Abstract
Acute treatment with positive allosteric modulators (PAMs) of mGlu1 and mGlu5 metabotropic glutamate receptors (RO0711401 and VU0360172, respectively) reduces the incidence of spike-and wave discharges in the WAG/Rij rat model of absence epilepsy. However, from the therapeutic standpoint, it was important to establish whether tolerance developed to the action of these drugs. We administered either VU0360172 (3 mg/kg, s.c.) or RO0711401 (10 mg/kg, s.c.) to WAG/Rij rats twice daily for ten days. VU0360172 maintained its activity during the treatment, whereas rats developed tolerance to RO0711401 since the 3rd day of treatment and were still refractory to the drug two days after treatment withdrawal. In response to VU0360172, expression of mGlu5 receptors increased in the thalamus of WAG/Rij rats after 1 day of treatment, and remained elevated afterwards. VU0360172 also enhanced mGlu5 receptor expression in the cortex after 8 days of treatment without changing the expression of mGlu1a receptors. Treatment with RO0711401 enhanced the expression of both mGlu1a and mGlu5 receptors in the thalamus and cortex of WAG/Rij rats after 3–8 days of treatment. These data were different from those obtained in non-epileptic rats, in which repeated injections of RO0711401 and VU0360172 down-regulated the expression of mGlu1a and mGlu5 receptors. Levels of VU0360172 in the thalamus and cortex remained unaltered during the treatment, whereas levels of RO0711401 were reduced in the cortex at day 8 of treatment. These findings suggest that mGlu5 receptor PAMs are potential candidates for the treatment of absence epilepsy in humans
Keywords: Absence epilepsy, Spike–wave discharges, WAG/Rij rats, VU0360172, RO0711401, Tolerance, Chronic treatment, Group I mGlu receptors
1. Introduction
About 20% of patients with absence epilepsy, particularly those with atypical absence seizures, are refractory to the currently used anti-absence drugs, such as ethosuximide, valproate, and clonazepam (Panayiotopoulos, 1999; Glauser et al., 2010). Moreover, in patients that are not refractory, antiepileptic drugs may cause class-related adverse effects, such as sedation, dizziness, amnesia and ataxia. Unfortunately, newer anti-absence drugs, such as lamotrigine and lacosemide, loose efficacy during chronic treatment (Glauser et al., 2010) or may have only marginal effects or paradoxically enhance absences seizures in animal models (van Rijn et al., 1994; van Luijtelaar et al., submitted for publication). Therefore, more effective antiepilepsy drugs are still needed.
Metabotropic glutamate (mGlu) receptors are potential targets for novel anti-absence drugs. mGlu receptors form a family of eight, G-protein-coupled subtypes (mGlu1-8). mGlu1 and mGlu5 receptors are coupled to Gq/G11, whereas the rest are coupled to Gi/Go. These receptors are strategically distributed at synapses of the cortico-thalamo-cortical network, which is the anatomical site of origin of spike–wave discharges (SWDs) underlying absence epilepsy (Ngomba et al., 2011a; van Luijtelaar et al., 2011). For example, mGlu1 and mGlu5 receptors are localized postsynaptically on neurons of ventrobasal thalamic nuclei (VB) (Romano et al., 1995; Liu et al., 1998), whereas mGlu4 receptors are localized presynaptically on cortical glutamatergic afferents onto thalamic reticular neurons (Ngomba et al., 2008). In the cerebral cortex, mGlu1 receptors are found on GABAergic interneurons (Stinehelfer et al., 2000), whereas mGlu5 receptors are expressed by pyramidal neurons postsynaptic to thalamo-cortical projections (Wijetunge et al., 2008), as well as by interneurons. The study of mGlu receptors in models of absence epilepsy has been facilitated by the recent availability of potent and systemically active subtype-selective ligands. Noteworthily, some of these drugs are now under clinical development for neurological and psychiatric disorders showing an overall good profile of safety and tolerability (reviewed by Nicoletti et al., 2011).
Ligands that bind to the glutamate recognition site of mGlu receptors behave as orthosteric agonists or antagonists. On the other hand, ligands that bind to an allosteric site typically localized on the seven-transmembrane domain, can act as either positive or negative allosteric modulators (PAMs and NAMs, respectively). By definition, PAMs amplify receptor function only in the presence of an orthosteric agonist, and therefore recruit only mGlu receptors activated by endogenous glutamate (reviewed Niswender and Conn, 2010). Acute systemic treatment with mGlu1 and mGlu5 receptor PAMs (RO0711401 and VU030172, respectively) reduces the incidence of SWDs in the WAG/Rij rat model (Ngomba et al., 2011b; D’Amore et al., 2013), a genetic model of absence epilepsy with excellent predictive validity (Coenen and van Luijtelaar, 2003; van Luijtelaar and Sitnikova, 2006). Because RO0711401 and VU030172 do not affect motor behaviour, there are interesting for the development as anti-absence drugs for humans. However, from a therapeutic standpoint it is important to establish whether the anti-absence activity of mGlu1 and mGlu5 receptors PAMs is retained or not in response to repeated drug administrations. In fact, the development of tolerance seriously limits the therapeutic use of clonazepam and other benzodiazepines currently used in the treatment of epilepsy (Peeters et al., 1990).
2. Materials and methods
2.1. Drugs
VU0360172 (N-clyclobutyl-6-[2-3(fluorophenyl) ethynyl] pyridine-3-carboxamine), a selective mGlu5 receptor PAM, was obtained from Vanderbilt University Medical Center (Williams et al., 2011). RO0711401 (9H-xanthene-9-carboxylic acid (4-trifluoromethyl-oxazol-2-yl) amide, a selective mGlu1 receptor PAM, was kindly provided by F. Hoffmann-La Roche (Basel, Switzerland). VU0360172 was dissolved in 10% Tween 80 and injected subcutaneously (s.c.). RO0711401 was dissolved in arachis oil (Sigma–Aldrich, Italy) and also injected s.c. All drug solutions were prepared freshly daily.
Control animals received equal volumes of 10% Tween 80 or arachis oil, s.c. RO0711401 was administered at 10 mg/kg because we previously found that this dose was effective at suppressing the number and mean duration of SWDs (Ngomba et al., 2011b). VU0360172 was administered at the centrally active dose of 3 mg/kg (Rodriguez et al., 2010), which was also effective in reducing SWD number and mean duration (D’Amore et al., 2013), taken into consideration that very high doses (30 mg/kg and more) of mGlu5 PAMs [e.g. 5PAM523 = (4-Fluorophenyl){(2R,5S)-5-[5-(5-fluoropyridin-2-yl)-1,2,4-oxadiazol-3-yl]-2-methylpiperidin-1-yl}methanone), has been reported to show neurotoxic and seizure side effects in Female Wistar Hannover rats (Parmentier-Batteur et al., 2013).
2.2. Animals
Thirty-six male WAG/Rij and six male ACI (Agouti Copenhagen Irish) rats were used for combined EEG–behavioural studies. These rats were born and raised at Radboud University Nijmegen, The Netherlands, and had a mean body weight of 350 g at 9 months of age. They were defined as being “symptomatic” at 6 months of age, when they develop about 16–20 SWDs per hour, or more than 200 SWDs per day (van Luijtelaar and Coenen, 1988). The animals were individually housed in Macrolon cages, kept under controlled conditions (20 °C, 60% humidity) in a room with a reversed light–dark cycle (white light on from 9 p.m. to 9 a.m.), with food and drinking water always available. Animals were handled regularly a few days before starting EEG registrations.
In addition, twelve 3-month-old male Wistar rats (mean body weight, 200–240 g; Charles River, Italy) and five 3 to 4-month-old male Wistar rats (mean body weight, 380–420 g; Charles River, Italy) were used for Western blot analysis and EEG recording respectively. These rats were housed under similar conditions of WAG/Rij and ACI rats in the animal facility at Neuromed Pozzilli, Italy.
Male adult cervelet-4 (crv4) homozygous mutant mice lacking mGlu1 receptors (Conti et al., 2006; kindly provided by Prof. Alda Maria Puliti, University of Genoa, Italy) and male adult mGlu5−/− mice (breeded at Neuromed Institute) were used to test the specificity of the antibodies used for the detection of mGlu1α and mGlu5 receptors in Western blot experiments.
The study was performed in accordance with the guidelines of the European Community for the use of experimental animals and was approved by local ethics committee for animal studies (RU-DEC). All efforts were made to reduce discomfort experienced by the animals and to keep the number of animals as low as acceptable.
