Abstract
Suppressing anxiety and fear memory relies on bidirectional projections between the medial prefrontal cortex and the amygdala. Positive allosteric modulators of mGluR5 improve cognition in animal models of schizophrenia and retrieval of newly formed associations such as extinction of fear-conditioned behaviour. The increase in neuronal network activities of the medial prefrontal cortex is influenced by both mGluR1 and mGluR5; however, it is not well understood how they modulate network activities and downstream information processing. To map mGluR5-mediated network activity in relation to its emergence as a viable cognitive enhancer, we tested group I mGluR compounds on medial prefrontal cortex network activity via multielectrode array neuronal spiking and whole-cell patch clamp recordings. Results indicate that mGluR5 activation promotes feed-forward inhibition that depends on recruitment of neuronal activity by carbachol-evoked up states. The rate of neuronal spiking activity under the influence of carbachol was reduced by the mGluR5 positive allosteric modulator, N-(1,3-Diphenyl-1H-pyrazolo-5-yl)-4-nitrobenzamide (VU-29), and enhanced by the mGluR5 negative allosteric modulator, 3-((2-methyl-1,3-thiazol-4-yl)ethynyl)pyridine hydrochloride (MTEP). Spontaneous inhibitory post-synaptic currents were increased upon application of carbachol and in combination with VU-29. These results emphasize a bias towards tonic mGluR5-mediated inhibition that might serve as a signal-to-noise enhancer of sensory inputs projected from associated limbic areas onto the medial prefrontal cortex neuronal microcircuit. Reprints and permissions: sagepub.co.uk/journalsPermissions.nav
Keywords: Cholinergic transmission, inhibitory postsynaptic currents, metabotropic glutamate receptors, multi-electrode recordings, positive allosteric modulator
Introduction
The most complex cognitive behaviours are controlled by the medial prefrontal cortex (mPFC), which has great impact in the pathology of many mental disorders. The mPFC is known to project to several downstream brain regions controlling executive function of motivated behaviour, anxiety and depression (Anderson and Green, 2001; Goncalves et al., 2009). For example, basal amygdala (BA) neurons that project to the mPFC respond to fear while those that respond to extinction of fear associations share reciprocal projections with the mPFC indicating top-down control of extinction learning (Herry et al., 2008). Moreover, ex vivo optogenetic stimulation of the ventral hippocampus (vHC) and mPFC results in activation of both principal cells and interneurons in the BA (Hübner et al., 2014). The identified innervated principal cells were shown to project back to the mPFC, indicating feedback from the BA and indirect, feed-forward processing from the vHC to the mPFC (Hübner et al., 2014). Coupling of activity between the prefrontal cortex and hippocampus is shown to occur during retrieval of spatial memory processing (Jones and Wilson, 2005) with inclusion of the amygdala for the retrieval of fear associations (Lesting et al., 2011). The thalamic input to the mPFC emanates mainly from the mediodorsal thalamus, which projects information concerning affective-motivational behaviours (Vertes, 2006). The prominent role of the medial thalamic nuclei in multisensory integration and information relay may partake in setting the state of cortical activation with regard to contextual information. Interestingly, the ability of thalamic projections to promote excitability in the ventral mPFC depends on the state of activity; in particular, cholinergic transmission (Gioanni et al., 1999).
The expression of cholinergic receptors is plentiful throughout the brain, yet only few cholinergic synapses exist in line with their presumed volume transmission of neurotransmitter release (Picciotto et al., 2012). This has implicated a modulatory role for cholinergic activation during arousal states. Indeed, it has been shown to enhance long-term potentiation (LTP) (Gioanni et al., 1999), although recent evidence suggests that it can also induce long-term depression (LTD; Caruana et al., 2011; Huang and Hsu, 2010). As has been the case for cholinergic receptors, mGluR5 activation is emerging as a viable cognitive enhancer based on rodent studies (Homayoun and Moghaddam, 2010). The peri-synaptic localization and G-protein coupled effector mechanisms of mGluR5 have largely accounted for their modulatory role and activation under specific conditions (Knopfel and Uusisaari, 2008). In particular, mGluR5 has been shown to enhance NMDAR-mediated currents (Awad et al., 2000), which mediate LTD during activation of muscarinic receptors in the mPFC (Caruana et al., 2011; Lopes-Aguiar et al., 2013). Evidence for mGluR5-mediated potentiation of NMDAR-mediated currents emerged when the NMDA receptor hypofunction hypothesis was the guiding principle accounting for all three symptoms of schizophrenia (Neill et al., 2010). The advantage of using positive allosteric modulators (PAMs) vs. conventional orthosteric agonists is that they only enhance currents when the endogenous neurotransmitter activates the receptor allowing for targeted activation (Stauffer, 2011). Accordingly, the mGluR5 PAMs proved beneficial in cognitive deficits in animal models of schizophrenia (Ayala et al., 2009; Balschun et al., 2006; Gastambide et al., 2012) as well as addiction (Gass and Olive, 2009). However, physiological actions of mGluR5 PAMs have shown dualistic modes in areas related to spatial memory and cognition. In the hippocampus, the mGluR5 PAM, VU-29 was shown to enhance both LTP and LTD (Ayala et al., 2009). In the mPFC, the mGluR5 PAM, 3-cyano-N-(1,3 diphenyl-1H-hyrazol-5-yl) benzamide (CDPPB) was shown to increase spontaneous spiking rate of both excitatory and inhibitory neurons as well as prevent further excessive spiking induced by NMDAR antagonism with MK-801 (Lecourtier et al., 2007).
We set out to investigate whether the dual effects of spiking rate in the mPFC occur with a more potent mGluR5 PAM, VU-29, and the extent of modulation by cholinergic and/or metabotropic glutamate neurotransmission, which are important in synaptic plasticity and cognition. Neuronal spiking output of the mPFC microcircuit is critical for top-down control resulting in coordinating activity of cortical and subcortical areas. Therefore, we performed multi-electrode array (MEA) recordings of network neuronal spiking in rat ventral mPFC acute slices during VU-29 in combination with or individual perfusion of carbachol, the group I mGluR agonist, (RS)-3,5-dihydroxyphenylglycine (DHPG) and the mGluR5 negative allosteric modulator, MTEP. Carbachol-mediated up-states encompassed synaptic and non-synaptic cholinergic neurotransmission (Picciotto et al., 2012) that, similar to DHPG, provided simultaneous activation of excitatory and inhibitory cells. Furthermore, we determined the occurrence of spontaneous, inhibitory post-synaptic currents (sIPSCs) during VU-29 with the above mediators using whole-cell voltage-clamp recordings of excitatory neurons in layer V rat ventral mPFC acute slices. Results implicate an involvement of VU-29 in enhancing the signal:noise ratio by reduction of spiking rates during up-states.
