Abstract
Background
Few studies have examined adherence to recommended guidelines for follow-up and outcomes after an unsatisfactory Pap test (UPT) with liquid-based technologies.
Methods
Within four US health plans median time to follow-up and percentage of patients with follow-up testing by 120 days was calculated following an UPT. Multivariable analyses evaluated the association of clinical factors on follow-up testing. We compared the risk of Cervical Intra-epithelial Neoplasia 2 or worse (CIN2+) diagnosis after UPT to risk after satisfactory Pap test while controlling for study site, test year, and other covariates.
Results
634,644 Pap Tests between 2004 and 2010 were included in the study.
Of 1,442 UPTs, 53.4% had follow-up testing within 120 days; follow-up differed across the health plans (P<.001); was higher with age <50 years old (57.2% vs. 48.8%, P=.01) and with positive Human Papilloma Virus (HPV) results (84.6% vs. 53.9, P<.01). CIN2+ risk was similar for both unsatisfactory and satisfactory Pap test. However, following an UPT, age <50 years, having no previous history of Pap testing, having a history of previous abnormal Pap test, and positive HPV status, were all risk factors for CIN2+; a positive HPV test was the strongest risk factor for development of CIN2+. Negative HPV test result was protective for CIN2+ diagnosis.
Conclusions
Various clinical factors associated with risk of CIN2+ appear to influence the receipt of follow-up after an UPT. HPV test results in UPTs might be used in follow-up strategies: specifically a negative test might reduce the urgency for repeat Pap testing.
Keywords: Pap Test, Liquid based, Unsatisfactory, Conventional, SurePath, ThinPrep
Background
Cervical cancer screening programs utilizing Papanicolaou (Pap) tests have been highly effective in reducing cervical cancer mortality1. Most failures of cervical cancer screening in organized screening programs are in under- or never-screened women2; however some failures due to false negative Pap tests. Various studies have also reported a substantial fraction of false negative Pap tests were felt to be unsatisfactory on retrospective review3–5 and that quality of slide preparation correlates with a higher rate of detection of squamous intra-epithelial lesions (SIL)6. In consideration of this evidence, The 2001 Bethesda System criteria for Pap test (Bethesda criteria) reporting included semi-quantitative criteria, which arbitrarily defined an “estimated minimum of approximately 8,000–12,000 or 5,000 well-preserved and well-visualized squamous cells” as adequate for evaluation with conventional smears and liquid-based preparations, respectively7. Pap tests with benign cells but insufficient squamous cellularity as defined by the Bethesda criteria are reported as unsatisfactory.
The American Society for Colposcopy and Cervical Pathology (ASCCP) recommends repeat testing within 2–4 months for women with an unsatisfactory Pap test (UPT)8. This recommendation is largely based on the previously mentioned studies of conventional smears that preceded the publication of the 2001 Bethesda System’s semi-quantitative criteria for adequacy3–5. Studies of the significance of UPTs since the 2001 Bethesda criteria publication have had conflicting results9–11. Two studies using conventional Pap tests after 2001 Bethesda criteria publication reported UPTs after applying the more stringent criteria were not associated with increased SILs relative to tests satisfactory and negative for intra-epithelial lesion or malignancy (NILM), suggesting that the 2001 Bethesda criteria cut-point of 10,000 cells for minimal acceptable cellularity for conventional Pap tests may be too high9, 10. Another study of Surepath™ liquid-based Pap tests reported significantly increased SILs in UPTs relative to Pap tests satisfactory and NILM11.
This study was conducted in four large health plans in the United States to determine clinical factors influencing timely follow-up of UPTs in liquid-based Pap tests. The clinical relevance of UPTs was assessed by follow-up cytology and histology.
Methods
Setting
We conducted this study within the National Cancer Institute-sponsored SEARCH (Screening Effectiveness And Research in Community-based Healthcare) study that included four health care systems from the HMO Cancer Research Network (CRN)12 (Group Health Cooperative, Washington; Reliant Medical Group, Massachusetts; and Kaiser Permanente Northwest (Oregon/SW Washington) and Hawaii). The Institutional Review Boards of participating sites approved the study.