2.3. Drug injection regimens
For EEG recordings and assessment of spontaneous motor activity, four separate groups of 8–9 WAG/Rij rats were treated twice daily (at 9 a.m. and 9 p.m.) for 10 days with RO0711401 (10 mg/kg, s.c.), VU0360172 (3 mg/kg, s.c.), or their respective vehicles (see above, s.c.). Drugs and vehicles were injected once more at 9 a.m. 2 days after withdrawal (day 13). Additional groups of WAG/Rij rats (n = 16, 4 rats per group) or non-epileptic Wistar rats (n = 12, 3 rats per group) were treated twice daily for 8 days with drugs or vehicles, and used for immunoblot analysis of mGlu1α and mGlu5 receptors in the thalamus and cerebral cortex. The same WAG/Rij rats treated with RO0711401 or VU0360172 were also used for measurements of drug levels in the thalamus, and cerebral cortex. These Wistar and WAG/Rij rats were killed 1 h after the morning injection (i.e. at 10 a.m.). Additional groups of non epileptic rats (Wistar or ACI rats) were injected with VU0360172, 3 mg/kg, s.c. or its vehicles.
2.4. In vivo recordings
2.4.1. EEG
A cortical tripolar electrode set was implanted via stereotactic surgery under isoflurane anaesthesia supplemented with pre- and postoperative Rimadyl as analgesic and lidocaine as local anaesthetic. One active electrode was implanted in the frontal region (coordinates with the skull surface flat and from bregma zero–zero, AP +2, 0: L −3, 5) with a second one in the parietal region (A −6, 0: L −4, 0) (Paxinos and Watson, 2005). The ground electrode was placed over the cerebellum. After surgery the rats had two weeks to recover, after which, they were moved into transparent EEG recording cages supplied sawdust and cage enrichment and with water and food ad libitum. WAG/Rij and ACI rats were connected to an EEG cable with a preamplifier and a swivel, which allowed free movement. Before recording the rats were habituated to the leads for at least 12 h. The differential recorded EEG was filtered (only frequencies between 1 and 100 Hz were allowed to pass) and were digitalized with a sample frequency of 512 Hz, and saved for an off-line analysis using Windaq system (DATAQ, Instruments, Akron, OH, USA).
Wistar rats were implanted with stainless-steel wire recording electrodes epidurally on the left and right parietal cortex under isofluorane anaesthesia supplemented with lidocaine local anaesthetic. EEG was recorded by means of Grass-Telefactor system and visually analysed to evaluate the occurrence of SWDs.
Baseline EEG recordings (Day 0) were carried out at day 0 during the first two hours of the dark period (i.e. 9 a.m. 11 a.m.). EEG and behavioural recordings were taken during the dark-phase, five hours post injection, because this is whenWAG/Rij rats have the greatest incidence of SWDs (van Luijtelaar and Coenen, 1988; Smyk et al., 2012).
SWDs were labelled visually using common criteria, regular trains of sharp spikes and slow waves lasting from of 1–10 s, spike–wave frequency of 7–10 Hz, a spikes amplitude at least twice the background signal and asymmetric appearance of the SWDs (van Luijtelaar and Coenen, 1986; Ovchinnikov et al., 2010).
2.4.2. Spontaneous motor activity
Spontaneous motor activity was recorded as previously reported (van Rijn et al., 2010); an analogic passive infrared detector (PIR) (Luna PR, Rokonet Electronics LTD, Rishon Le Tzion, Israel) was fixed to a semi-open lid on top of the each rat’s EEG recording cage. The analogue signal was digitalized simultaneously with the EEG signal. Movements were quantified by calculating the mean of the absolute value of the PIR signal per hour. The values of each individual rat were analysed to investigate if there were any differences in motor activity between baseline- and post injection periods to see if there were any drug effects.
2.5. Detection of RO0711401 and VU0360172 in cortex and thalamus of WAG/Rij rats
2.5.1. Sample preparation
WAG/Rij rats treated with RO0711401 or VU0360172 were killed by decapitation on days 1, 3 or 8 as described above. The brains were rapidly removed, and the left portion of the thalamus and cerebral cortex were dissected and immediately frozen at −80 °C. Tissue samples were homogenized with 1 ml of 0, 1% aqueous formic acid. The weight of each sample was recorded. 30 μl of tissue homogenate was added to 150 μl of internal standard working solution (1 mM Dansilnorvaline in 100% acetonitrile). After extensive vortex (60 s), samples were centrifuged at 14,000 rpm for 5 min. 40 μl of supernatant was then mixed with 160 μl of 0.1% aqueous formic acid and transferred to an autosampler vial for injection into the chromatographic system.
2.5.2. Liquid Chromatography–tandem mass spectrometry analysis
HPLC analysis was performed with an Agilent Liquid Chromatography System series 1100 (Agilent Technologies, USA), with a binary pump, an autosampler, a solvent degasser and a column oven. Chromatographic separation was performed with a 50 × 2.0 mm, Luna C18, 5 μm,100 Å pore size column (Phenomenex, Torrance, CA, USA), equipped with a Security guard precolumn (Phenomenex, Torrance, CA, USA), containing the same packing material. The column was maintained at room temperature. The mobile phase was a solution of 0.1% aqueous formic acid (eluent A) and 100% acetonitrile (eluent B). The injection volume was 20 μl; elution was performed at a flow rate of 300 μl/min, using 10% solvent B for 1 min, linear gradient to 90% solvent B for 3 min, 90% solvent B for 2 min and afterwards re-equilibrating with 90% solvent A for 4 min. The total analysis run time was 10 min.
Mass spectrometry was performed with a 3200 Triple Quadrupole system, equipped with a Turbo Ion Spray source (Applied Biosystems, Foster City, CA). Data were acquired and processed with Analyst 1.4.2 software. The detector was set in the positive ion mode. The ion spray voltage was set at 5000 V and the source temperature was 300 °C. The collision activation dissociation (CAD) gas was set at medium value and nitrogen was used as the collision gas. The Q1 and Q3 quadrupoles were tuned for the unit mass resolution. The transitions of the precursor ions to the product ions were monitored with a dwell time of 100 ms for each analyte. The instrument was set in the multiple reaction monitoring (MRM) modes and mass spectrometer parameters were optimized to maximize sensitivity for all transitions.
Data analysis were carried out using a calibration curve with established known concentrations (0, 6.25, 12.5, 25, 50, 100, 250, 500, and 1000 ng/ml) of RO0711401 and VU0360172 dissolved in 0.1% aqueous formic acid and processed in the same way as of tissue samples. The equation of linear regression obtained for this value range was y = 0.000204x + 0.0033 (r = 0.9982). Mass spectrometer parameters were optimized to maximize sensitivity for each transition of both drugs (internal standard) and the instrument was set in the multiple-reaction monitoring mode.
2.6. Western blot analysis of mGlu1α and mGlu5 receptors
This analysis was carried out in the right portions of the thalamus and cerebral cortex dissected from WAG/Rij and Wistar rats treated for 1, 3 or 8 days with RO0711401, VU0360172, or their respective vehicles. Samples from crv4 mutant mice (cerebellum) and mGlu5−/− mice (cerebral cortex) were used to verify the identity of the mGlu1α and mGlu5 receptor bands, respectively. Tissue samples were homogenized at 4 °C in 50 mM Tris–HCl buffer, pH 7.4, containing 1 mM EDTA, 1% Triton X-100, 1 mM PMSF, 1 μg/ml aprotinin, 1 μg/ml pepstatin, and 1 μg/ml leupeptin. Proteins were resuspended in SDS-bromophenol blue reducing buffer with 40 mM DTT. Western blot analyses were carried out using 8% SDS polyacrylamide gels, which were electroblotted onto immunoblot PVDF membranes (BioRad, Milano, Italy); filters were blocked overnight in TTBS buffer containing 5% non-fatty milk.
Specific rabbit polyclonal antibodies for mGlu1α or mGlu5 receptor were used (1:500 and 1:1,000, respectively, Upstate Biotechnology, Lake Placid, NY, USA). Blots were incubated for 1 h with primary polyclonal antibodies or a mouse monoclonal antibody to label β-actin (1:100,000, Sigma, St. Louis, MO, USA) and then incubated for 1 h with secondary antibodies (peroxidase-coupled anti-rabbit or anti-mouse, Amersham, Piscataway, NJ, USA) diluted 1:7000 with TTBS. Immunostaining was revealed by enhanced ECL (Amersham, Piscataway, NJ, USA).