Materials and methods
Slice preparation
Coronal slices (300 μm) of the mPFC were prepared from male Sprague-Dawley rats (post-natal 42–49 days) housed in a regulated onsite animal facility with 12 hour/12 hour light/dark cycles and ad libitum food and water. Rats were anaesthetized with isoflurane prior to decapitation and the brain was quickly removed from the skull and placed in ice-cold artificial cerebrospinal fluid (aCSF) that contained (mM): 124 NaCl; 1.25 NaH2PO4·H2O; 8.3 MgSO4·7 H2O; 2.7 KCl; 26 NaHCO3; 2 CaCl2·2 H2O; 18 D(+)-glucoseH2O; 2 L(+)-ascorbic acid adjusted to pH 7.2 with KOH, yielding 315 mOsm and bubbled with 95% O2-5% CO2. Slices were prepared using a vibrating microtome (Leica VT1200S, Nussloch, Germany) and transferred to an incubation chamber containing bubbled aCSF with lower Mg2+ (1.3 mM) for 30 min at 37°C followed by 1 hour at room temperature prior to recording. All experiments using animal subjects were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and were approved by the animal care and use committee of Johnson and Johnson Pharmaceutical Research and Development.
Drug treatment
All agonists and antagonists were prepared as stocks in dH2O apart from N-(1,3-Diphenyl-1H-pyrazolo-5-yl)-4-nitrobenzamide (VU-29; Tocris Bioscience, UK), which was dissolved in 0.12% dimethylsulfoxide in dH2O. Stock solutions were stored at −20°C and diluted to final concentrations just before application. Final concentrations were determined with regard to established EC50 and IC50 values as well as slice perfusion considerations obtained from the literature. All chemicals for the aCSF and internal solution were purchased from Sigma-Aldrich NV/SA, Belgium as well as carbamoylcholine chloride (carbachol, CCH) and (RS)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP). Drugs purchased from Tocris were as follows: DHPG; MTEP; 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX); [R-(R*,S*)]-5-(6,8-dihydro-8-oxofuro[3,4-e]-1,3-benzodioxol-6-yl)-5,6,7,8-tetrahydro-6,6-dimethyl-1,3-dioxolo[4,5-g]isoquinolinium iodide (BMI); (RS)-3-amino-2-(4-chlorophenyl)-2-hydroxypropyl-sulfonic acid (2-HS).
Electrophysiological recordings
Each mPFC slice was placed in a MEA chip (Qwane Biosciences SA, Switzerland), arranged in an 8×8, 3D configuration of 60 platinum electrodes (each 40 μm in diameter, separated by 200 μm centre to centre) with one channel serving as ground. Extracellular spiking was recorded at a bath temperature of 25°C via a temperature feedback controller (TC02, Multi-Channel Systems, Germany) using the MC_Rack 4.4.8 software interfaced with the USB-ME64-System (gain 1200; band width 10 kHz; Multi Channel Systems). We opted to record at this lower temperature to be able to detect any small increases in the spike rates upon drug application. Thus, avoiding reaching saturated high spike rates at higher temperature. Each slice was submerged in a MEA chip and perfused at 3 mL/min (Minipuls 2; Gilson Inc., WI, USA) for 5 min with bubbled aCSF as a control solution prior to baseline recording for 1 min. After baseline recording, each drug or combination tested was perfused for 5 min and then recorded for 1 min. Perfusion of control aCSF or drug solutions was continuous during recordings. Recordings were high pass filtered (200 Hz; Bessel 4th order) and spikes were collected by threshold into 1 second bins (spike rate) and saved as a DAT file with MC_Rack. The DAT files for control and subsequent to drug application were imported into Excel, where a template was created to designate channels to responses. Total averages in 1 min recording were calculated for spike rate per slice; spike rate per channel and number of active channels determined by a minimum of one spike recorded. Averages represent active channels and percent changes were calculated with regard to control aCSF. Surface maps were generated to designate the layer of activity in the mPFC. Layers were determined from the interhemispheric fissure with reference to stereotaxic coordinates (Paxinos et al., 1980) using a graticule scale. Data are presented as mean ± SEM of the percent differences between drug and baseline aCSF recordings in each slice. A Student’s t-test or one-way analysis of variance with Tukey’s post hoc test at p<0.05 was used for statistical significance.
Whole-cell recordings were performed in submerged mPFC slices using standard wall (0.64 mm) borosilicate capillary glass (Harvard Apparatus Ltd., UK) that was pulled to resistances of 4–5 MΩ using a Flaming/Brown P-87 puller (Sutter Instruments Co., Ca, USA). The internal solution contained (mM): 126 KCl; 10 NaCl; 1 MgCl2; 11 ethylene glycol tetraacetic acid (EGTA); 10 (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES); 2 Mg-ATP; 0.25 Na3-GTP adjusted to 7.2 pH with KOH, yielding 289 mOsm. This high Cl− solution facilitated the recordings of sIPSCs at a holding potential of −70 mV in voltage clamp (Edwards et al., 1990). The high concentration of EGTA was used to minimize polysynaptic events based on the reference used for the internal solution (Edwards et al., 1990). It should be noted that fast calcium sequestration by 1,2-bis(o-aminophenoxy) ethane-N,N,N’,N’-tetraacetic acid (BAPTA) remained unaltered, thus allowing for involvement of downstream effects by calcium during agonist applications. A glass micropipette filled with internal solution was inserted into a 1-HL-U holder containing Ag/ AgCl wire (Molecular Devices Ltd., UK). The holder was connected to the CV-7B headstage (Molecular Devices) and bath ground followed by amplification (voltage-clamp gain 0.5 V/nA; current-clamp gain 10) and low pass filtering (2 kHz) using Multiclamp 700B (Molecular Devices). Clampex 10.2 software (Molecular Devices) was used to control triggering and acquisition of responses by interfacing with the Multiclamp 700B via the Digidata 1440 A/D converter digitized at 10 kHz (Molecular Devices). Liquid junction potentials were calculated from the Clampex built-in JPCalcW program and subtracted online. Cells were viewed via DIC infrared on an Olympus BX51W1 upright fixed-stage microscope (Olympus, Belgium) and captured by a CCD, Retiga Exi camera onto a computer display running QCapture Software 2.98 (QImaging, BC, Canada). The pipette was manoeuvred (Patchstar stepper motor with LinLab software control; Scientifica Ltd., UK) through the slice towards the cell. Once in whole-cell, dialysing of intracellular pipette contents were equilibrated over 5 min of continuous perfusion with bubbled aCSF at 1.5 mL/min (sciQ400; Watson-Marlow NV, Belgium). The temperature of the bath was maintained at 25°C via a temperature feedback controller interfaced with LinLab software (ALA Scientific Instruments Inc., NY, USA; Scientifica Ltd., UK). The solution was then switched to aCSF containing 10 μM NBQX and 20 μM CPP to block fast excitatory neurotransmission and perfused for 5 min. In current-clamp mode, the bridge and fast capacitance transients were balanced before recording 10 sweeps every 2 seconds of current-evoked steps (–90 pA; Δ 65 pA; 500 ms duration). Recordings were switched back to voltage-clamp where whole-cell capacitance and series resistance was compensated for by 70% at 2 kHz prior to recording a short hyperpolarizing transient for passive membrane property calculations followed by sIPSCs every second for 1 min. Spontaneous IPSCs recordings were repeated for every solution tested and at the end of each experiment, 5 μM BMI and 20 μM 2-HS were perfused in the aCSF for verification. Cells were included for analysis if series resistance was less than 20 MΩ and did not change by 20%. Event templates of sIPSCs shapes were produced for each cell recorded to capture sIPSCs for measurements of peak, rise slope, rise time and instantaneous frequency. Spontaneous IPSCs within 1 min were averaged and presented as mean ± SEM for control and drug. Statistical analysis was performed using the Student’s t-test at p < 0.05. All electrophysiological recordings were performed in the ventral mPFC consisting of the prelimbic and infralimbic areas. Slices were used once throughout and (n) refers to the number of slices (MEA recordings) or individual cells (sIPSCs) in each experimental group. A minimum of five rats were used in each experimental group.
