Abstract
Background
The aggressive and heterogeneous nature of lung cancer has thwarted efforts to reduce mortality from this cancer through the use of screening. The advent of low-dose helical computed tomography (CT) altered the landscape of lung-cancer screening, with studies indicating that low-dose CT detects many tumors at early stages. The National Lung Screening Trial (NLST) was conducted to determine whether screening with low-dose CT could reduce mortality from lung cancer.
Methods
From August 2002 through April 2004, we enrolled 53,454 persons at high risk for lung cancer at 33 U.S. medical centers. Participants were randomly assigned to undergo three annual screenings with either low-dose CT (26,722 participants) or single-view posteroanterior chest radiography (26,732). Data were collected on cases of lung cancer and deaths from lung cancer that occurred through December 31, 2009.
Results
The rate of adherence to screening was more than 90%. The rate of positive screening tests was 24.2% with low-dose CT and 6.9% with radiography over all three rounds. A total of 96.4% of the positive screening results in the low-dose CT group and 94.5% in the radiography group were false positive results. The incidence of lung cancer was 645 cases per 100,000 person-years (1060 cancers) in the low-dose CT group, as compared with 572 cases per 100,000 person-years (941 cancers) in the radiography group (rate ratio, 1.13; 95% confidence interval [CI], 1.03 to 1.23). There were 247 deaths from lung cancer per 100,000 person-years in the low-dose CT group and 309 deaths per 100,000 person-years in the radiography group, representing a relative reduction in mortality from lung cancer with low-dose CT screening of 20.0% (95% CI, 6.8 to 26.7; P = 0.004). The rate of death from any cause was reduced in the low-dose CT group, as compared with the radiography group, by 6.7% (95% CI, 1.2 to 13.6; P = 0.02).
Conclusions
Screening with the use of low-dose CT reduces mortality from lung cancer. (Funded by the National Cancer Institute; National Lung Screening Trial ClinicalTrials.gov number, NCT00047385.)
Lung Cancer Is An Aggressive And Heterogeneous disease.1,2 Advances in surgical, radiotherapeutic, and chemotherapeutic approaches have been made, but the long-term survival rate remains low.3 After the Surgeon General's 1964 report on smoking and health, mortality from lung cancer among men peaked and then fell; among women, the peak occurred later and a slight decline has occurred more recently.4 Even though the rate of heavy smoking continues to decline in the United States,5 94 million current or former smokers remain at elevated risk for the disease,6 and lung cancer remains the leading cause of death from cancer in this country.3 The prevalence of smoking is substantially higher in developing countries than in the United States, and the worldwide burden of lung cancer is projected to rise considerably during the coming years.7
Although effective mass screening of high-risk groups could potentially be of benefit, randomized trials of screening with the use of chest radiography with or without cytologic analysis of sputum specimens have shown no reduction in lung-cancer mortality.8 Molecular markers in blood, sputum, and bronchial brushings have been studied but are currently unsuitable for clinical application.8 Advances in multidetector computed tomography (CT), however, have made high-resolution volumetric imaging possible in a single breath hold at acceptable levels of radiation exposure,9 allowing its use for certain lung-specific applications. Several observational studies have shown that low-dose helical CT of the lung detects more nodules and lung cancers, including early-stage cancers, than does chest radiography.8 Therefore, the National Cancer Institute (NCI) funded the National Lung Screening Trial (NLST), a randomized trial, to determine whether screening with low-dose CT, as compared with chest radiography, would reduce mortality from lung cancer among high-risk persons. The NLST was initiated in 2002.10 In October 2010, the available data showed that there was a significant reduction with low-dose CT screening in the rates of both death from lung cancer and death from any cause. We report here the findings of the NLST, including the performance characteristics of the screening techniques, the approaches used for and the results of diagnostic evaluation of positive screening results, the characteristics of the lung-cancer cases, and mortality. A comprehensive description of the design and operations of the trial, including the collection of the data and the acquisition variables of the screening techniques, has been published previously.10
Methods
Trial Oversight
The NLST, a randomized trial of screening with the use of low-dose CT as compared with screening with the use of chest radiography, was a collaborative effort of the Lung Screening Study (LSS), administered by the NCI Division of Cancer Prevention, and the American College of Radiology Imaging Network (ACRIN), sponsored by the NCI Division of Cancer Treatment and Diagnosis, Cancer Imaging Program. Chest radiography was chosen as the screening method for the control group because radiographic screening was being compared with community care (care that a participant usually receives) in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial (ClinicalTrials.gov number, NCT00002540).11 The NLST was approved by the institutional review board at each of the 33 participating medical institutions. The study was conducted in accordance with the protocol; both the protocol and the statistical analysis plan are available with the full text of this article at NEJM.org.
Participants
We enrolled participants from August 2002 through April 2004; screening took place from August 2002 through September 2007. Participants were followed for events that occurred through December 31, 2009 (Fig. 1 in the Supplementary Appendix, available at NEJM.org).