2.7. Statistical analysis
It was first tested whether the two vehicles differed from each other or were associated with hourly or daily EEG or behavioural parameters. Since this was not the case, it was decided to pool the data of the two control groups. The effects of chronic administration of VU0360172 or RO0711401 on incidence and mean duration of SWDs as a percentage of the base-line data as well as the behavioural activity of animals were tested in three separate repeated-measures mixed-design ANOVA with incidence and mean duration of SWDs or amplitude of the PIR as dependent variables. For all three analyses, the time of EEG recording (5 h) and day of experiment (day 1, day 10, day 13) were used as the within-subjects factors, the administered compound (vehicle(s), VU0360172 and RO0711401) was used as the between-subjects factors. Bonferroni’s test was used to isolate the differences between drugs, days and hours. Two-way mixed-design ANOVA was used for the analyses of the day effect across all injection days (1–10 and 13) in order to establish more clearly the development of tolerance and the effects of interruption of treatment. The incidence in the first hour post-drug was the dependent variable.
Statistical analysis of HPLC and Western blot data for thalamus and cortex separately were evaluated using a one-way ANOVA with drug treatment between days (day 1, day 3, day 8) as within-subjects factor, followed by the post-hoc analysis (Bonferroni’s multiple comparison test). Data are expressed as means ± SEM. Where values are means ± S.E.M. of 3 determinations per group with *P < 0.05 vs. Control and #P < 0.05 vs. day 1 or § P < 0.05 vs. day 3 treatment.
3. Results
3.1. Acute and chronic administration of mGlu1 and mGlu5 receptor PAMs in WAG/Rij rats: effects on SWDs
Symptomatic 6–7 months old WAG/Rij rats were treated twice daily for 10 days with RO0711401 (10 mg/kg, s.c.), VU0360172 (3 mg/kg, s.c.) or their respective vehicles. SWDs were analysed every day during the treatment and then after 3 days of withdrawal in response to drug rechallenge. Sample recordings of EEG activities from a representative WAG/Rij rat and a non epileptic Wistar rat are shown in Fig. 1A, B.
Fig. 1.
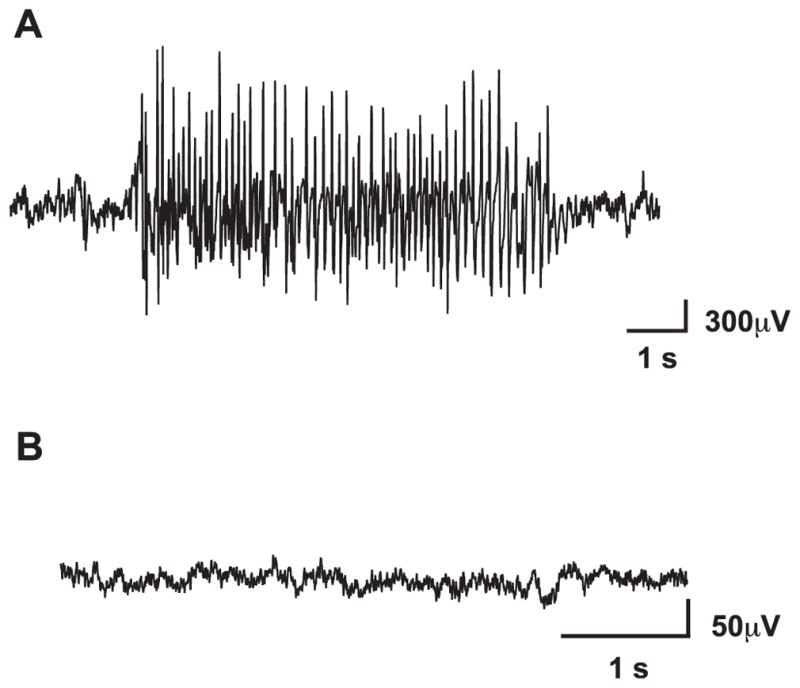
Representative EEG recording from symptomatic WAG/Rij rats and age-matched Wistar rats. A typical SWDs in the EEG recording of WAG/Rij rats is shown in (A). SWDs had a frequency of 7–10 Hz, an amplitude of at least twice the background, and a minimal duration of 1 s. Note the absence of SWDs in control Wistar rats (B).
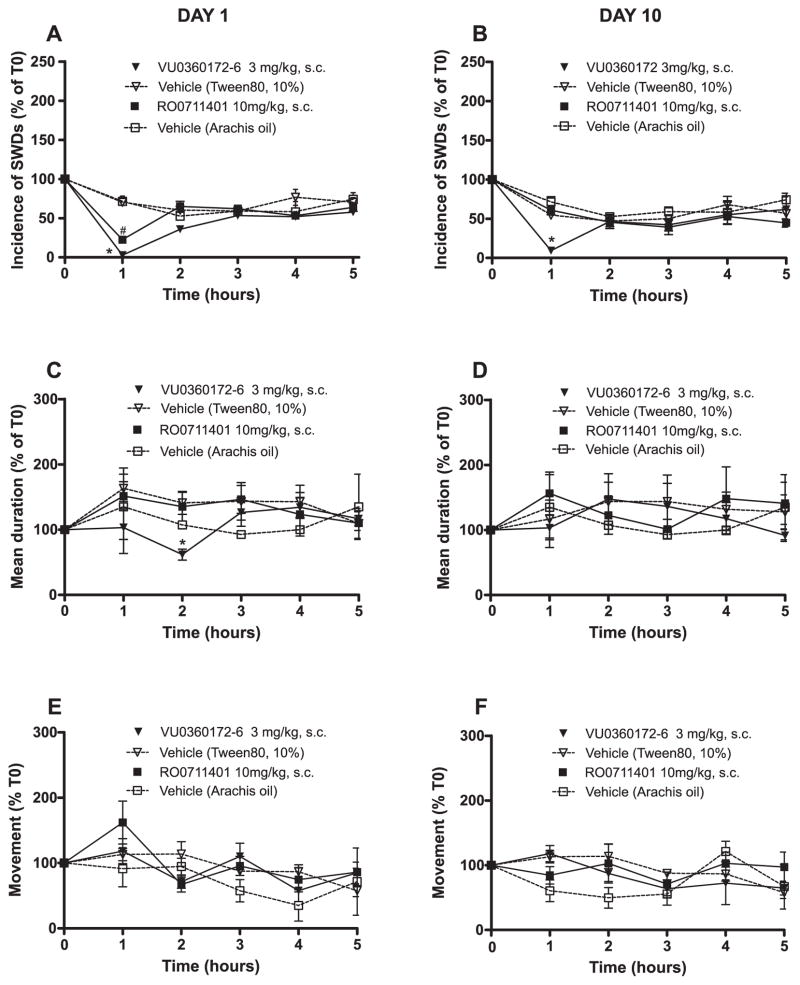
The analysis of the incidence of SWDs during the first 5 h post injection (data on incidence and duration for day 1 and 10 are shown in Fig. 2) showed a drug effect (F = 14.12, df 1.24, P < 0.001, η2 = .54; VU0360172 and RO0711401 groups had less SWDs than vehicle), an “hour” effect (F = 22.40, df 4.96, P < 0.001, η2 = 0.48; hour 1 < hours 2–5), and interactions between hour × drug (F = 7.13, df 8.96, P < 0.001; η2 = 0.38) and between day × drug (F = 10.20, df 4.48, P < 0.001, η2 = 0.46). Post hoc analysis on day 1 showed that RO0711401 and VU0360172 reduced the incidence of SWDs in the 1st post injection hour (Fig. 2A), with VU0360172 being more effective than RO0711401. WAG/Rij rats treated with VU0360172 (but not with RO0711401) also showed a trend to a reduction in the incidence of SWDs at the 2nd hour post injection (P > 0.05). Post-hoc analyses on the incidence of SWDs on day 10 showed that VU0360172 retained its anti-absence activity at the 1st hour post injection whereas RO0711401 became inactive. Fig. 2B shows the incidence of SWDs on day 10 of treatment. Post hoc analyses on day 13 showed exactly the same group differences as on day 10, with VU0360172, but not RO0711401, being effective as an anti-absence drug in the 1st hour post-injection. However, RO0711401 significantly reduced the incidence of SWDs in the second hour post injection on day 13 as compared to VU0360172 and vehicle (P < 0.05; not shown), suggesting that RO0711401 had regained some of its effects after 3 days of withdrawal.
Fig. 2.
Differential effects of RO0711401 and VU0360172 on the incidence of SWDs on days 1 and 10 of treatment in WAG/Rij rats. Rats were treated s.c. twice daily with RO0711401 (10 mg/kg), VU0360172 (3 mg/kg) or their respective vehicles. SWD incidence measured at baseline (time 0) and in the first 5 h post injection on day 1 and 10 of treatment are shown in (A) and (B), respectively. Mean duration of SWDs is shown in (C) and (D); spontaneous motor activity is shown in (E) and (F). Values are means + S.E.M. P < 0.05 vs. the respective vehicles (*) or vs. VU0360172 (#). Statistical analysis is detailed in the Results session.