Results
Effects of carbachol or group I mGluR activation in the ventral mPFC
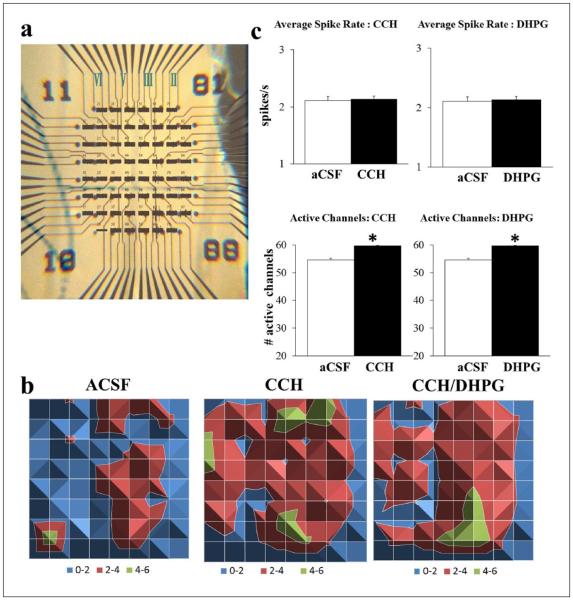
Carbachol (CCH) is a cholinergic agonist that is resistant to breakdown by cholinesterases and activates both muscarinic and nicotinic acetylcholine receptors (mAchRs, nAchRs). The pre- or post-synaptic location of these receptors on excitatory and inhibitory cells dictates whether there is suppression or increased activation. We tested the effects of CCH in the ventral mPFC, an area known to regulate higher-order cognitive functions. CCH (20 μM) caused a small, insignificant increase in the spike rate (7.56 ± 0.03%; p = 0.06) and a significant increase in the number of activated channels from layers II/III to V/VI (11.45 ± 0.04%; p < 0.05; n = 80; Figure 1). The increased number of activated channels depicts an increase in the number of cells activated that may occur randomly or with regard to cortical layer. The increased spread to layers V/VI was barely reflected by a paired t-test of spike rate per channel (p = 0.0543) indicating a lack of location specificity. Prior to examining mGluR5 neurotransmission for its role as a cognitive enhancer, we tested the effects of activating both mGluR1 and mGluR5 due to their mechanistic differences in synaptic depression (Lüscher and Huber, 2010; Volk et al., 2006). At a similar concentration (100 μM) and perfusion duration (5 min) shown to induce LTD in the hippocampus (Lüscher and Huber, 2010; Volk et al., 2006), DHPG increased the recruitment of activity (9.17 ± 0.01%; p < 0.05; n = 85) without affecting the spike rate (1.26 ± 0.013%; Figure 1(b)) irrespective of location.
Figure 1.
CCH (20 μM) and DHPG (100 μM) induced recruitment of neuronal activity. (a) Example mPFC slice overlaid on recording channels marked for prelimbic (PL; top), infralimbic (IL; bottom) and layers II–VI (right to left) using a graticule scale. (b) Surface maps display the average spike rate per channel in 1 min (colour-coded legend) spreading from layer II/III to layer V/VI following CCH. When combined with DHPG (n = 25), there was a similar increase in the number of active channels and a reduction in spike rate per channel without a change in the total spike rate compared to CCH alone. (c) Either CCH (left; n = 80) or DHPG (right; n = 55) on its own did not affect total spike rate (top) but increased the number of active channels (bottom; *p < 0.05; open: control aCSF; filled: drug).
Combined effects of carbachol and DHPG in the ventral mPFC
As a result of their similar increases in the recruitment of neuronal activity, we tested whether the combined effects of DHPG and CCH lead to changes in spike rate or maintained baseline levels of network output. DHPG enhanced the effects of CCH (n = 25) by increasing the number of active channels (CCH: 48.19 ± 0.12%; CCH/DHPG: 60.59 ± 0.10%; p < 0.05) yet significantly decreased the spike rate per channel (Figure 1(b)). The overall rate irrespective of channel location was not significantly different between the two (CCH: 4.78 ± 0.06%; CCH/DHPG: –5.10 ± 0.06%). It should be noted that the percent changes were larger in this smaller batch of experiments (n = 25 vs. n = 80 above), likely due to the variability of activated cells between slices during baseline conditions. This variability was taken into account by normalizing all drug effects throughout to baseline aCSF for each slice prior to averaging.
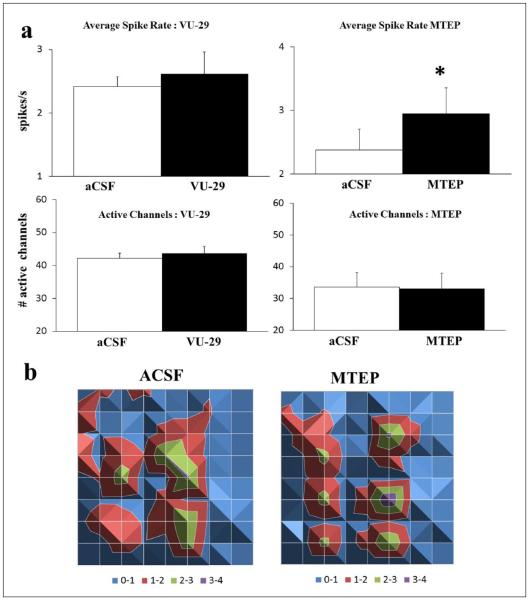
Effects of an mGluR5 positive and negative allosteric modulator in the ventral mPFC
Next, we tested the effects of the specific mGluR5 PAM, VU-29, shown to facilitate synaptic plasticity in the hippocampus and enhance spatial learning (Ayala et al., 2009). As mGluR5 are predominantly expressed in excitatory cells of the mPFC (Lopez-Bendito et al., 2002), any effects of VU-29 would shed light on whether excitation dominates under baseline conditions. VU-29 (1 μM) had a small and insignificant effect on spike rate (7.40 ± 0.09%; p = 0.23) as well as no effect on the number of active channels (3.20 ± 0.03%; n = 30; Figure 2(a)). The lack of effect on baseline activity by VU-29 implied that ongoing baseline activity was not mediated through mGluR5. To test this, we measured the effects on baseline activity by the specific, mGluR5 negative allosteric modulator, MTEP. MTEP (10 μM) caused a significant and location specific increase in layer V spike rate (23.77 ± 0.02%; p < 0.05) without any change in the number of active channels (–2.4 ± 0.04%; n = 20; Figure 2). These results indicated ongoing spontaneous mGluR5-mediated synaptic transmission in the mPFC without further effect by VU-29.
Figure 2.
mGluR5 antagonism increases spiking rate. (a) VU-29 (1 μM; left; n = 30) did not alter total spike rate (top) or number of active channels (bottom). MTEP (10 μM; right; n = 20) increased spike rate (*p < 0.05) without changing the number of active channels (open: control aCSF; filled: drug). (b) Surface maps display the average spike rate per channel in 1 min (colour-coded legend) and are arranged similar to Figure 1(a). Depicted is the increased rate of activity by MTEP in the same activated layers as compared to control aCSF.