Eligible patients included women age 21 to 65 years who were enrolled in one of the health plans during time periods that varied by site: 2004–2010 (2 sites); 2004–2007 (1 site); 2006–2010 (1 site). At each site, liquid-based Pap tests results were retrieved. Women who had a history of total hysterectomy or known cervical cancer before Pap receipt were excluded. Results from conventional Pap tests were excluded. Continuous enrollment in the patient’s health care plan for 2 years after the Pap test was required for inclusion. There were no enrollment criteria prior to the Pap test. A consort diagram is presented in Appendix figure 1.
Data collection
Demographics information, enrollment, history of cervical cancer, hysterectomy status, and Pap and HPV molecular testing were collected from the HMO Research Network’s Virtual Data Warehouse13 and operational laboratory information systems. Pap test results, reason(s) for reports of UPTs and cytologic methods were collected from semi-structured text cytology reports at Sites 1, 3, and 4; at Site 2 this information was extracted from a coded cytology dataset to classify using 2001 Bethesda criteria as either normal, atypical squamous cells of uncertain significance (ASCUS), atypical squamous cells cannot exclude a high-grade SIL (ASC-H), atypical glandular cells, endocervical adenocarcinoma in-situ, low-grade SIL (LSIL), high-grade SIL (HSIL), squamous cell carcinoma, any malignancy other than squamous cell carcinoma or unsatisfactory. Through chart review at Sites 1, 3 and 4 we mapped results for cervical biopsy results using decision rules developed by two investigators (AK and CO, a cytopathologist); at Site 2 this information was extracted from a coded data set and mapped into: normal, cervical intraepithelial neoplasia 1 (CIN1), cervical intraepithelial neoplasia 2 (CIN2), cervical intraepithelial neoplasia 3 (CIN3), endocervical adenocarcinoma in-situ or any primary invasive cervical malignancy. Processing method was further stratified by Thinprep or Surepath methodology. High risk HPV molecular test results performed on the UPT (co-tested specimens) and previous HPV testing for up to 2 years prior to the UPT were retrieved. Patients were classified as HPV positive or HPV negative based on the results of the test(s). Patients with multiple HPV tests having both positive and negative results were classified as HPV positive.
All laboratories maintained standards established by Clinical Laboratory Improvement Amendments and processed specimens as per manufacturers recommendations14. Sites 1, 2 and 3 processed Pap tests at a central laboratory (unique for each site) and site 4 used multiple laboratories, although most Pap tests at Site 4 (>95% of Pap tests) were processed at a single laboratory. Sites 1, 2 and 3 used Surepath methodology and all laboratories from site 4 used ThinPrep methodology.
Histologic and cytology follow-up data were recorded for each index UPT for a period of two years. After the index UPT (figure 1), subsequent UPT(s) within two years was considered a follow-up study. A woman could contribute a second index UPT to the study if it occurred greater than 2 years after the first index UPT. We collected time to the first follow-up study, either repeat cytology or histology specimen for each index UPT. The most severe histologic lesion within the 2 year follow-up period was recorded (Cancer > Endocervical adenocarcinoma in-situ > CIN3 > CIN2 > CIN1 > Benign) and the proportion of patients with CIN2+ (Cancer, CIN3, endocervical adenocarcinoma in-situ or CIN2) was calculated.
Figure 1.