Analysis of mean duration of SWDs at days 1, 10, and 13 showed a day effect (F = 9.39, df = 2.46, P < 0.000, η2 = 0.290) and a significant second-order interaction between day × hour × drug (F = 3.54, df = 16.184, P < 0.000, η2 = 0.235). Post hoc tests on day 1 showed that mean duration of SWDs was reduced by VU0360172 at the 2nd hour post injection (see Fig. 2C) compared to all other groups. VU0360172 had lost its effect on the mean duration of SWD at day 10 and 13. Mean duration was not affected by treatment with RO0711401 (Fig. 2C, D).
To exclude that SWDs data could be biased by a potential behavioural effect of the two drugs, motor behaviour was measured with PIR at day 1, 10 and 13 during the first 5 h post injection. Statistical analysis showed neither drug effect nor interactions effects between drug and any other independent variable (Fig. 2E, F).
The incidence of SWDs and their mean duration were exclusively analysed at 1 h post injection on all treatment days in order to inspect the changes in drug efficacy over time. Two-way ANOVA analysis showed a drug effect (F = 591.90, df 2.15, P < 0.001, η2 = 0.99; VU0360172 < RO0711401 < vehicle), a day effect (F = 7.63, df 10.150, P < 0.001, η2 = 0.34) and an interaction between day × drug (F = 9.80, df 20.150, P < 0.001, η2 = 0.57). Post hoc analysis showed that VU0360172 retained its anti-absence action across all 10 days of treatment, and kept also its anti-absence action at the rechallenge on day 2 after withdrawal. In contrast, RO0711401 showed its anti-absence effect only during the first 2 days of treatment, and failed to reduce the incidence of SWDs afterwards. The anti-absence activity of RO0711401 was not restored after 2 days of drug withdrawal (P > 0.05; Fig. 3). There was no effect of the two drugs on the mean duration of SWDs at 1 h post injection at any day of the treatment (not shown).
Fig. 3.
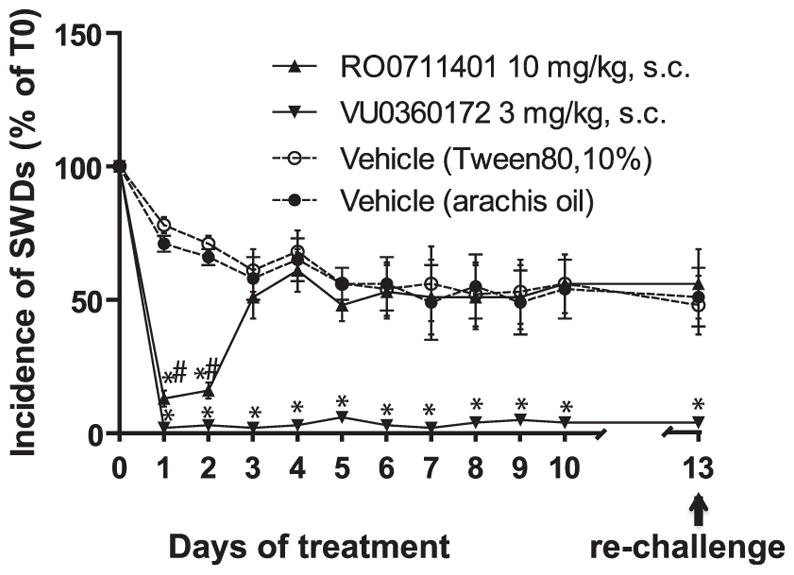
Development of tolerance to the anti-absence activity of RO0711401 after the first 3 days of treatment. WAG/Rij rats were treated s.c. twice daily for 10 days with RO0711401 (10 mg/kg), VU0360172 (3 mg/kg), or the respective vehicles. A re-challenge with each of the two drugs was performed after 3 days of withdrawal. The incidence of SWDs at 1 h after the morning injection is shown. Values are means + S.E.M. P < 0.05 vs. the respective vehicles (*) or vs. VU0360172 (#).
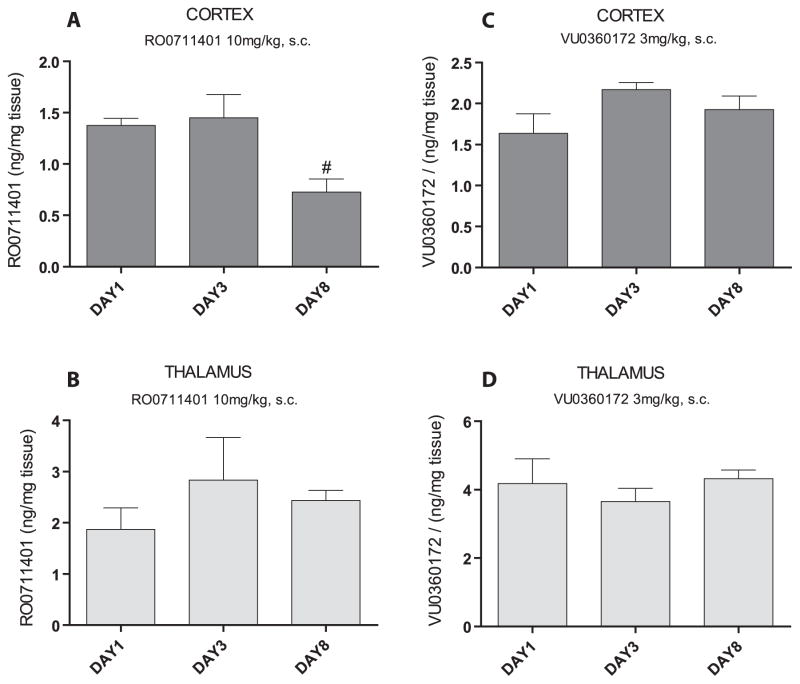
3.2. Measurements of thalamic and cortical drug levels during repeated administrations of RO0711401 or VU0360172
To examine whether the development of tolerance to RO0711401 was associated with time-dependent changes in central bioavailability of the drug, we measured RO0711401 levels in the thalamus and cortex in three separate groups of WAG/Rij rats treated with the drug as mentioned above and killed at day 1, 3 or 8 at one hour after the morning injection. One-way ANOVA did not reveal any significant effect of day of treatment in the thalamus (Fig. 4B, P > 0.05) while a significant day effect was observed in the cortex (Fig. 4A, F = 1,501, df = 1.4, P < 0.05, η2 = 0.273), with levels of RO0711401 being reduced on day 8 of treatment. However, RO0711401 levels were unchanged on day 3 of treatment, when tolerance to the anti-absence effect begun to develop (Fig. 4A). We also measured VU0360172 levels and found no significant day effect, neither in cortex nor in thalamus (Fig. 4C, D, P > 0.05).
Fig. 4.
Drug levels in the thalamus and cortex during 8-day treatment with RO0711401 or VU0360172 in WAG/Rij rats. Rats were treated s.c. twice daily for 8 days with RO0711401 (10 mg/kg) or VU0360172 (3 mg/kg). Values are means + S.E.M. #P < 0.05 vs. the respective values on day 1 or 3 of treatment.
3.3. Measurements of mGlu1α and mGlu5 expression in the thalamus and somatosensory cortex after treatment with RO0711401 or VU0360172
Next it was examined whether repeated administrations of RO0711401 or VU0360172 induced any adaptive changes in the expression of mGlu1α and mGlu5 receptors in WAG/Rij rats that could correlate with the temporal profile of drug response on SWD incidence.
Immunoblot analysis revealed bands at 140 kDa and 130 kDa, which, that correspond to the deduced molecular sizes of the receptor’s monomers (arrowheads in the representative immunoblots). A faint higher molecular weight band corresponding to dimers of mGlu1α and mGlu5 receptors was also visible in some of the immunoblots (not shown) but was not included in our densitometric analysis. The identity of the bands was verified using the cerebellum of crv4 mutant mice lacking mGlu1 receptors (Conti et al., 2006) or the cerebral cortex of mGlu5 receptor knockout mice as negative controls. The lower band present in the representative mGlu1α and mGlu5 immunoblots was non-specific because it was still visible in samples from crv4 and mGlu5−/− mice, respectively (Fig. 5).
Fig. 5.