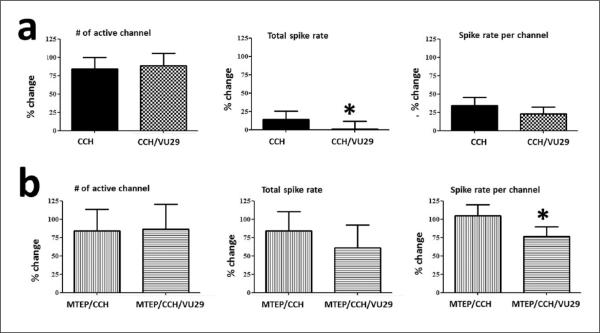
Combined effects of carbachol, VU-29 and MTEP in the ventral mPFC
We next tested if the lack of effect by VU-29 depended on the amount of activation as mGluR5 is located at peri-synaptic sites (Lopez-Bendito et al., 2002). In the presence of CCH, VU-29 significantly decreased the spike rate by half (CCH: 14.11 ± 0.11%; VU-29/CCH: 7.48 ± 0.11%; p < 0.05) but not the recruitment of activity as indicated by the changes in number of active channels (CCH: 83.88 ± 0.16%; VU-29/CCH: 88.25 ± 0.17%; n = 35; Figure 3(a)). This effect was partially antagonized by MTEP by enhancing the spike rate during CCH activation in the absence (MTEP/CCH: 84.18 ± 0.27%; p < 0.05 unpaired) or presence of VU-29 (MTEP/VU-29/CCH: 61.26 ± 0.31%; p < 0.05 unpaired). Nevertheless, the spike rate was reduced when VU-29 was added in the presence of MTEP and CCH and this was dependent on location, i.e. layer II and V (p < 0.05). The lack of antagonism is consistent with the known effects of VU-29 overcoming blockade by similar MTEP analogues that all bind to the same allosteric site (Chen et al., 2008). As above, MTEP did not have any effect on the recruitment of activity during CCH (MTEP/CCH: 84.10 ± 0.30%; MTEP/VU-29/CCH: 86.77 ± 0.34%; n = 20; Figure 3(b)). Whether the reduction in spiking rate by VU-29 resulted from indirect feed-forward inhibition or a direct reduction in excitatory neurotransmission remained to be determined.
Figure 3.
CCH (20 μM) evoked activity is reduced by VU-29 (1 μM). Percent changes from control aCSF are shown for the effects of VU-29 during CCH (a; n = 35) and its partial antagonism by MTEP (10 μM; b; n = 20). Left to right: VU-29 in combination with CCH (grid) did not alter the number of active channels but decreased the total spike rate by half (*p < 0.05) irrespective of channel location compared to CCH on its own (filled). In the presence of MTEP (vertical bars), the effects were not fully antagonized and depended on location as evidenced by decreases in spike rate per channel (horizontal bars; *p < 0.05). The partial antagonism by MTEP resulted by enhancing CCH-induced increases in spiking rate in a location specific manner (*p < 0.05, unpaired).
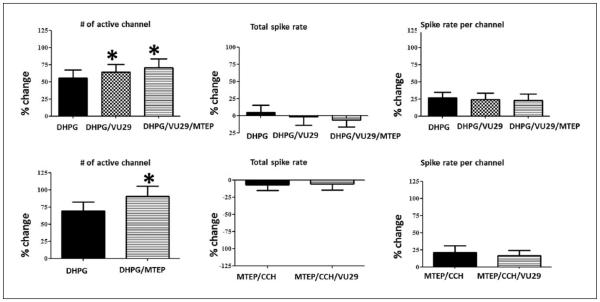
Combined effects of DHPG, VU-29 and MTEP in the ventral mPFC
As mGluR1 is predominantly expressed in interneurons (Lopez-Bendito et al., 2002), we investigated whether the decrease in spike rate by VU-29/CCH depended on the recruitment of mGluR1 mediated inhibition by DHPG. Confirmation of these results would support VU-29-mediated enhancement of excitatory to inhibitory synapses in promoting divergent feed-forward inhibition and a reduction in spike rate. The increased recruitment of activity caused by DHPG was significantly increased by VU-29 (DHPG: 55.15 ± 0.12%; VU-29/DHPG: 64.00 ± 0.12%; n = 30; p < 0.05) and significantly enhanced by MTEP (DHPG: 69.29 ± 0.13%; MTEP/DHPG: 90.61 ± 0.15%; n = 30; p < 0.05). However, there were no changes in the spike rate in the presence of VU-29 (DHPG: 4.9 ± 0.11%; VU-29/DHPG: –1.32 ± 0.13%) or MTEP (DHPG: –7.21 ± 0.08%; MTEP/DHPG: –5.93 ± 0.09%; Figure 4). The enhanced recruitment of activity by either activating or blocking mGluR5 implied that recruitment of mGluR1-mediated inhibition superseded excitation at similar spiking rates.
Figure 4.
DHPG-induced (100 μM) recruitment of activity is enhanced by VU-29 (1 μM) or MTEP (10 μM). Percent changes are shown for the effects of VU-29 and/or MTEP during DHPG (n = 30). During DHPG (filled), increases in the recruitment of activity were observed (*p < 0.05) for VU-29 (top; grid) or MTEP (bottom; horizontal bars) without any effect on spike rate. The effects of VU-29 were not antagonized by MTEP but further increased (top; *p < 0.05).