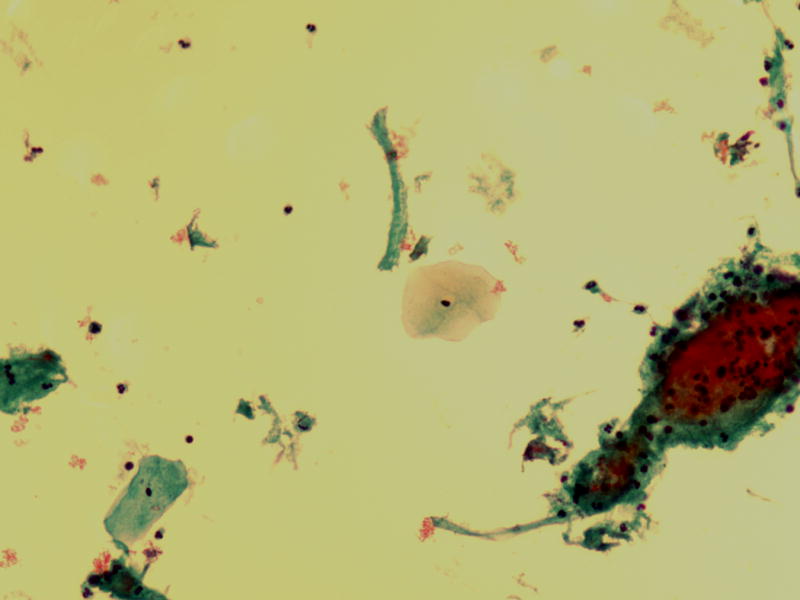
Unsatisfactory Pap test due to insufficient squamous cellularity (ThinPrep, 400×)
Analysis
Median time to the first follow-up study after all UPT was calculated by site. We calculated percentage of UPTs followed up within 120 days, approximately the end of the 2–4 month period recommended by ASCCP for repeat testing following an UPT. We calculated the percentage of tests followed up within 120 days for all liquid UPTs stratified by whether or not the woman later developed CIN2+.
We examined the association between each of the following factors and the completion of follow-up within 120 days among those with UPTs using Chi-Square: age (<50 vs. ≥50 years old), HPV status, history of CIN 2+, type of Pap test (screening vs. diagnostic), time since last Pap test, and result of previous Pap test(s). Our age categorization approximated menopausal status15. Diagnostic Pap test was defined as a test within 9 months of a previous Pap test or cervical biopsy; all others were considered screening tests. Odds ratios and 95% confidence intervals of the associations with completion of follow-up within 120 days were generated using multivariable logistic regression controlling for study site, year of test and repeated measures.
Hazard ratios and 95% confidence intervals for the relationship between an UPT and future risk of CIN2+ were estimated using multivariable Cox proportional hazards models controlling for potential confounding variables and adjusting for repeated measures for women with multiple Pap tests using generalized estimating equations. Follow-up ended at CIN2+ diagnosis or censoring, defined as death, disenrollment from the health plan, or end of the follow-up period at that site. In separate models, we compared the risk of CIN2+ following UPTs to two control groups: control group A was defined as all satisfactory liquid-based Pap tests; control group B was defined as all satisfactory liquid-based Pap tests excluding diagnoses of HSIL, endocervical adenocarcinoma, squamous cell carcinoma and any malignancy other than squamous cell carcinoma. Proportional hazards assumptions were tested and met for the main predictor variables in both models.
We have previously shown in this cohort significantly higher fraction of unsatisfactory results using Thinprep platform as compared to Surepath platform16. To assess the possibility that the relationship between an UPT and CIN2+ differed by type of liquid-based technology, we performed a sensitivity analysis by including an interaction term between UPT and the site using Thinprep technology in a proportional hazards model. All analyses were conducted using SAS for Windows, version 9.2.
Results
A total of 634,644 Pap tests from 351,877 women were included in the study. Of 1,442 total UPTs with liquid based technologies from all four sites 1,153 (80%) had a follow-up study of some type within the two year period following the UPT and 289 (20%) did not have a follow-up study of any kind. Of those with a follow-up study 1,096 had normal Pap test(s) and 57 had Pap test follow-up that was either abnormal or unsatisfactory. Of those with follow-up 55 (5%) had a cervical biopsy or excision. The percentage of liquid-based UPTs with documented follow-up by 120 days was significantly different across study sites and varied from 41.1% to 65.6 % (P <.001, Table 1).