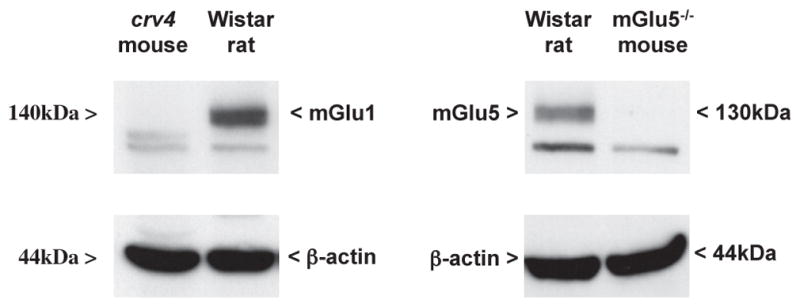
Specificity of the antibodies used for immunoblot analysis of mGlu1α and mGlu5 receptors. Western blot analysis was performed in protein extracts from the cerebellum of crv4 mutant mice (left) and the cerebral cortex of mGlu5−/− mice (right) to verify the identity of the bands corresponding to mGlu1α and mGlu5 receptors, respectively. mGlu1α and mGlu5 receptor labelling are also shown in the cortex and thalamus of Wistar rats, respectively, for comparison. The upper band at 140 and 130 kDa corresponds to mGlu1α and mGlu5 receptor monomers, respectively. The lower band was non-specific because it was still present in crv4 and mGlu5−/− mice.
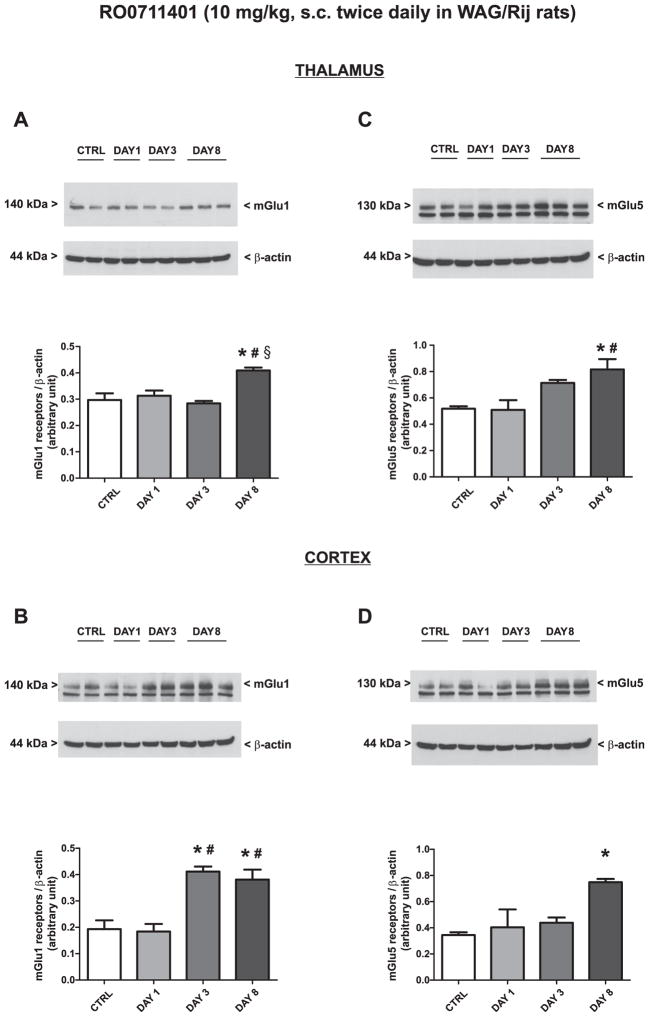
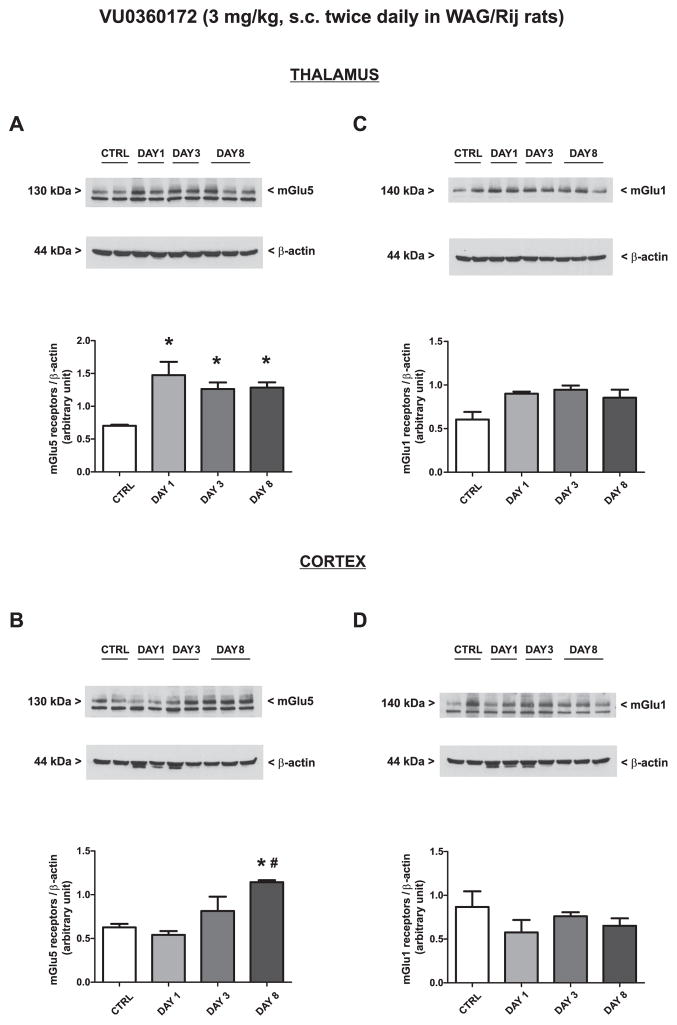
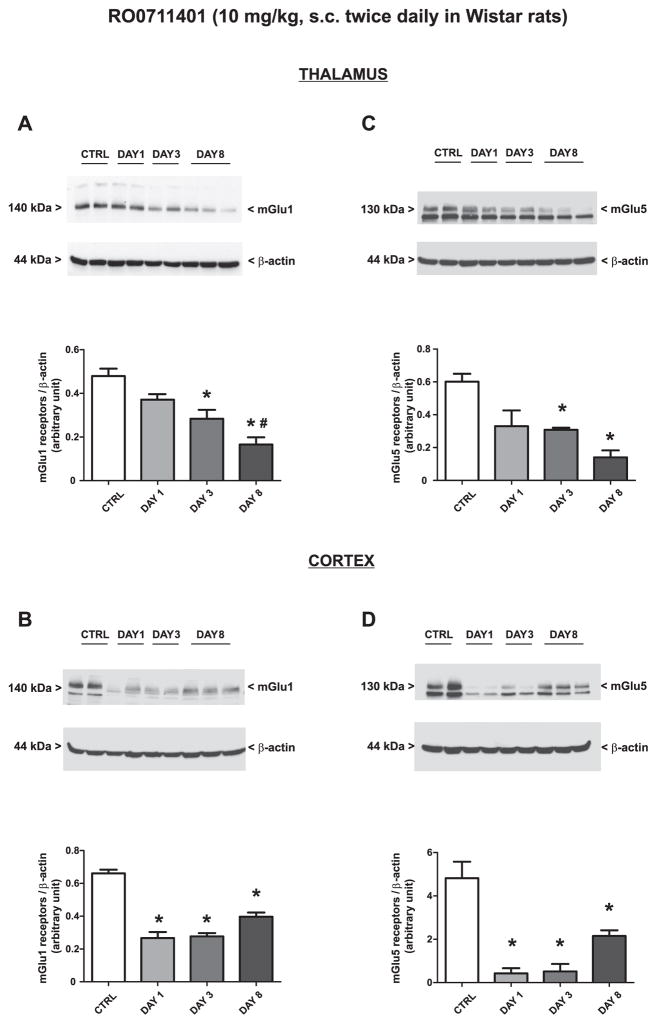
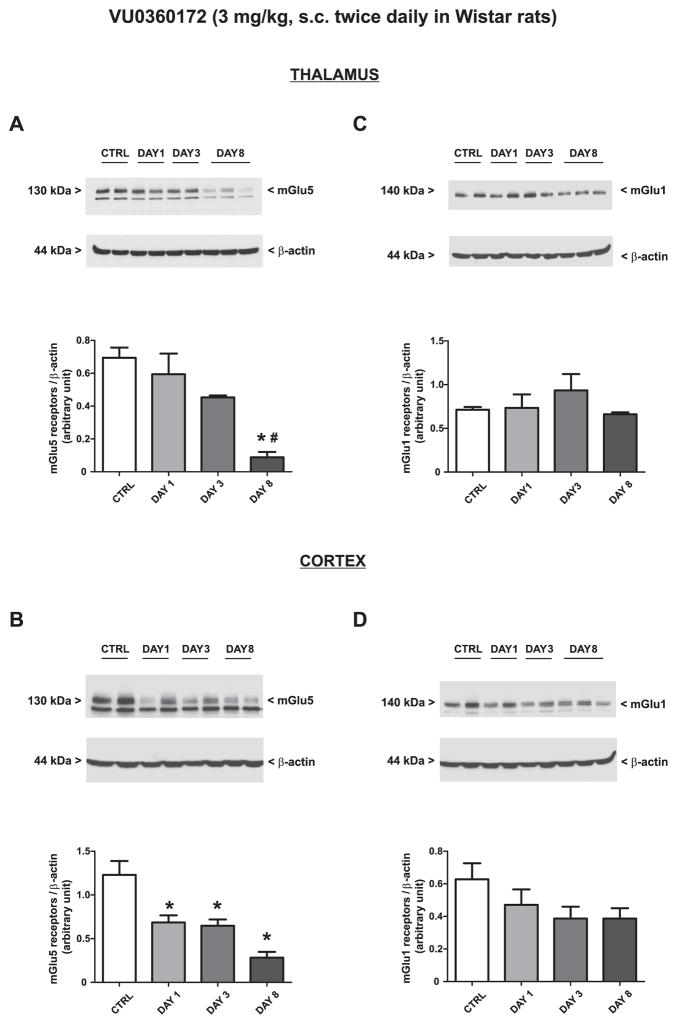
Treatment of WAG/Rij rats with vehicle twice daily for 8 days did not change the expression of mGlu1α or mGlu5 receptors in the thalamus or cortex (only values at day 8 are shown and indicated as “controls”). Treatment with RO0711401 caused an increase in both mGlu1α and mGlu5 receptor protein levels on day 8. The drug also enhanced mGlu1α receptor levels on day 3 in the cortex [Fig. 6A–D; mGlu1α (F = 10.36, P = 0.0039 and F = 15.50, P = 0.011; for the thalamus and cortex respectively) and mGlu5 (F = 7.33, P = 0.011 and F = 6.15, P = 0.018; for the thalamus and cortex respectively)]. In contrast, treatment with VU0360172 in WAG/Rij rats caused an early and persistent increase in mGlu5 receptor expression in the thalamus (Fig. 7A: F = 7.56, P = 0.0042) a late increase of mGlu5 receptor expression in the cortex (Fig. 7B: F = 9.17, P = 0.0057) and no changes in mGlu1α receptor levels in any of the two brain regions (Fig. 7C, D).