Eligible participants were between 55 and 74 years of age at the time of randomization, had a history of cigarette smoking of at least 30 pack-years, and, if former smokers, had quit within the previous 15 years. Persons who had previously received a diagnosis of lung cancer, had undergone chest CT within 18 months before enrollment, had hemoptysis, or had an unexplained weight loss of more than 6.8 kg (15 lb) in the preceding year were excluded. A total of 53,454 persons were enrolled; 26,722 were randomly assigned to screening with low-dose CT and 26,732 to screening with chest radiography. Previously published articles describing the NLST10,12 reported an enrollment of 53,456 participants (26,723 in the low-dose CT group and 26,733 in the radiography group). The number of enrolled persons is now reduced by 2 owing to the discovery of the duplicate randomization of 2 participants.
Participants were enrolled at 1 of the 10 LSS or 23 ACRIN centers. Before randomization, each participant provided written informed consent. After the participants underwent randomization, they completed a questionnaire that covered many topics, including demographic characteristics and smoking behavior. The ACRIN centers collected additional data for planned analyses of cost-effectiveness, quality of life, and smoking cessation. Participants at 15 ACRIN centers were also asked to provide serial blood, sputum, and urine specimens. Lung-cancer and other tissue specimens were obtained at both the ACRIN and LSS centers and were used to construct tissue microarrays. All biospecimens are available to researchers through a peer-review process.
Screening
Participants were invited to undergo three screenings (T0, T1, and T2) at 1-year intervals, with the first screening (T0) performed soon after the time of randomization. Participants in whom lung cancer was diagnosed were not offered subsequent screening tests. The number of lung-cancer screening tests that were performed outside the NLST was estimated through self-administered questionnaires that were mailed to a random subgroup of approximately 500 participants from LSS centers annually. Sample sizes were selected to yield a standard error of 0.025 for the estimate of the proportion of participants undergoing lung-cancer screening tests outside the NLST in each group. For participants from ACRIN centers, information on CT examinations or chest radiography performed outside the trial was obtained, but no data were gathered on whether the examinations were performed as screening tests.
All screening examinations were performed in accordance with a standard protocol, developed by medical physicists associated with the trial, that specified acceptable characteristics of the machine and acquisition variables.10,13,14 All low-dose CT scans were acquired with the use of multidetector scanners with a minimum of four channels. The acquisition variables were chosen to reduce exposure to an average effective dose of 1.5 mSv. The average effective dose with diagnostic chest CT varies widely but is approximately 8 mSv.10,13,14 Chest radiographs were obtained with the use of either screen-film radiography or digital equipment. All the machines used for screening met the technical standards of the American College of Radiology.10 The use of new equipment was allowed after certification by medical physicists.
NLST radiologists and radiologic technologists were certified by appropriate agencies or boards and completed training in image acquisition; radiologists also completed training in image quality and standardized image interpretation. Images were interpreted first in isolation and then in comparison with available historical images and images from prior NLST screening examinations. The comparative interpretations were used to determine the outcome of the examination. Low-dose CT scans that revealed any non-calcified nodule measuring at least 4 mm in any diameter and radiographic images that revealed any noncalcified nodule or mass were classified as positive, “suspicious for” lung cancer. Other abnormalities such as adenopathy or effusion could be classified as a positive result as well. Abnormalities suggesting clinically significant conditions other than lung cancer also were noted, as were minor abnormalities. At the third round of screening (T2), abnormalities suspicious for lung cancer that were stable across the three rounds could, according to the protocol, be classified as minor abnormalities rather than positive results.
Results and recommendations from the interpreting radiologist were reported in writing to the participant and his or her health care provider within 4 weeks after the examination. Since there was no standardized, scientifically validated approach to the evaluation of nodules, trial radiologists developed guidelines for diagnostic follow-up, but no specific evaluation approach was mandated.
Medical-Record Abstraction
Medical records documenting diagnostic evaluation procedures and any associated complications were obtained for participants who had positive screening tests and for participants in whom lung cancer was diagnosed. Pathology and tumor-staging reports and records of operative procedures and initial treatment were also obtained for participants with lung cancer. Pathology reports were obtained for other reported cancers to exclude the possibility that such tumors represented lung metastases. Histologic features of the lung cancer were coded according to the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3),15 and the disease stage was determined according to the sixth edition of the Cancer Staging Manual of the American Joint Committee on Cancer.16 At ACRIN sites, additional medical records were also obtained for a number of substudies, including studies of health care utilization and cost-effectiveness.10
Vital Status
Participants completed a questionnaire regarding vital status either annually (LSS participants) or semiannually (ACRIN participants). The names and Social Security numbers of participants who were lost to follow-up were submitted to the National Death Index to ascertain probable vital status. Death certificates were obtained for participants who were known to have died. An end-point verification team determined whether the cause of death was lung cancer. Although a distinction was made between a death caused by lung cancer and a death that resulted from the diagnostic evaluation for or treatment of lung cancer, the deaths from the latter causes were counted as lung-cancer deaths in the primary end-point analysis. The members of the team were not aware of the group assignments (see Section 2 in the Supplementary Appendix).