Table 1.
Summary of Follow-up testing by 120 after an unsatisfactory Liquid-based Pap test according to study site and Pap testing technology
| Study site | Pap test technology | n | Percentage of patients with follow-up by day 120 | Median days to follow-up* (25th, 75th percentile) |
P-value |
|---|---|---|---|---|---|
| 1 | Sure-Path | 640 | 65.6 | 32 (12, 133) | |
| 2 | Sure-Path | 415 | 45.3 | 50 (21, 190) | |
| 3 | Sure-Path | 129 | 41.1 | 128 (71, 378) | |
| 4 | Thin-Prep | 258 | 42.2 | 106 (41, 363) | <.001 |
| All sites | 1,442 | 53.4 | 55 (19, 209) |
The time to follow-up testing for unsatisfactory results was calculated using data on completed tests during the study period.
Following an UPT, women <50 years of age were significantly more likely to have follow-up by 120 days than women ≥50 (57.2% vs. 48.8, P=.01) (Table 2). Women with positive HPV status were more likely than those without HPV testing to have follow-up within 120 days (84.6% vs. 53.9%, P<.01) and women with negative HPV status were less likely than those without HPV testing to receive follow-up within 120 days (42.1% vs. 53.9%, P=.02). Percentage with follow-up by 120 days did not differ by Pap indication (screening or diagnostic) or by results of previous Pap testing. After multivariable adjustment, only positive HPV status and age <50 years remained significant.
Table 2.
Factors Associated with Follow-up of Liquid-Based Unsatisfactory Pap Tests. N = 1,442
| Variable | N | Follow-up testing by day 120, % | Unadjusted P-value* | Adjusted Odds ratio (95% CI)** |
|---|---|---|---|---|
| Age, years | ||||
| <50 | 786 | 57.2 | .01 | 1.3 (1.1–1.6) |
| ≥50 | 656 | 48.8 | referent | |
|
| ||||
| HPV status | ||||
| HPV negative | 126 | 42.1 | .02 | 1.0 (0.65–1.6) |
| HPV positive | 26 | 84.6 | <.01 | 6.1 (2.0–18.5) |
| No HPV testing | 1290 | 53.9 | referent | |
|
| ||||
| Prior cervical biopsy results | ||||
| Prior biopsy with CIN2+ | 17 | 76.5 | .09 | 2.7 (.89–8.2) |
| No known CIN2+ | 1425 | 53.1 | referent | |
|
| ||||
| Indication for Pap testing | ||||
| Diagnostic Pap | 129 | 51.9 | .39 | 0.8 (0.52–1.3) |
| Screening Pap | 1313 | 53.5 | referent | |
|
| ||||
| Results of prior Pap test(s) | ||||
| Any prior result abnormal | 15 | 60.0 | .46 | 1.2 (0.31–4.8) |
| No prior results | 978 | 56.2 | <.01 | 1.1 (0.70–1.8) |
| All prior results normal | 449 | 47.0 | referent | |
|
| ||||
| Time since prior Pap | ||||
| 0–18 months | 347 | 48.1 | .01 | 0.9 (0.57–1.5) |
| 19+ months | 1068 | 55.2 | referent | |
Note.