Fig. 6.
Adaptive changes in the expression of mGlu1α and mGlu5 receptors caused by 8-day treatment with RO0711401 in WAG/Rij rats. Rats were treated s.c. twice daily for 8 days with RO0711401 (10 mg/kg) or the respective vehicle. Values are means + S.E.M. P < 0.05 (One-way ANOVA + Bonferroni’s test) vs. the respective control (*), day 1 (#) or day 3 (§) values.
Fig. 7.
Adaptive changes in the expression of mGlu5 receptors caused by 8-day treatment with VU0360172 in WAG/Rij rats. Rats were treated s.c. twice daily for 8 days with VU0360172 (3 mg/kg) or the respective vehicle. Values are means + S.E.M. P < 0.05 (One-way ANOVA + Bonferroni’s test) vs. the respective control (*) or day 1 (#) values.
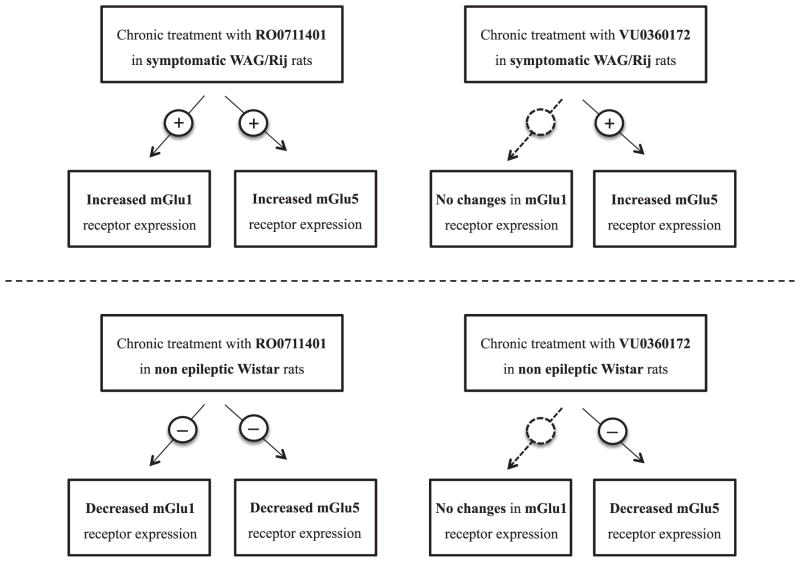
To examine whether these adaptive changes in receptor expression represented a general response to mGlu1 and mGlu5 receptor PAMs or were peculiar to epileptic WAG/Rij rats, the analysis was extended to non-epileptic age-matched Wistar rats treated with vehicles, RO0711401 or VU0360172 for 1, 3 and 8 days. It was confirmed in these rats that treatment with RO0711401 caused changes in both mGlu1α and mGlu5 receptors whereas treatment with VU0360172 caused selective changes in mGlu5 receptors. However, the direction of these changes was opposite to that observed in WAG/Rij rats. Wistar rats treated with RO0711401 showed an early and persistent down-regulation of mGlu1α and mGlu5 receptors in the thalamus and cortex [Fig. 8A–D; mGlu1α (F = 18.51, P = 0.0020 and F = 46.19, P = 0.0001; for the thalamus and cortex respectively) and mGlu5 (F = 10.76, P = 0.0035 and F = 21.00, P = 0.0026; for the thalamus and cortex respectively)]. In contrast, Wistar rats treated with VU0360172 showed a late reduction in mGlu5 receptor expression in the thalamus (F = 10.75, P = 0.0035) and an early and persistent reduction of mGlu5 receptor expression in the cortex (F = 14.82, P = 0.0012) with no changes in the expression of mGlu1α receptors (Fig. 9A–D). Thus, interestingly, epileptic WAG/Rij rats and non-epileptic Wistar rats showed opposite manifestations of receptor adaptation in response to prolonged mGlu1 and mGlu5 receptor enhancement. A synopsis on the effect of chronic treatment with RO0711401 or VU0360172 on the thalamic/cortical expression of mGlu1α and mGlu5 receptors is shown in Fig. 10.
Fig. 8.
Adaptive changes in the expression of mGlu1α and mGlu5 receptors caused by 8-day treatment with RO0711401 in non-epileptic Wistar rats. Rats were treated s.c. twice daily for 8 days with RO0711401 (10 mg/kg) or the respective vehicle. Values are means + S.E.M. P < 0.05 (One-way ANOVA + Bonferroni’s test) vs. the respective control (*) or day 1 (#) values.
Fig. 9.
Adaptive changes in the expression of mGlu5 receptors caused by 8-day treatment with VU0360172 in non-epileptic Wistar rats. Rats were treated s.c. twice daily for 8 days with VU0360172 (3 mg/kg) or the respective vehicle. Values are means + S.E.M. P < 0.05 (One-way ANOVA + Bonferroni’s test) vs. the respective control (*) or day 1 (#) values.
Fig. 10.
Schematic diagram illustrating mGlu1/mGlu5 receptor responses in symptomatic WAG/Rij and non-epileptic control Wistar rats following chronic treatment with either mGlu1 or mGlu5 receptor PAMs. Detailed results of western blot analysis of mGlu1 and mGlu5 receptors are provided in Figs. 6–9.
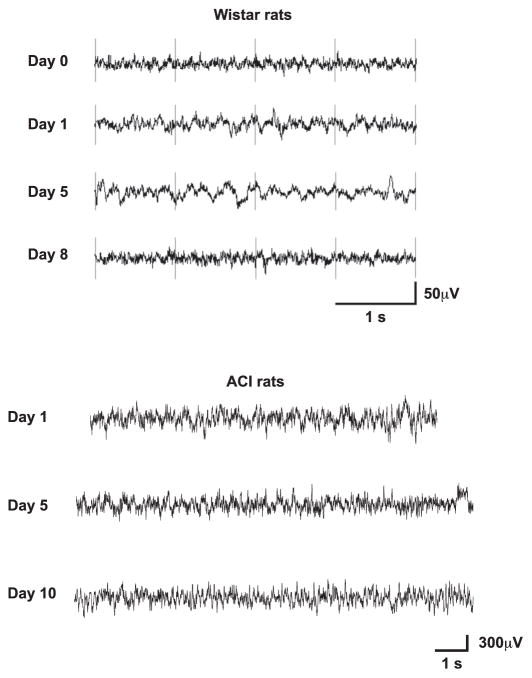
Finally, we examined whether chronic treatment with VU0360172 in Wistar rats could induce the appearance of SWDs as a result of the down-regulation of mGlu5 receptors in the thalamus (see D’Amore et al., 2013). No SWDs were recorded in Wistar rats treated for 8 days with VU0360172 (3 mg/kg, s.c. twice daily). Similarly, no SWDs were induced by repeated VU0360172 injections (3 mg/kg, s.c., twice daily for 10 days) in non-epileptic ACI rats, used as additional controls (Fig. 11).