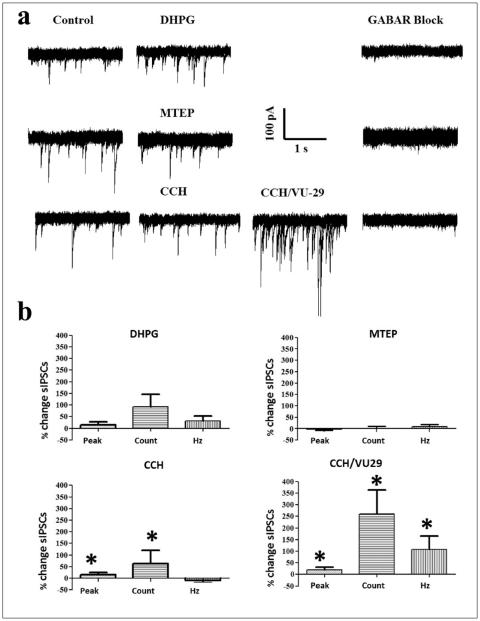
Spontaneous IPSCs are influenced by VU-29, CCH and DHPG in the ventral mPFC
We next asked if the decrease in rate of activity by VU-29 during CCH activation could result from an increase in inhibition. Also, if the increased rate of activity by MTEP was due to a decrease in inhibition. Therefore, we measured sIPSCs for 1 min in layer V ventral mPFC by whole-cell voltage clamp of excitatory cells at −70 mV (Figure 5(a)). Layer V was chosen for recording as it is the main target of information relay from thalamic input, which drives excitation through nAChRs (Gioanni et al., 1999). Based on the size of the ventral mPFC and the larger pyramidal cells in deep layers, the location of layer V was determined to be between 600–800 μm lateral to the interhemispheric fissure using a graticule scale (Paxinos et al., 1980). Excitatory cells were visualized and designated by typical spiking properties during current-evoked steps at the beginning of experiments. Measurements of peak, slope, rise time, number of sIPSCs and instantaneous frequency were analysed (Table 1). Although our measurements of sIPSCs occurred during a holding potential close to reversal of potassium currents, it is not possible to exclude the influence of leak K+ channels on sIPSCs from distal dendrites. Responses that fell within 1 SE of the rise time were included in the analysis to avoid outliers pertaining to slower excitatory events that may not have been blocked by glutamatergic antagonists. As expected, CCH significantly increased the number of sIPSCs (62.51 ± 57.09%; p < 0.05), which in combination with VU-29 was enhanced by an unexpectedly large amount (259.41 ± 104.52%; n = 17; p < 0.05). In contrast, MTEP did not alter the number of sIPSCs (0.49 ± 9.81%; n = 20) and while DHPG substantially increased the number of sIPSCs (DHPG: 93.57 ± 51.81%; n = 26), it was not statistically significant. The GABAA and GABAB receptor antagonists BMI (5 μM) and 2-HS (20 μM), respectively blocked the sIPSCs in all three groups (Figure 5(a)). The largest increases in the number of sIPSCs were accompanied by increases in the instantaneous frequency (CCH: –8.50 ± 11.08 %; CCH/VU-29: 107.78 ± 56.42 %; MTEP: 8.28 ± 9.59 %; DHPG: 32.72 ± 20.07 %) and again only the effects following CCH/ VU-29 were statistically significant (p < 0.05; Figure 5(b)). The resting membrane potential following CCH/VU-29 (–64.41 ± 2.16 mV) and DHPG (–64.23 ± 1.80 mV) became significantly depolarized compared to control (–67.88 ± 0.94 mV; p < 0.05) without an effect on input resistance (216.20 ± 17.79 MΩ), although all treatments tended towards a decrease. The small depolarization of resting membrane potential could reflect the known effects of muscarinic acetylcholine and metabotropic glutamate receptors in closing leak K+ channels and opening non-selective cation channels (Krause et al., 2002). Pearson’s correlations showed that the changes in the number of sIPSCs were independent of amplitude, rise time or instantaneous frequency suggestive of a lack of pathway specificity. There was a near positive correlation (r = 0.85) between the number of sIP-SCs occurring before and after MTEP suggesting that further increases in the number of sIPSCs might have occurred during increased episodes of inhibition during baseline. Such a scenario highlights the flexibility of circuits depending on the strength of inputs to promote excitation or inhibition.
Figure 5.
VU-29 (1 μM) enhanced CCH-mediated (20 μM) increases in sIPSCs. (a) Raw traces (5 second sweeps overlaid) of control (left), during DHPG (100 μM); MTEP (10 μM); CCH or CCH combined with VU-29 (centre) and together with BMI (5 μM) and 2-HS (20 μM; right). (b) Percent changes in peak, number of sIPSCs and instantaneous frequency from left to right. Top: DHPG resulted in substantial increases in the peak and total number of sIPSCs but this was not significant (left; n = 26) while MTEP resulted in minimal change (right; n = 20). Significant increases in the peak and total number of sIPSCs following CCH were considerably enhanced when combined with VU-29 (bottom; n = 17; *p < 0.05). In addition, the combination of CCH and VU-29 resulted in significant increases in instantaneous frequency (*p < 0.05).
Table 1.
Spontaneous IPSC parameters averaged (mean ± SE) for each treatment group.
| Treatment | Peak (pA) | Slope (pA/ms) | Rise time (ms) | Counts | Instantaneous frequency (Hz) |
|---|---|---|---|---|---|
| Control (n = 63) | −73.88 ± 3.87 | −68.42 ± 5.33 | 1.48 ± 0.07 | 80.90 ± 8.03 | 9.13 ± 0.43 |
| DHPG (n = 26) | −68.40 ± 4.15 | −59.63 ± 6.45 | 1.53 ± 0.08 | 76.81 ± 8.81 | 9.21 ± 0.46 |
| MTEP (n = 20) | −81.32 ± 7.91 | −80.63 ± 9.06 | 1.29 ± 0.08 | 81.55 ± 13.50 | 9.51 ± 0.74 |
| CCH (n = 17) | −74.93 ± 7.49 | −66.87 ± 8.05 | 1.49 ± 0.14 | 97.29 ± 27.85 | 9.35 ± 1.17 |
| CCH/VU-29 (n = 17) | −82.37 ± 5.49 | −75.27 ± 6.59 | 1.44 ± 0.12 | 221.94 ± 47.26 | 13.23 ± 0.99 |
Discussion
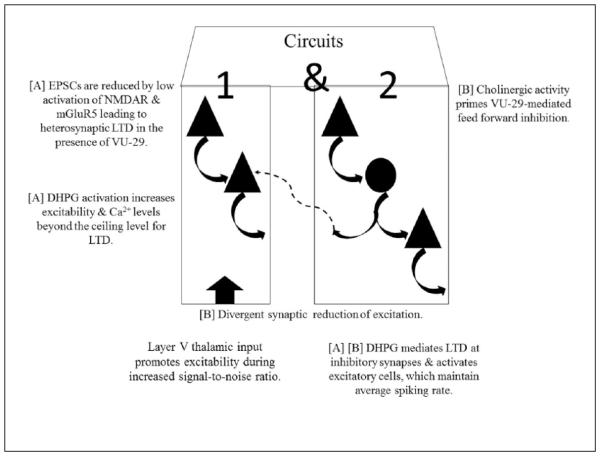
The main finding of the current study is that mGluR5-mediated transmission in the ventral mPFC shows prevalence for suppression of network excitability level. This observation is supported by several of our experimental results. First, the mGluR5 PAM, VU-29, resulted in decreases in spiking rate when combined with the cholinergic agonist, CCH, known to increase the activity of both excitatory and inhibitory neurons through nicotinic receptors (Poorthuis et al., 2013) and reduce excitation via muscarinic receptors (Caruana et al., 2011; Huang and Hsu, 2010). Second, VU-29 enhanced the recruitment of neuronal activity in the presence of the mGluR1 agonist, DHPG, previously shown to facilitate both excitation and, to a greater extent, inhibition via mGluR1 (Sun and Neugebauer, 2011). Third, VU-29 remarkably enhanced sIPSCs compared to CCH. Fourth, DHPG in combination with CCH reduced spiking rate with regard to channel location. Fifth, blocking tonic activation with the specific mGluR5 negative allosteric modulator, MTEP, resulted in an increase in spiking rate. The last point also indicates that, under baseline conditions, mGluR5 acts to maintain a low level spiking rate that cannot be further decreased by VU-29 unless there is further recruitment of neuronal activity. It should be noted that VU-29 can overcome antagonist effects of MTEP analogues as evidenced by a rightward shift in concentration curves without alterations in the maximum glutamate-evoked response by VU-29 (Chen et al., 2008). Moreover, VU-29 has been shown to potentiate mGluR5-mediated intracellular calcium signalling cascades at concentrations that only partially occupy the same binding site as MTEP and analogues (Chen et al., 2008). Therefore, we discuss the effects of enhancing mGluR5 activation by VU-29 without its antagonism by MTEP. Our schematic representation based on spiking rate across channels depicts feed-forward inhibitory circuits as a relevant factor leading to the discrepancy between the known effects of mGluR5-mediated excitation on single cells vs. inhibition during network output (Figure 6). Taken together, these results show the role of mGluR5 in maintaining the signal:noise ratio, thereby increasing the responsiveness of the network by promoting inhibition during baseline conditions and upstates induced by CCH. This effect may allow a high awareness and vigilance state for incoming sensory inputs during learning processes such as fear conditioning acquisition when the amygdala activation leads to inhibition of the mPFC (Milad and Quirk, 2002).