Univariate testing compared to referent group;
Adjusted for all variables listed in table, study site, test year and repeated measures
HPV = Human Papilloma Virus; CIN2+ = Cervical Intra-epithelial Neoplasia 2 or worse
Multivariable adjusted risk of CIN2+ diagnosis after an UPT compared to our two control groups is shown in Table 3. UPTs were not associated with increased CIN2+ diagnoses as compared to control group A or B. Positive HPV status was by far the strongest predictor of CIN2+ with hazard ratios of 14.5 (95% CI 12.6–16.6) and 17.8 (95% CI 15.5–20.6) compared to control group A and B, respectively. Age <50 years and history of prior abnormal Pap testing showed significantly increased risk of CIN2+ following an UPT. Diagnostic Pap tests versus screening Pap tests showed significantly increased risk of CIN2+ following an UPT as compared to control group A but not control group B. Negative HPV status was protective for the diagnosis of CIN2+ with hazard ratios of 0.48 (95% CI 0.35–0.66) and 0.56 (95% CI 0.40–0.78) for the two control groups. Of the 126 cases with negative HPV status, 98 of these had a negative result on the UPT (e.g. co-tested specimens). Of these 98 cases, none were diagnosed with CIN2+ in the follow-up period of the study. Women who were diagnosed with CIN2+ in the follow-up period were more likely to be followed up by 120 days (80% vs. 53.3%, P<.01) than women who were not diagnosed with CIN2+ (Table 4). Sensitivity analyses showed no statistically significant interaction between Thinprep versus Surepath platform and diagnosis of CIN2+ in the follow-up period after an UPT.
Table 3.
Factors Associated with Risk of Development of CIN2+ Following an Unsatisfactory Liquid- Based Pap Test
| Variable | Control group A* Hazard Ratio (95% CI) |
Control group B** Hazard Ratio (95% CI) |
|---|---|---|
|
| ||
| Pap test result | ||
| Unsatisfactory | 0.91 (0.50–1.7) | 1.2 (0.63–2.1) |
| Satisfactory | referent | referent |
|
| ||
| Age, years | ||
| <50 | 4.1 (3.6–4.7) | 4.4 (3.8–5.1) |
| ≥ 50 | referent | referent |
|
| ||
| Indication for Pap testing | ||
| Diagnostic Pap test | 1.6 (1.2–2.1) | 1.1 (0.82–1.5) |
| Screening Pap test | referent | referent |
|
| ||
| Time since prior Pap | ||
| 0–18 months | 0.76 (0.60–0.96) | 0.88 (0.69–1.1) |
| 19+ months | referent | referent |
|
| ||
| Results of prior Pap test(s) | ||
| Previous Abnormal Pap test | 2.1 (1.5–2.9) | 2.2 (1.6–3.1) |
| No Previous Pap test | 1.4 (1.1–1.6) | 1.2 (1.0–1.5) |
| Previous Pap test Normal | referent | referent |
|
| ||
| Previous Biopsy results | ||
| Previous biopsy CIN2+ | 1.5 (1.1–1.9) | 1.2 (0.94–1.7) |
| No known CIN2+ | referent | referent |
|
| ||
| HPV status | ||
| HPV negative | 0.48 (0.35–0.66) | 0.56 (0.40–0.78) |
| HPV positive | 14.5 (12.6–16.6) | 17.8 (15.5–20.6) |
| No testing done | referent | referent |
Note. CIN2+ = Cervical Intra-epithelial Neoplasia 2 or higher
Adjusted for all factors in table, study site, year of test, repeated measures
Control group A was comprised of all satisfactory Pap tests
Control group B was comprised of all satisfactory Pap tests excluding Pap tests with high grade squamous intra-epithelial lesions, endocervical adenocarcinoma in-situ, squamous cell carcinoma and any malignancy other than squamous cell carcinoma
Table 4.
Summary of women with and without CIN2+ following an Unsatisfactory Liquid-Based Pap Test
| Variable | Women with CIN2+ (n=10) | Women without CIN2+ (n=1429) | p-value |
|---|---|---|---|
|
| |||
| Number (%) FU by 120 days | 8 (80.0) | 762 (53.3) | <.01 |
| Number (%)FU by 180 days | 8 (80.0) | 827 (57.9) | <.01 |
| Median days to FU | 28 | 55 | .13 |
| Number (%) history of CIN 2+ | 2 (20.0) | 15 (1.1) | <.01 |
Note. CIN2+ = Cervical Intra-epithelial Neoplasia 2 or higher
Discussion
This multisite study of 634,644 Pap tests provides frequencies of women receiving guideline-recommended follow-up after an UPT, clinical factors influencing follow-up and histologic outcomes following UPTs. Although ASCCP guidelines recommend promptly recalling all patients with an UPT without considering clinical factors, our study suggests clinical factors are influencing the proportion with guideline-recommended follow-up. Several of the factors we examined increased the proportion of women followed-up by 120 days and were risk factors for the diagnosis of CIN2+ following an UPT. Our data suggests clinicians are correctly recognizing patients with a higher risk of CIN2+ diagnosis and are more aggressive in recalling these patients with UPTs.