Fig. 11.
EEG records showing no SWDs in Wistar or ACI rats treated chronically with VU0360172. Wistar or ACI rats (n = 3–6 per group) were treated with VU0360172 (3 mg/kg s.c.) or vehicle twice daily for 8–10 days, and recorded at baseline (day 0) and at the indicated days of treatment. These treatments did not cause the appearance of SWDs in the two strains of rats. Representative EEG recordings of rats treated with VU0360172 are shown.
4. Discussion
The main objective of the study was to establish whether tolerance develops to the SWD-suppressing activity of mGlu1 and mGlu5 receptor PAMs and to unravel the molecular nature of tolerance (if any). This is a necessary step for the preclinical and clinical development of mGlu receptor PAMs for the treatment of absence epilepsy in humans.
As expected (Ngomba et al., 2011b; D’Amore et al., 2013), both mGlu1 and mGlu5 receptor PAMs were able to suppress SWDs in WAG/Rij rats in the early phases of the treatment. However, a large difference between the two drugs was apparent when the response was monitored on a daily basis during chronic administration. The anti-absence effect of the mGlu5 receptor PAM, VU0360172, on the incidence of SWDs largely persisted over time, with only small signs of tolerance. More precisely, treatment with VU0360172 caused a significant reduction in the incidence of SWDs at the 1st hour and a reduction trend at the 2nd hour on day 1. The effect at the 1st hour was fully maintained on day 10. On the other hand, the mean duration of SWDs was reduced at the 2nd hour post injection on day 1 but not on day 10. In contrast, the mGlu1 receptor PAM, RO0711401, lost its anti-absence effects on the incidence of SWDs on day 10.
In order to disclose when precisely tolerance to RO0711401 began to develop, we analysed the incidence of SWDs in the first hour of injection of all ten consecutive days of treatment. The outcomes showed that the anti-absence action of RO0711401 remained unchanged from day 1 to day 2, diminished on day 3, and completely disappeared on day 4, remaining absent throughout the rest of the experiment. Therefore, tolerance to RO0711401 began to develop after relatively short time, i.e., after only four injections and was complete after two additional injections. The small and incomplete tolerance to the mGlu5 receptor PAM, VU0360172, is somehow consistent with data obtained with CDPPB another mGlu5 receptor PAM, in a mouse model of audiogenic seizures (Pacey et al., 2011).
Therefore, it seems that the two drugs differ with respect to the development of tolerance, with VU0360172 retaining most of the “therapeutic” effect when repeatedly administered to WAG/Rij rats, and RO0711401 inducing fast and complete tolerance to the anti-absence effect.
We have collected only few pharmacokinetic data on the two PAMs in this study. VU0360172 shows high plasma protein-binding and little or no first-pass metabolism (Rodriguez et al., 2010). R00711401 has a short elimination half-life (<2 h), but no data on liver metabolism are available (Vieira et al., 2009). Whether or not the two drugs may inhibit or accelerate their own metabolism with time is unknown. We found that thalamic levels of the two drugs remained stable during eight consecutive days of treatment in WAG/Rij rats, while a decrease over the days (day 3 vs. day 8) found in the cortex might indicate a contribution of pharmacokinetic tolerance to the loss of effects of RO0711401 on day 8 (but not on day 3).
We next measured the expression of mGlu1α and mGlu5 receptors in the thalamus and cortex of WAG/Rij rats on different days of drug treatment, as compared to treatments with the respective vehicles. This analysis was also carried out on non epileptic Wistar rats treated with the two drugs. We were surprised to find that adaptive changes in receptor expression induced by the two PAMs were highly divergent between WAG/Rij and Wistar rats. In Wistar rats, both drugs substantially reduced the expression of the respective mGlu receptor subtype particularly in the cerebral cortex. Interestingly, repeated administrations of RO0711401 also reduced the expression of the mGlu5 receptors whereas VU0360172 has no effect on the expression of the mGlu1α receptors. This particular type of receptor regulation was completely disrupted in WAG/Rij rats, where the two PAMs rather enhanced the expression of the respective subtype, although with different temporal and regional profile. However, also in this case, RO0711401 changed the expression of both the mGlu1α and the mGlu5 receptors, whereas VU0360172 selectively changed the expression of the mGlu5 receptors only.
In an attempt to explain the different receptor selectivity in the adaptive changes induced by the two PAMs, we wish to highlight that mGlu receptor subtypes can form both homodimers (e.g., either mGlu1-mGlu1 or mGlu5-mGlu5 dimers) and intra-group heterodimers (e.g., mGlu1-mGlu5 heterodimers), at least in heterologous expression systems (Doumazane et al., 2011). In addition, only one PAM molecule per dimer is sufficient to fully amplify receptor activity (Kniazeff et al., 2004). We speculate that in the thalamus and cortex of both WAG/Rij and Wistar rats there is a prevalence of mGlu1-mGlu1 homodimers and mGlu1-mGlu5 heterodimers over mGlu5-mGlu5 homodimers. This explains why the mGlu1 receptor PAM, RO0711401, induced adaptive changes in both mGlu1 and mGlu5 receptors, whereas the mGlu5 receptor PAM, VU0360172, selectively induced changes in mGlu5 receptors. The reason why receptor regulation caused by chronic PAM treatment is qualitatively different in WAG/Rij rats is unknown. Under normal conditions, prolonged agonist exposure causes mGlu receptor desensitization (i.e., uncoupling from G proteins), followed by receptor internalization that may lead to reducing receptor expression (i.e., receptor down-regulation) if de novo synthesis of receptor protein does not compensate for receptor degradation (for reviews, see Reiter and Lefkowitz, 2006; Whalen et al., 2011). It is possible that mechanisms that lie at the core of receptor desensitization and down-regulation are distorted in WAG/Rij rats because of the hypersynchronous activity of the cortico-thalamic-cortical network. This encourages the study of molecules involved in desensitization and internalization of mGlu1 and mGlu5 receptors, such as G-protein coupled receptor kinases and β-arrestin (reviewed by Iacovelli et al., 2013) in WAG/Rij rats and other animal models of absence epilepsy.
In conclusion, our data support the development of mGlu5 receptor PAMs as potential symptomatic drugs for the chronic treatment of absence epilepsy. These drugs may not cause pharmacodynamic tolerance perhaps because they fail to induce adaptive changes in the cognate mGlu1 receptor. We wish to highlight that VU0360172 (and also the mGlu1 PAM, RO0711401) did not affect motor behaviour and did not cause gross signs of toxicity in either WAG/Rij or Wistar rats. mGlu5 receptor PAMs are already under development for the treatment of schizophrenia with the only concern of neurotoxicity and convulsive seizures induced by very high doses of these compounds (Parmentier-Batteur et al., 2013). It remains to be established whether mGlu5 receptor PAMs can be safely associated with other drugs used in the treatment of absence epilepsy in patients that are resistant to conventional antiepileptic medication.
Acknowledgments
We wish to thank Elly Willems-van Bree, Hans Krijnen, Saskia Hermeling, Gerard van Oijen and Michelle Huismans for technical support and Silvia Gatti (F. Hoffmann-La Roche) for providing RO0711401.
