Dear editor
In a recent edition of Clinical Ophthalmology, Zlotcavitch et al presented a case of progressive diabetic traction retinal detachment in the fellow eye 1 week after vitrectomy with intravitreal bevacizumab.1 This interesting observation extends previous original work by the same authors in which proliferative diabetic retinopathy was noted to regress following a bevacizumab injection into the fellow eye.2 Several points pertaining to this thought-provoking report deserve further discussion.
Bevacizumab exits the eye through the trabecular meshwork and choroidal circulation, and enters the bloodstream unchanged. Since the intravitreal half-life of bevacizumab in human eyes is considerably shorter than the intravascular half-life (9.8 days3 vs 20 days4), the drug accumulates in the circulation. Concentrations increase initially, peak at approximately 2 weeks, and then decrease exponentially as intraocular concentrations fall further. Bevacizumab circulates to the fellow eye and enters both the vitreous and anterior chamber, although it remains unclear whether intravitreal or intravascular drug is primarily responsible for vascular inhibition. Since intravascular bevacizumab contacts neovascular endothelium directly, the blood concentration of bevacizumab, and not the intravitreal concentration, may be the primary determinant of contralateral effects.
Rabbit5 and monkey6 models, along with a small human study,7 show that bevacizumab exits the eye more rapidly following vitrectomy. The magnitude of the intravitreal half-life reduction varies between reports, but the 46% decrease contended by Zlotcavitch et al resulting in a human half-life of 5.3 days, is a reasonable assumption. With these rates in mind, we mathematically modeled the time-dependent intravitreal and intravascular bevacizumab concentrations in patients before and after vitrectomy. Using the half-lives mentioned above, the concentrations of bevacizumab following a 1.25 mg intravitreal injection are as follows:
where [B]V is the intravitreal concentration of bevacizumab and [B]S is the serum concentration of bevacizumab. The time-dependent concentrations of bevacizumab in both vitreous and serum can be seen in Figure 1.
Figure 1.
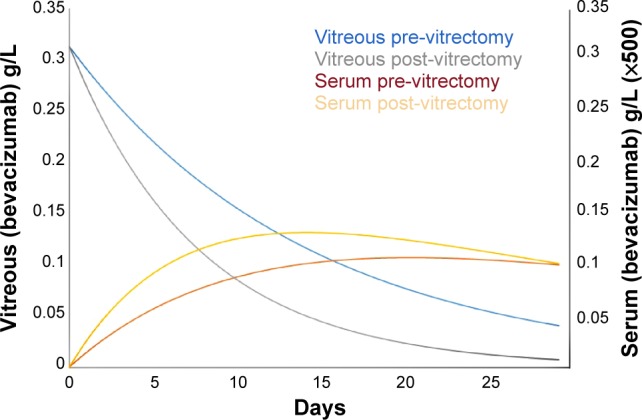
Time-dependent vitreous and serum bevacizumab concentrations in patients before and after vitrectomy.
Notes: In post-vitrectomy patients, the vitreous concentration falls more rapidly, leading to faster accumulation in the serum. The serum concentrations are amplified by a factor of 500 to fit the graph.
Several important observations regarding serum concentrations and the resultant exposure of the fellow eye to bevacizumab can be made from the graph. In a post-vitrectomy patient, the serum concentration rises faster and peaks earlier than in a pre-vitrectomy patient, with maximum concentrations at 14 days and 20 days, respectively. More importantly, the serum concentration at 7 days in a post-vitrectomy patient is 1.53 times that in a pre-vitrectomy patient and the area under the curve ratio through 7 days is 1.6 times. Therefore, a vitrectomy significantly increases the exposure of the fellow eye to bevacizumab during the first week, which helps to explain the observation made by Zlotcavitch et al.
As a monoclonal antibody against vascular endothelial growth factor (VEGF), bevacizumab works by decreasing the concentration of unbound (metabolically active) VEGF. In eyes with proliferative diabetic retinopathy, the degree of fibrosis depends upon the relative amounts of connective tissue growth factor and VEGF. The introduction of bevacizumab alters the ratio of connective tissue growth factor to VEGF in favor of fibrosis,8 as occurred in this case.
Footnotes
Disclosure
The authors report no conflicts of interest in this communication.
References
- 1.Zlotcavitch L, Flynn HW, Jr, Avery RL, Rachitskaya A. Progression to macula-off tractional retinal detachment after a contralateral intraoperative intravitreal bevacizumab injection for proliferative diabetic retinopathy. Clin Ophthalmol. 2015;9:69–71. doi: 10.2147/OPTH.S69466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695.e1–e15. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 3.Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol. 2008;146:508–512. doi: 10.1016/j.ajo.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 4.Avastin. Bevacizumab solution for intravenous infusion prescribing information. [Accessed January 21, 2015]. Available from: http://www.gene.com/download/pdf/avastin_prescribing.pdf.
- 5.Christoforidis JB, Xie Z, Jiang A, et al. Serum levels of intravitreal bevacizumab after vitrectomy, lensectomy and non-surgical controls. Curr Eye Res. 2013;38:761–766. doi: 10.3109/02713683.2013.763988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kakinoki M, Sawada O, Sawada T, Saishin Y, Kawamura H, Ohji M. Effect of vitrectomy on aqueous VEGF concentration and pharmacokinetics of bevacizumab in macaque monkeys. Invest Ophthalmol Vis Sci. 2012;53:5877–5880. doi: 10.1167/iovs.12-10164. [DOI] [PubMed] [Google Scholar]
- 7.Beer PM, Wong SJ, Hammad AM, Falk NS, O’Malley MR, Khan S. Vitreous levels of unbound bevacizumab and unbound vascular endothelial growth factor in two patients. Retina. 2006;26:871–876. doi: 10.1097/01.iae.0000233327.68433.02. [DOI] [PubMed] [Google Scholar]
- 8.Van Geest RJ, Lesnik-Oberstein SY, Tan HS, et al. A shift in the balance of vascular endothelial growth factor and connective tissue growth factor by bevacizumab causes the angiofibrotic switch in proliferative diabetic retinopathy. Br J Ophthalmol. 2012;96:587–590. doi: 10.1136/bjophthalmol-2011-301005. [DOI] [PMC free article] [PubMed] [Google Scholar]