Our study adds to the literature in several important ways. Many of the studies that have investigated the risk of UPT used conventional Pap tests and occurred prior to publication of the 2001 semi-quantitative Bethesda criteria for adequacy. Thus, ASCCP recommendations for UPTs using liquid-based platforms are largely based on data from studies of conventional Pap tests using non-standardized criteria for specimen adequacy. The follow-up piece of the current study includes only liquid-based Pap tests done performed well after publication of the 2001 Bethesda criteria and therefore more applicable to current practice. Our data suggests that HPV test results, when available, are useful for stratifying the risk of the severe forms of cervical neoplasia, and thus the urgency of follow-up of an UPT. Positive HPV status had the highest hazard ratio for diagnosis of CIN2+ of all variables analyzed and negative HPV status was protective. This is not surprising considering the role of HPV infection in cervical cancer development and primary screening for cervical cancer with HPV molecular testing is being considered. Future recommendations on management of UPTs might take HPV molecular data into consideration for best follow-up. For example a patient with an UPT, particularly if the cellularity is near the minimal acceptable level, with negative HPV testing might not need to be recalled in the 2–4 month window recommended by ASCCP. Similarly the risk associated with a positive HPV test in certain patients with an UPT might justify referral to colposcopy. Age less than 50, diagnostic Pap tests, previous abnormal Pap test, no previous Pap test, and previous biopsy of CIN2+ were all associated with significantly increased risk of CIN2+ following an UPT albeit it with much lower hazard ratio estimates as compared to HPV status. The increased risk seen in UPTs in women younger than 50 years of age probably relates to the fact that the peak incidence of pre-cancerous cervical lesions occurs well before 50 and benign, physiologic cervical atrophy leads to many UPTs in postmenopausal women. The other factors associated with increased risk of CIN2+ are not surprising and probably reflect the higher risk seen in women who have a personal history of CIN2+ or prior positive Pap tests.
In our study, just over 50% of women with an UPT received a follow-up study within 120 days, approximately the end of the 2–4 month window recommended by ASCCP8. We found the proportion of women with follow-up within 120 days differed according to study site, HPV status and patient’s age. Surprisingly, follow-up rates for unsatisfactory results did not differ by indication for exam.
For our longitudinal analyses for development of CIN2+ following an UPT with liquid based tests, we chose to compare risk of CIN2+ to two control groups: all satisfactory Pap tests (control group A) and all satisfactory Pap tests excluding high risk cytology diagnoses (control group B). The most appropriate comparison to establish the risk of an UPT would be a control group of satisfactory and benign Pap tests with an adequately long interval of follow-up after the index Pap test. Comparing outcomes for UPT to all satisfactory Pap tests suffers because all satisfactory studies includes high grade SILs and even cancers. Our control group B excludes Pap tests with high risk lesions but does include some abnormal Pap tests that would generate more work-up and cervical biopsies. Interestingly, we found no increase in CIN2+ diagnoses in UPTs compared to either control group. The risk of CIN2+ for UPTs in the era of liquid-based Pap testing may be lower than with conventional Paps based on differences in specimen preparation. Liquid-based Pap tests effectively remove obscuring elements leaving insufficient quantity of squamous cells as the predominant cause of UPTs16, 17. Obscuring blood and elements from tumor diathesis in a patient with invasive cervical cancer is thus less likely to lead to an unsatisfactory study with liquid-based Pap tests as compared to conventional smears. This could explain a lower risk following an UPTs processed with liquid-based technologies.