References
- Coenen AM, van Luijtelaar EL. Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav Genet. 2003;33:635–655. doi: 10.1023/a:1026179013847. [DOI] [PubMed] [Google Scholar]
- Conti V, Aghaie A, Cilli M, Martin N, Caridi G, Musante L, Candiano G, Castagna M, Fairen A, Ravazzolo R, Guenet JL, Puliti A. crv4, a mouse model for human ataxia associated with kyphoscoliosis caused by an mRNA splicing mutation of the metabotropic glutamate receptor 1 (Grm1) Int J Mol Med. 2006;18:593–600. [PubMed] [Google Scholar]
- D’Amore V, Santolini I, van Rijn CM, Biagioni F, Molinaro G, Prete A, Conn PJ, Lindsley CW, Zhou Y, Vinson PN, Rodriguez AL, Jones CK, Stauffer SR, Nicoletti F, van Luijtelaar G, Ngomba RT. Potentiation of mGlu5 receptors with the novel enhancer, VU0360172, reduces spontaneous absence seizures in WAG/Rij rats. Neuropharmacology. 2013;66:330–338. doi: 10.1016/j.neuropharm.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumazane E, Scholler P, Zwier JM, Trinquet E, Rondard P, Pin JP. A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J. 2011;25:66–77. doi: 10.1096/fj.10-163147. [DOI] [PubMed] [Google Scholar]
- Glauser TA, Cnaan A, Shinnar S, Hirtz DG, Dlugos D, Masur D, Clark PO, Capparelli EV, Adamson PC. Childhood absence epilepsy study group. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N Engl J Med. 2010;362:790–799. doi: 10.1056/NEJMoa0902014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovelli L, Nicoletti F, De Blasi A. Molecular mechanisms that desensitize metabotropic glutamate receptor signaling: an overview. Neuropharmacology. 2013;66:24–30. doi: 10.1016/j.neuropharm.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Kniazeff J, Bessis AS, Maurel D, Ansanay H, Prézeau L, Pin JP. Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nat Struct Mol Biol. 2004;11:706–713. doi: 10.1038/nsmb794. [DOI] [PubMed] [Google Scholar]
- Liu XB, Muñoz A, Jones EG. Changes in subcellular localization of metabotropic glutamate receptor subtypes during postnatal development of mouse thalamus. J Comp Neurol. 1998;395:450–465. doi: 10.1002/(sici)1096-9861(19980615)395:4<450::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Ngomba RT, Ferraguti F, Badura A, Citraro R, Santolini I, Battaglia G, Bruno V, De Sarro G, Simonyi A, van Luijtelaar G, Nicoletti F. Positive allosteric modulation of metabotropic glutamate 4 (mGlu4) receptors enhances spontaneous and evoked absence seizures. Neuropharmacology. 2008;54:344–354. doi: 10.1016/j.neuropharm.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Ngomba RT, Santolini I, Salt TE, Ferraguti F, Battaglia G, Nicoletti F, van Luijtelaar G. Metabotropic glutamate receptors in the thalamocortical network: strategic targets for the treatment of absence epilepsy. Epilepsia. 2011a;52:1211–1222. doi: 10.1111/j.1528-1167.2011.03082.x. [DOI] [PubMed] [Google Scholar]
- Ngomba RT, Santolini I, Biagioni F, Molinaro G, Simonyi A, van Rijn CM, D’Amore V, Mastroiacovo F, Olivieri G, Gradini R, Ferraguti F, Battaglia G, Bruno V, Puliti A, van Luijtelaar G, Nicoletti F. Protective role for type-1 metabotropic glutamate receptors against spike and wave discharges in the WAG/Rij rat model of absence epilepsy. Neuropharmacology. 2011b;60:1281–1291. doi: 10.1016/j.neuropharm.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology. 2011;60:1017–1041. doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov A, Lüttjohann A, Hramov A, van Luijtelaar G. An algorithm for real-time detection of spike–wave discharges in rodents. J Neurosci Methods. 2010;194:172–178. doi: 10.1016/j.jneumeth.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Pacey LK, Tharmalingam S, Hampson DR. Subchronic administration and combination metabotropic glutamate and GABAB receptor drug therapy in fragile X syndrome. J Pharmacol Exp Ther. 2011;338:897–905. doi: 10.1124/jpet.111.183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayiotopoulos CP. Typical absence seizures and their treatment. Arch Dis Child. 1999;81:351–355. doi: 10.1136/adc.81.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier-Batteur S, Hutson PH, Menzel K, Uslaner JM, Mattson BA, O’Brien JA, Magliaro BC, Forest T, Stump CA, Tynebor RM, Anthony NJ, Tucker TJ, Zhang XF, Gomez R, Huszar SL, Lambeng N, Fauré H, Le Poul E, Poli S, Rosahl TW, Rocher JP, Hargreaves R, Williams TM. Mechanism based neurotoxicity of mGlu5 positive allosteric modulators – development challenges for a promising novel antipsychotic target. Neuropharmacology. 2013:1–13. doi: 10.1016/j.neuropharm.2012.12.003. http://dx.doi.org/10.1016/j.neuropharm.2012.12.003. [DOI] [PubMed]
- Paxinos G, Watson C. The Rat Brain in the Stereotaxic Coordinates. Academic Press Ltd; London: 2005. [Google Scholar]
- Peeters BW, van Rijn CM, Nutt DJ, Titulaer MN, Vossen JM, Coenen AM. Diazepam and Ro 15-1788 increase absence epilepsy in WAG/Rij rats chronically exposed to diazepam. Eur J Pharmacol. 1990;178:111–114. doi: 10.1016/0014-2999(90)94801-4. [DOI] [PubMed] [Google Scholar]
- Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Rodriguez AL, Grier MD, Jones CK, Herman EJ, Kane AS, Smith RL, Williams R, Zhou Y, Marlo JE, Days EL, Blatt TN, Jadhav S, Menon UN, Vinson PN, Rook JM, Stauffer SR, Niswender CM, Lindsley CW, Weaver CD, Conn PJ. Discovery of novel allosteric modulators of metabotropic glutamate receptor subtype 5 reveals chemical and functional diversity and in vivo activity in rat behavioral models of anxiolytic and anti-psychotic activity. Mol Pharmacol. 2010;78:1105–1123. doi: 10.1124/mol.110.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O’Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Smyk MK, Coenen A, Lewandowski MH, van Luijtelaar G. Internal desynchronization facilitates seizures. Epilepsia. 2012;53:1511–1518. doi: 10.1111/j.1528-1167.2012.03577.x. [DOI] [PubMed] [Google Scholar]
- Stinehelfer S, Vruwink M, Burette A. Immunolocalization of mGluR1alpha in specific populations of local circuit neurons in the cerebral cortex. Brain Res. 2000;861:37–44. doi: 10.1016/s0006-8993(00)01952-1. [DOI] [PubMed] [Google Scholar]
- van Luijtelaar EL, Coenen AM. Two types of electrocortical paroxysms in an inbred strain of rats. Neurosci Lett. 1986;70:393–397. doi: 10.1016/0304-3940(86)90586-0. [DOI] [PubMed] [Google Scholar]
- van Luijtelaar G, Sitnikova E. Global and focal aspects of absence epilepsy: the contribution of genetic models. Neurosci Biobehav Rev. 2006;30:983–1003. doi: 10.1016/j.neubiorev.2006.03.002. [DOI] [PubMed] [Google Scholar]
- van Luijtelaar EL, Coenen AM. Circadian rhythmicity in absence epilepsy in rats. Epilepsy Res. 1988;2:331–336. doi: 10.1016/0920-1211(88)90042-3. [DOI] [PubMed] [Google Scholar]
- van Luijtelaar G, Sitnikova E, Littjohann A. On the origin and suddenness of absences in genetic absence models. Clin EEG Neurosci. 2011;42:83–97. doi: 10.1177/155005941104200209. [DOI] [PubMed] [Google Scholar]
- van Luijtelaar G, Bikbaev A, LeClerck K, Mantagne A, Kaminski R. The effects of lacosamide on absence seizures in the WAG/Rij and GAERS models. Epilepsia. (submitted for publication) [Google Scholar]
- van Rijn CM, Weyn Banningh EW, Coenen AM. Effects of lamotrigine on absence seizures in rats. Pol J Pharmacol. 1994;46:467–470. [PubMed] [Google Scholar]
- van Rijn CM, Gaetani S, Santolini I, Badura A, Gabova A, Fu J, Watanabe M, Cuomo V, van Luijtelaar G, Nicoletti F, Ngomba RT. WAG/Rij rats show a reduced expression of CB1 receptors in thalamic nuclei and respond to the CB1 receptor agonist, R(+)WIN55,212-2, with a reduced incidence of spike–wave discharges. Epilepsia. 2010;51:1511–1521. doi: 10.1111/j.1528-1167.2009.02510.x. [DOI] [PubMed] [Google Scholar]
- Vieira E, Huwyler J, Jolidon S, Knoflach F, Mutel V, Wichmann J. Fluorinated 9H-xanthene-9-carboxylic acid oxazol-2-yl-amides as potent, orally available mGlu1 receptor enhancers. Bioorg Med Chem Lett. 2009;19:1666–1669. doi: 10.1016/j.bmcl.2009.01.108. [DOI] [PubMed] [Google Scholar]
- Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of β-arrestin-and G protein-biased agonists. Trends Mol Med. 2011;17:126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijetunge LS, Till SM, Gillingwater TH, Ingham CA, Kind PC. mGluR5 regulates glutamate-dependent development of the mouse somatosensory cortex. J Neurosci. 2008;28:13028–13037. doi: 10.1523/JNEUROSCI.2600-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Manka JT, Rodriguez AL, Vinson PN, Niswender CM, Weaver CD, Jones CK, Conn PJ, Lindsley CW, Stauffer SR. Synthesis and SAR of centrally active mGlu5 positive allosteric modulators based on an aryl acetylenic bicyclic lactam scaffold. Bioorg Med Chem Lett. 2011;21:1350–1353. doi: 10.1016/j.bmcl.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]