: 10.1002/9780470151808.sc01e06s13. [DOI] [PubMed] [Google Scholar]
- Ilancheran S, et al. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77(3):577–588. doi: 10.1095/biolreprod.106.055244. [DOI] [PubMed] [Google Scholar]
- Fliniaux I, Viallet JP, Dhouailly D, Jahoda CA. Transformation of amnion epithelium into skin and hair follicles. Differentiation. 2004;72(9-10):558–565. doi: 10.1111/j.1432-0436.2004.07209009.x. [DOI] [PubMed] [Google Scholar]
- Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23(10):1549–1559. doi: 10.1634/stemcells.2004-0357. [DOI] [PubMed] [Google Scholar]
- Avila M, Espana M, Moreno C, Pena C. Reconstruction of ocular surface with heterologous limbal epithelium and amniotic membrane in a rabbit model. Cornea. 2001;20(4):414–420. doi: 10.1097/00003226-200105000-00016. [DOI] [PubMed] [Google Scholar]
- Sankar V, Muthusamy R. Role of human amniotic epithelial cell transplantation in spinal cord injury repair research. Neuroscience. 2003;118(1):11–17. doi: 10.1016/s0306-4522(02)00929-6. [DOI] [PubMed] [Google Scholar]
- Yuge I, et al. Transplanted human amniotic epithelial cells express connexin 26 and Na-K-adenosine triphosphatase in the inner ear. Transplantation. 2004;77(9):1452–1454. doi: 10.1097/00007890-200405150-00023. [DOI] [PubMed] [Google Scholar]
- Li H, et al. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(3):900–907. doi: 10.1167/iovs.04-0495. [DOI] [PubMed] [Google Scholar]
- Liu T, et al. Human amniotic epithelial cells ameliorate behavioral dysfunction and reduce infarct size in the rat middle cerebral artery occlusion model. Shock. 2008;29(5):603–611. doi: 10.1097/SHK.0b013e318157e845. [DOI] [PubMed] [Google Scholar]
- Venkatachalam S, et al. Novel neurotrophic factor secreted by amniotic epithelial cells. Biocell. 2009;33(2):81–89. [PubMed] [Google Scholar]
- Manuelpillai U, et al. Human amniotic epithelial cell transplantation induces markers of alternative macrophage activation and reduces established hepatic fibrosis. PLoS One. 2012;7(6):e38631. doi: 10.1371/journal.pone.0038631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei JP, et al. Human amnion-isolated cells normalize blood glucose in streptozotocin-induced diabetic mice. Cell Transplant. 2003;12(5):545–552. doi: 10.3727/000000003108747000. [DOI] [PubMed] [Google Scholar]
- Murphy S, et al. Human amnion epithelial cells prevent bleomycin-induced lung injury and preserve lung function. Cell Transplant. 2011;20(6):909–923. doi: 10.3727/096368910X543385. [DOI] [PubMed] [Google Scholar]
- Murphy SV, et al. Human amnion epithelial cells induced to express functional cystic fibrosis transmembrane conductance regulator. PLoS One. 2012;7(9):e46533. doi: 10.1371/journal.pone.0046533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SV, et al. Human amnion epithelial cells do not abrogate pulmonary fibrosis in mice with impaired macrophage function. Cell Transplant. 2012;21(7):1477–1492. doi: 10.3727/096368911X601028. [DOI] [PubMed] [Google Scholar]
- Hodges RJ, Lim R, Jenkin G, Wallace EM. Amnion epithelial cells as a candidate therapy for acute and chronic lung injury. Stem cells Int. 2012;2012:709763. doi: 10.1155/2012/709763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JL, Chan ST, Wallace EM, Lim R. Human amnion epithelial cells mediate lung repair by directly modulating macrophage recruitment and polarization. Cell Transplant. 2013. [DOI] [PubMed]
- Litvinov SV, et al. Epithelial cell adhesion molecule (Ep-CAM) modulates cell-cell interactions mediated by classic cadherins. J Cell Biol. 1997;139(5):1337–1348. doi: 10.1083/jcb.139.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter MJ, Nagtegaal ID, van Krieken JH, Litvinov SV. The epithelial cell adhesion molecule (Ep-CAM) as a morphoregulatory molecule is a tool in surgical pathology. The American journal of pathology. 2003;163(6):2139–2148. doi: 10.1016/S0002-9440(10)63570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musina RA, Bekchanova ES, Sukhikh GT. Comparison of mesenchymal stem cells obtained from different human tissues. Bull Exp Biol Med. 2005;139(4):504–509. doi: 10.1007/s10517-005-0331-1. [DOI] [PubMed] [Google Scholar]
- Park A, et al. Newborn Stem Cells: Identity, Function, and Clinical Potential. John Wiley & Sons, Inc; 2013. pp. 119–137. [Google Scholar]