Our study has limitations including the non-randomized design. The cytologic diagnoses were conducted in a clinical setting, whereby reflecting use in community settings. We do not know the fraction of UPTs that were reprocessed, so we cannot control for variability in the threshold to reprocess. Our study consisted of many different clinical sites within 4 health care systems and we do not have details of the nature and duration of training of clinical and laboratory personnel transitioning to liquid-based Pap tests. Also the HPV testing platforms was not standard among the labs contributing cases to the studies. Guidelines regarding optimal intervals between screening Pap tests are evolving and the impact of an UPT could be different with a longer interval between screening Pap tests.
In conclusion, just over half of all women with an unsatisfactory liquid based Pap test had a follow-up study within 120 days. Several clinical factors influenced the percentage of patients with adequate follow-up and most factors that were associated with increased risk of pre-cancerous cervical lesions hastened follow-up. A positive or negative HPV test result shows promise for determining the urgency of follow-up after an unsatisfactory Pap test.
Acknowledgments
Funding Source: Data collection for this work was supported by an award from the National Cancer Institute at the National Institutes of Health (SEARCH: Cancer Screening Effectiveness and Research in Community-based Healthcare, RC2 CA148576) to Buist/Doubeni. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Note: Elements of the data infrastructure were developed for the HMO Cancer Research Network Virtual Data Warehouse (U19 CA 79689, to Wagner/Hornbrook/Kushi).
Appendix: Figure 1.
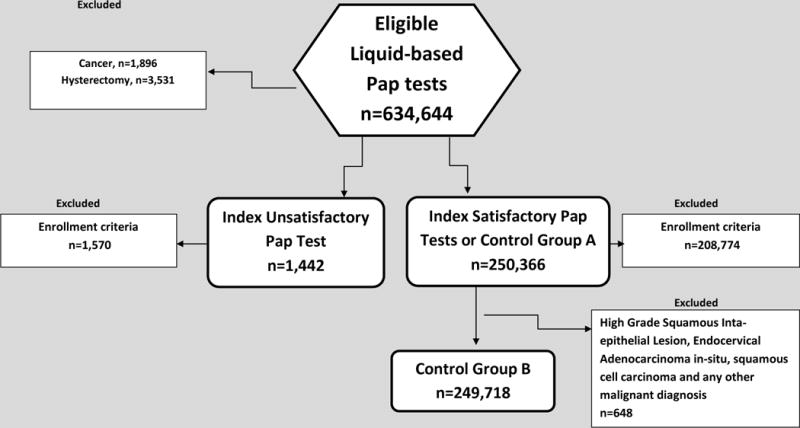
Consort Diagram
Footnotes
Role of sponsor: The sponsor did not participate in: the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Information on author access to data: “All authors had full access to all the data and played and active role in writing of the manuscript.” “Christopher L. Owens, Daniel Peterson, and Terry S. Field conducted and are responsible for the data analysis”.