Figure 6.
mPFC circuit schematic based on neuropharmacology. Excitatory post-synaptic current (EPSC) (Hypothesis [A]: circuit 1). mGluR5-mediated reduction in EPSCs is present during baseline conditions but additional recruitment of cells is needed (circuit 2) to cause further reductions by VU-29. Reductions in EPSCs can be due to lower activation of both NMDARs and intracellular Ca2+ release responsible for promoting heterosynaptic LTD (Nishiyama, et al., 2000) or by uncoupling of mGluR5 effectors via Homer1a (Kammermeier and Worley, 2007). Circuit 2, CCH or DHPG recruits both circuit 1 and 2 but DHPG directly activates inhibition via mGluR1 that may lead to LTD at inhibitory synapses. Feed-forward inhibition (Hypothesis [B]: circuit 1). Spiking rate is dictated by the amount of feed-forward inhibition. Circuit 2, CCH or DHPG recruits both 1 and 2. In the case of CCH, excitation is balanced with inhibition via post-synaptic nAChRs and presynaptic mAChRs. However, more excitation is available via CCH for VU-29 to exert its effects by promoting feed-forward inhibition in circuit 2, which predominates due to divergent inhibitory synapses. As average spiking rate was unaltered by the recruitment of inhibition via DHPG, direct mGluR1-mediated LTD of inhibition may have accounted for maintaining control levels of spiking rate at excitatory cells. Triangle: excitatory cell; Circle: inhibitory cell.
Medial prefrontal cortical circuit dynamics and group I metabotropic glutamate receptors
At the single cell level and specifically in response to evoked synaptic activity in the rat mPFC, the mGluR5 PAM, N-Cyclobutyl-6-[2-(3-fluorophenyl)ethynyl]-3-pyridinecarboxamide hydrochloride (VU0360172), has been shown to increase EPSCs by a post-synaptic mechanism and decrease IPSCs via presynaptic retrograde activation of endocannabinoid subtype 1 (CB1) receptors (Kiritoshi et al., 2013). Moreover, the mGluR5 agonist, RS)-2-Chloro-5-hydroxyphenylglycine (CHPG), induces spontaneous EPSCs but not miniature EPSCs in layer V of the prefrontal cortex (Marek and Zhang, 2008), indicating the importance of activation strength. In our study, VU-29 had no effect on baseline but increased the recruitment of neuronal activity in combination with DHPG and decreased spike rate in combination with CCH. Compared to the previous study (Kiritoshi et al., 2013), our results show different effects of an mGluR5 PAM with regard to the whole network activity as opposed to individual recordings of evoked activity at specific stimulated inputs within a microcircuit. In behaving rats, multi-channel recordings also resulted in increases in mPFC spiking rate following dosing with the mGluR5 PAM, CDPPB (Lecourtier et al., 2007) and a reduction with the mGluR5 negative allosteric modulator, 2-Methyl-6-(phenylethynyl)pyridine (MPEP) (Homayoun and Moghaddam, 2006). However, CDPPB prevented excessive spiking rate caused by blocking NMDARs in support of a role of mGluR5 PAMs in maintaining a balance in excitation and inhibition. These dual effects of mGluR5 PAMs highlight the relevance of pathway specificity, either by NMDA receptor activation or indirectly via other limbic projections as compounds were applied systemically in the in vivo studies. In addition to its role in mGluR1-mediated inhibition, DHPG also induced an increase in spontaneous excitatory transmission in layer V mPFC pyramidal cells which, in parallel with MPEP, could be blocked by the mGluR1 antagonist, (S)-(+)-α-Amino-4-carboxy-2-methylbenzeneacetic acid (LY367385, Melendez et al., 2005). This would allude to the possibility of an mGluR1-mediated disinhibitory effect in the mPFC alongside that of feed-forward inhibition. In support of this, it was shown that, compared to excitation, DHPG caused greater increases in synaptic inhibition of layer V mPFC pyramidal cells evoked by presumed amygdala afferents (Sun and Neugebauer, 2011). Our results dictate a similar scenario where network excitation is limited by mGluR5 activation and dependent upon neuronal circuitry; in particular, feed-forward inhibition. Moreover, the significant increases in frequency of sIPSCs during CCH/VU-29 could allude to a summation of convergent inhibitory synaptic activity onto pyramidal neurons. Although, mGluR5 is found predominantly in excitatory cells, some expression on interneurons (Lopez-Bendito et al., 2002) could have also accounted for inhibitory influences in network spiking. A presynaptic mechanism via mGluR5-mediated retrograde signalling is not considered here as this would lead to a reduction in GABAergic neurotransmitter release.
Synergistic effects of carbachol and group I metabotropic glutamate receptors in the mPFC
Presynaptic muscarinic AChR activation has been shown to suppress synaptic transmission in layer II/III prefrontal cortex (Vidal and Changeux, 1993). Post-synaptic muscarinic AChR activation was shown to result in tonic firing of layer V pyramidal cells, which performed as high-pass filters to promote bursting during activation of presynaptic muscarinic AChRs in the same cells (Carr and Surmeier, 2007). Also, the activation of interneurons by nicotinic AChRs and their lack in pyramidal cells of the same layers (Poorthuis et al., 2013) promotes net inhibition in layer II/III of the mPFC. In contrast, direct glutamatergic enhancement by nicotinic AChRs has been observed for thalamocortical inputs to layer V of the prefrontal cortex (Gioanni et al., 1999). Our results demonstrate a dramatic increase in sIPSCs in layer V excitatory cells following VU29/ CCH. The recruitment of neuronal activity caused by CCH in our results may have primed inhibitory synaptic efficacy. Although not significant, it was noted that CCH caused a spread of activity from superficial to deep layers. Therefore, it is plausible that the additional recruitment of inhibition in the deep layers was necessary to promote reduced spiking rates via enhanced activation of mGluR5-mediated excitation by VU-29. The fact that VU-29 decreased spiking rate during CCH but not DHPG application would allude to DHPG-mediated LTD of inhibitory transmission. In the context of learning and cognition, suppression of intrinsic synaptic transmission may promote information relay from extrinsic thalamic inputs including, among others, the amygdala glutamatergic projections, which primarily terminate in layer V and layer II mPFC pyramidal neurons (Cassell et al., 1989) as well as parvalbumin-positive interneurons throughout layers II-VI (Gabbott et al., 2006). Indeed, it has been shown that suppression of synaptic transmission by muscarinic AChR activation also increases the amplitude of LTP in neocortical structures (Lin and Phillis, 1991). Moreover, encoding of learning and consolidation (Giocomo and Hasselmo, 2007), for example, of fear conditioning was blocked by the muscarinic AChR antagonist (Young et al., 1995), scopolamine. In contrast, the retrieval of memories (Giocomo and Hasselmo, 2007) such as fear associations (Rogers and Kesner, 2004) was blocked by the acetylcholinesterase inhibitor physostigmine. A hypothesis based on these results postulates that elevated levels of ACh facilitates encoding while lower levels are necessary for correct retrieval of information (Giocomo and Hasselmo, 2007). The decrease in spiking rate by VU-29/CCH may thus provide benefits during acquisition of fear associations when the amygdala is active. During increased activity of the mPFC, top-down control of the amygdala is in place resulting in extinction of fear-associated memories (Likhtik et al., 