Conflict of Interest Statement: “The authors have no conflicts of interest to disclose”
References
- 1.Bray F, Loos AH, McCarron P, et al. Trends in cervical squamous cell carcinoma incidence in 13 European countries: changing risk and the effects of screening. Cancer Epidemiol Biomarkers Prev. 2005 Mar;14(3):677–686. doi: 10.1158/1055-9965.EPI-04-0569. [DOI] [PubMed] [Google Scholar]
- 2.Leyden WA, Manos MM, Geiger AM, et al. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J Natl Cancer Inst. 2005 May 4;97(9):675–683. doi: 10.1093/jnci/dji115. [DOI] [PubMed] [Google Scholar]
- 3.Pairwuti S. False-negative Papanicolaou smears from women with cancerous and precancerous lesions of the uterine cervix. Acta Cytol. 1991 Jan-Feb;35(1):40–46. [PubMed] [Google Scholar]
- 4.Paterson ME, Peel KR, Joslin CA. Cervical smear histories of 500 women with invasive cervical cancer in Yorkshire. Br Med J (Clin Res Ed) 1984 Oct 6;289(6449):896–898. doi: 10.1136/bmj.289.6449.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Graaf Y, Vooijs GP, Gaillard HL, Go DM. Screening errors in cervical cytologic screening. Acta Cytol. 1987 Jul-Aug;31(4):434–438. [PubMed] [Google Scholar]
- 6.Henry JA, Wadehra V. Influence of smear quality on the rate of detecting significant cervical cytologic abnormalities. Acta Cytol. 1996 May-Jun;40(3):529–535. doi: 10.1159/000333910. [DOI] [PubMed] [Google Scholar]
- 7.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002 Apr 24;287(16):2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 8.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013 Apr;17(5 Suppl 1):S1–S27. doi: 10.1097/LGT.0b013e318287d329. [DOI] [PubMed] [Google Scholar]
- 9.Adams AL, Gidley J, Roberson J, Wang W, Eltoum I, Chhieng DC. Clinical significance of unsatisfactory conventional pap smears owing to inadequate squamous cellularity defined by the Bethesda 2001 criterion. Am J Clin Pathol. 2005 May;123(5):738–743. [PubMed] [Google Scholar]
- 10.Fidda N, Miron J, Rodgers WH, Rader A. Impact of the new Bethesda System 2001 on specimen adequacy of conventional cervicovaginal smears. Diagn Cytopathol. 2004 Apr;30(4):235–239. doi: 10.1002/dc.10408. [DOI] [PubMed] [Google Scholar]
- 11.Alsharif M, McKeon DM, Gulbahce HE, Savik K, Pambuccian SE. Unsatisfactory SurePath liquid-based Papanicolaou tests: causes and significance. Cancer. 2009 Feb 25;117(1):15–26. doi: 10.1002/cncy.20009. [DOI] [PubMed] [Google Scholar]
- 12.Wagner EH, Greene SM, Hart G, et al. Building a research consortium of large health systems: the Cancer Research Network. J Natl Cancer Inst Monogr. 2005;(35):3–11. doi: 10.1093/jncimonographs/lgi032. [DOI] [PubMed] [Google Scholar]
- 13.Hornbrook MC, Hart G, Ellis JL, et al. Building a virtual cancer research organization. Journal of the National Cancer Institute Monographs. 2005;(35):12–25. doi: 10.1093/jncimonographs/lgi033. [DOI] [PubMed] [Google Scholar]
- 14.Medicare, Medicaid and CLIA programs; revision of the laboratory regulations for the Medicare, Medicaid, and Clinical Laboratories Improvement Act of 1967 programs–HCFA. Final rule with comment period. Fed Regist. 1990 Mar 14;55(50):9538–9610. [PubMed] [Google Scholar]
- 15.Phipps AI, Ichikawa L, Bowles EJ, et al. Defining menopausal status in epidemiologic studies: A comparison of multiple approaches and their effects on breast cancer rates. Maturitas. 2010 Sep;67(1):60–66. doi: 10.1016/j.maturitas.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owens CL, Peterson D, Kamineni A, et al. Effects of transitioning from conventional methods to liquid-based methods on unsatisfactory Papanicolaou tests: results from a multicenter US study. Cancer Cytopathol. 2013 Oct;121(10):568–575. doi: 10.1002/cncy.21309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siebers AG, Klinkhamer PJ, Vedder JE, Arbyn M, Bulten J. Causes and relevance of unsatisfactory and satisfactory but limited smears of liquid-based compared with conventional cervical cytology. Arch Pathol Lab Med. 2012 Jan;136(1):76–83. doi: 10.5858/arpa.2011-0113-OA. [DOI] [PubMed] [Google Scholar]