2005; Maren and Quirk, 2004; Pape and Paré, 2010; Sah and Westbrook, 2008). It is noteworthy that the mGluR5 PAM, CDPPB enhanced extinction of drug-seeking behaviour (Cleva et al., 2011) while mGluR5 was shown to mediate memory for fear extinction via infralimbic activation (Fontanez-Nuin et al., 2011). As MTEP increased spiking rate in the ventral mPFC, it is possible that synaptic transmission is maintained at relatively low levels during baseline conditions by tonically active feed-forward inhibition. We observed increases in sIPSCs in layer V ventral mPFC excitatory cells during DHPG as well as CCH adding credence to both direct activation of inhibition via mGluR1 and nAChRs or an indirect mGluR5-mediated activation of excitatory onto inhibitory synapses and a presumed reduction in excitation by presynaptic mAChRs. As neither DHPG nor CCH reduced total spiking rate, it is possible that the combined effects of mGluR1 and mGluR5 or nAChR and mAChR maintained the balance in excitation and inhibition towards baseline levels. The difference being that this balance was more susceptible following CCH when combining with VU-29. In our plausible model (Figure 6), either a reduction of EPSCs (Kammermeier and Worley, 2007; Nishiyama, et al., 2000) or feed-forward inhibition is hypothesized to explain the reduction in spike rate and increases in sIP-SCs by VU-29/CCH. The latter requires the assumption that few, low-frequency spiking inhibitory cells are needed in order to exert profound effects on network activity. Feed-back inhibition cannot be excluded, although it may not figure prominently in the present results as adequate activation of mGluR5 reduces presynaptic GABA release through retrograde activation of endocannabinoid receptors in the mPFC (Kiritoshi et al., 2013; Wedzony and Chocyk, 2009) leading to increases or no change in neuronal spiking. The last point takes note that all neurons immunopositive for CB1 receptors were shown to be GABAergic cells in the mPFC (Wedzony and Chocyk, 2009), similar to observations in the hippocampus (Hajos et al., 2000).
In light of the potential for mGluR5 PAMs as cognitive enhancers, our results provide mechanistic insights into the synaptic influences of mGluR1 and mGluR5 during baseline conditions as well as CCH activated up-states. These results are relevant for validation of mGluR5 PAM analogues as well as comparison with models of psychiatric disorders. Chemical induction of LTD by DHPG is mediated post-synaptically via mGluR1 and involves presynaptic endocannabinoid receptors and reduction in neurotransmitter release via mGluR5 (Lüscher & Huber, 2010; Volk et al., 2006). mGluR1 and mGluR5 are predominantly expressed in inhibitory and excitatory cells in the mPFC, respectively (Lopez-Bendito et al., 2002) and endocannabinoid receptors are located on GABAergic presynaptic terminals (Lafourcade et al., 2007; Wedzony and Chocyk, 2009). Therefore, group I mGluRs are in a position to lead to long-lasting depression at inhibitory to excitatory synapses, albeit in the presence of DHPG and in the mPFC. Increasing mPFC excitability leads to inhibition of amygdala output and thereby extinction (Quirk et al., 2003) and retrieval of extinction was shown to be blocked by an mGluR5 antagonist (Fontanez-Nuin et al., 2011). Whether the reduced spiking rate by VU-29, in the presence of CCH in the mPFC, resulted in postsynaptic decreases in EPSCs as observed in autaptic excitatory synapses (Kammermeier and Worley, 2007) and/or indirectly via feed-forward inhibition remains to be determined. Based on our findings, VU-29 may act as cognitive enhancer during the acquisition phase but also might affect the executive role of mPFC in controlling top-down subcortical structures such as the amygdala during conditions of arousal. Similarly, elevated and lower levels of ACh neurotransmission have been linked to encoding and retrieval of memories, respectively (Giocomo and Hasselmo, 2007). Thus, during arousal states, VU-29 may exert its beneficial effects by increasing the signal:noise ratio and enhance acquisition of new learning.
Acknowledgements
The authors would like to acknowledge Dr John Kemp for insightful comments and Erik De Prins for technical assistant.
Funding
This work was supported by an IWT Flander’s Research Grant (00000300661).
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Anderson MC, Green C. Suppressing unwanted memories by executive control. Nature. 2001;410:366–369. doi: 10.1038/35066572. [DOI] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, et al. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000;20:7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Chen Y, Banko JL, et al. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 2009;34:2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Zuschratter W, Wetzel W. Allosteric enhancement of metabotropic glutamate receptor 5 function promotes spatial memory. Neuroscience. 2006;142:691–702. doi: 10.1016/j.neuroscience.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Carr DB, Surmeier DJ. M1 muscarinic receptor modulation of Kir2 channels enhances temporal summation of excitatory synaptic potentials in prefrontal cortex pyramidal neurons. J Neurophysiol. 2007;97:3432–3438. doi: 10.1152/jn.00828.2006. [DOI] [PubMed] [Google Scholar]
- Caruana DA, Warburton EC, Bashir ZI. Induction of activity-dependent LTD requires muscarinic receptor activation in medial prefrontal cortex. J Neurosci. 2011;31:18464–18478. doi: 10.1523/JNEUROSCI.4719-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell MD, Chittick CA, Siegel MA, et al. Collateralization of the amygdaloid projections of the rat prelimbic and infralimbic cortices. J Comp Neurol. 1989;279:235–248. doi: 10.1002/cne.902790207. [DOI] [PubMed] [Google Scholar]
- Chen Y, Goudet C, Pin JP, et al. N-{4-Chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl}-2-hydroxybe nzamide (CPPHA) acts through a novel site as a positive allosteric modulator of group 1 metabotropic glutamate receptors. Mol Pharmacol. 2008;73:909–918. doi: 10.1124/mol.107.040097. [DOI] [PubMed] [Google Scholar]
- Cleva RM, Hicks MP, Gass JT, et al. mGluR5 positive allosteric modulation enhances extinction learning following cocaine self-administration. Behav Neurosci. 2011;125:10–19. doi: 10.1037/a0022339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanez-Nuin DE, Santini E, Quirk GJ, et al. Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons. Cereb Cortex. 2011;21:727–735. doi: 10.1093/cercor/bhq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Busby SJ. Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience. 2006;139:1039–1048. doi: 10.1016/j.neuroscience.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol Psychiatry. 2009;65:717–720. doi: 10.1016/j.biopsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastambide F, Cotel MC, Gilmour G, et al. Selective remediation of reversal learning deficits in the neurodevelopmental MAM model of schizophrenia by a novel mGlu5 positive allosteric modulator. Neuropsychopharmacology. 2012;37:1057–1066. doi: 10.1038/npp.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioanni Y, Rougeot C, Clarke PB, et al. Nicotinic receptors in the rat prefrontal cortex: increase in glutamate release and facilitation of mediodorsal thalamo-cortical transmission. Eur J Neurosci. 1999;11:18–30. doi: 10.1046/j.1460-9568.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Giocomo LM, Hasselmo ME. Neuromodulation by glutamate and acetylcholine can change circuit dynamics by regulating the relative influence of afferent input and excitatory feedback. Mol Neurobiol. 2007;36:184–200. doi: 10.1007/s12035-007-0032-z. [DOI] [PubMed] [Google Scholar]
- Goncalves L, Nogueira MI, Shammah-Lagnado SJ, et al. Prefrontal afferents to the dorsal raphe nucleus in the rat. Brain Res Bull. 2009;78:240–247. doi: 10.1016/j.brainresbull.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, et al. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, et al. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Bursting of prefrontal cortex neurons in awake rats is regulated by metabotropic glutamate 5 (mGlu5) receptors: rate-dependent influence and interaction with NMDA receptors. Cereb Cortex. 2006;16:93–105. doi: 10.1093/cercor/bhi087. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Group 5 metabotropic glutamate receptors: role in modulating cortical activity and relevance to cognition. Eur J Pharmacol. 2010;639:33–39. doi: 10.1016/j.ejphar.2009.12.042. [DOI] [PubMed] [Google Scholar]
- Huang CC, Hsu KS. Activation of muscarinic acetylcholine receptors induces a nitric oxide-dependent long-term depression in rat medial prefrontal cortex. Cereb Cortex. 2010;20:982–996. doi: 10.1093/cercor/bhp161. [DOI] [PubMed] [Google Scholar]
- Hübner C, Bosch D, Gall A, et al. Ex vivo dissection of optogenetically activated mPFC and hippocampal inputs to neurons in the basolateral amygdala: implications for fear and emotional memory. Front Behav Neurosci. 2014 doi: 10.3389/fnbeh.2014.00064. doi: 10.3389/fnbeh.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatical memory task. PLoS Biol. 2005 doi: 10.1371/journal.pbio.0030402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ, Worley PF. Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effectors. Proc Natl Acad Sci U S A. 2007;104:6055–6060. doi: 10.1073/pnas.0608991104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiritoshi T, Sun H, Ren W, et al. Modulation of pyramidal cell output in the medial prefrontal cortex by mGluR5 interacting with CB1. Neuropharmacology. 2013;66:170–178. doi: 10.1016/j.neuropharm.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopfel T, Uusisaari M. Modulation of excitation by metabotropic glutamate receptors. Results Probl Cell Differ. 2008;44:163–175. doi: 10.1007/400_2007_035. [DOI] [PubMed] [Google Scholar]
- Krause M, Offermanns S, Stocker M, et al. Functional specificity of G alpha q and G alpha 11 in the cholinergic and glutamatergic modulation of potassium currents and excitability in hippocampal neurons. J Neurosci. 2002;22:666–673. doi: 10.1523/JNEUROSCI.22-03-00666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade M, Elezgarai I, Mato S, et al. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PloS One. 2007;2:e709. doi: 10.1371/journal.pone.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L, Homayoun H, Tamagnan G, et al. Positive allosteric modulation of metabotropic glutamate 5 (mGlu5) receptors reverses N-Methyl-D-aspartate antagonist-induced alteration of neuronal firing in prefrontal cortex. Biol Psychiatry. 2007;62:739–746. doi: 10.1016/j.biopsych.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesting J, Narayanan RT, Kluge C, et al. Patterns of coupled theta activity inamygdala-hippocampal-prefrontal cortical circuits during fear extinction. PLoS One. 2011 doi: 10.1371/journal.pone.0021714. doi: 10.1371/journal.pone.0021714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, et al. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Phillis JW. Muscarinic agonist-mediated induction of long-term potentiation in rat cerebral cortex. Brain Res. 1991;551:342–345. doi: 10.1016/0006-8993(91)90955-u. [DOI] [PubMed] [Google Scholar]
- Lopes-Aguiar C, Bueno-Junior LS, Ruggiero RN, et al. NMDA receptor blockade impairs the muscarinic conversion of sub-threshold transient depression into long-lasting LTD in the hippocampus-prefrontal cortex pathway in vivo: correlation with gamma oscillations. Neuropharmacology. 2013;65:143–155. doi: 10.1016/j.neuropharm.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Shigemoto R, Fairen A, et al. Differential distribution of group I metabotropic glutamate receptors during rat cortical development. Cereb Cortex. 2002;12:625–638. doi: 10.1093/cercor/12.6.625. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ, Zhang C. Activation of metabotropic glutamate 5 (mGlu5) receptors induces spontaneous excitatory synaptic currents in layer V pyramidal cells of the rat prefrontal cortex. Neurosci Lett. 2008;442:239–243. doi: 10.1016/j.neulet.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev. Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extra-cellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther. 2005;314:139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Neill JC, Barnes S, Cook S, et al. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther. 2010;128:419–432. doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Hong K, Mikoshiba K, et al. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature. 2000;408:584–588. doi: 10.1038/35046067. [DOI] [PubMed] [Google Scholar]
- Pape HC, Paré D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosc Methods. 1980;3:129–149. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–129. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorthuis RB, Bloem B, Schak B, et al. Layer-specific modulation of the prefrontal cortex by nicotinic acetylcholine receptors. Cereb Cortex. 2013;23:148–161. doi: 10.1093/cercor/bhr390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, et al. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, Kesner RP. Cholinergic modulation of the hippocampus during encoding and retrieval of tone/shock-induced fear conditioning. Learning and Memory. 2004;11:102–107. doi: 10.1101/lm.64604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Westbrook RF. Behavioural neuroscience: The circuit of fear. Nature. 2008;454:589–590. doi: 10.1038/454589a. [DOI] [PubMed] [Google Scholar]
- Stauffer SR. Progress toward positive allosteric modulators of the metabotropic glutamate receptor subtype 5 (mGluR5) ACS Chem Neurosci. 2011;2:450–470. doi: 10.1021/cn2000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Neugebauer V. mGluR1, but not mGluR5, activates feed-forward inhibition in the medial prefrontal cortex to impair decision making. J Neurophysiol. 2011;106:960–973. doi: 10.1152/jn.00762.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;141:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Vidal C, Changeux JP. Nicotinic and muscarinic modulations of excitatory synaptic transmission in the rat prefrontal cortex in vitro. Neuroscience. 1993;56:23–32. doi: 10.1016/0306-4522(93)90558-w. [DOI] [PubMed] [Google Scholar]
- Volk LJ, Daly CA, Huber KM. Differential roles for group 1 mGluR subtypes in induction and expression of chemically induced hippocampal long-term depression. J Neurophysiol. 2006;95:2427–2438. doi: 10.1152/jn.00383.2005. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Chocyk A. Cannabinoid CB1 receptors in rat medial prefrontal cortex are colocalized with calbindin- but not parvalbumin- and calretinin-positive GABA-ergic neurons. Pharmacol Reports. 2009;61:1000–1007. doi: 10.1016/s1734-1140(09)70161-6. [DOI] [PubMed] [Google Scholar]
- Young SL, Bohenek DL, Fanselow MS. Scopolamine impairs acquisition and facilitates consolidation of fear conditioning: differential effects for tone vs context conditioning. Neurobiol Learning and Memory. 1995;63:174–180. doi: 10.1006/nlme.1995.1018. [DOI] [PubMed] [Google Scholar]