Statistical Analysis
The primary analysis was a comparison of lung-cancer mortality between the two screening groups, according to the intention-to-screen principle. We estimated that the study would have 90% power to detect a 21% decrease in mortality from lung cancer in the low-dose CT group, as compared with the radiography group. Secondary analyses compared the rate of death from any cause and the incidence of lung cancer in the two groups.
Event rates were defined as the ratio of the number of events to the person-years at risk for the event. For the incidence of lung cancer, person-years were measured from the time of randomization to the date of diagnosis of lung cancer, death, or censoring of data (whichever came first); for the rates of death, person-years were measured from the time of randomization to the date of death or censoring of data (whichever came first). The latest date for the censoring of data on incidence of lung cancer and on death from any cause was December 31, 2009; the latest date for the censoring of data on death from lung cancer for the purpose of the primary end-point analysis was January 15, 2009. The earlier censoring date for death from lung cancer was established to allow adequate time for the review process for deaths to be performed to the same, thorough extent in each group. We calculated the confidence intervals for incidence ratios assuming a Poisson distribution for the number of events and a normal distribution of the logarithm of the ratio, using asymptotic methods. We calculated the confidence intervals for mortality ratios with the weighted method that was used to monitor the primary end point of the trial,17 which allows for a varying rate ratio and is adjusted for the design. The number needed to screen to prevent one death from lung cancer was estimated as the reciprocal of the reduction in the absolute risk of death from lung cancer in one group as compared with the other, among participants who had at least one screening test. The analyses were performed with the use of SAS/STAT18 and R19 statistical packages.
Interim analyses were performed to monitor the primary end point for efficacy and futility. The analyses involved the use of a weighted log-rank statistic, with weights increasing linearly from no weight at randomization to full weight at 4 years and thereafter. Efficacy and futility boundaries were built on the Lan–DeMets approach with an O'Brien–Fleming spending function.20 Interim analyses were performed annually from 2006 through 2009 and semiannually in 2010.
An independent data and safety monitoring board met every 6 months and reviewed the accumulating data. On October 20, 2010, the board determined that a definitive result had been reached for the primary end point of the trial and recommended that the results be reported.21 The board's decision took into consideration that the efficacy boundary for the primary end point had been crossed and that there was no evidence of unforeseen screening effects that warranted acting contrary to the trial's prespecified monitoring plan. The NCI director accepted the recommendation of the data and safety monitoring board, and the trial results were announced on November 4, 2010.
Results
Characteristics of the Participants
The demographic characteristics and smoking history of the participants were virtually identical in the two groups (Table 1). As compared with respondents to a 2002–2004 U.S. Census survey of tobacco use22 who met the NLST eligibility criteria for age and smoking history, NLST participants were younger, had a higher level of education, and were more likely to be former smokers.12 As of December 31, 2009, vital status was known for 97% of the participants in the low-dose CT group and 96% of those in the radiography group. The median duration of follow-up was 6.5 years, with a maximum duration of 7.4 years in each group.
Table 1. Selected Baseline Characteristics of the Study Participants*.
| Characteristic | Low-Dose CT Group (N = 26,722) | Radiography Group (N = 26,732) |
|---|---|---|
| number (percent) | ||
| Age at randomization | ||
| <55 yr† | 2 (<0.1) | 4 (<0.1) |
| 55–59 yr | 11,440 (42.8) | 11,420 (42.7) |
| 60–64 yr | 8,170 (30.6) | 8,198 (30.7) |
| 65–69 yr | 4,756 (17.8) | 4,762 (17.8) |
| 70–74 yr | 2,353 (8.8) | 2,345 (8.8) |
| ≥75 yr† | 1 (<0.1) | 3 (<0.1) |
| Sex | ||
| Male | 15,770 (59.0) | 15,762 (59.0) |
| Female | 10,952 (41.0) | 10,970 (41.0) |
| Race or ethnic group‡ | ||
| White | 24,289 (90.9) | 24,260 (90.8) |
| Black | 1,195 (4.5) | 1,181 (4.4) |
| Asian | 559 (2.1) | 536 (2.0) |
| American Indian or Alaska Native | 92 (0.3) | 98 (0.4) |
| Native Hawaiian or other Pacific Islander | 91 (0.3) | 102 (0.4) |
| More than one race or ethnic group | 333 (1.2) | 346 (1.3) |
| Data missing | 163 (0.6) | 209 (0.8) |
| Hispanic ethnic group‡ | ||
| Hispanic or Latino | 479 (1.8) | 456 (1.7) |
| Neither Hispanic nor Latino | 26,079 (97.6) | 26,039 (97.4) |
| Data missing | 164 (0.6) | 237 (0.9) |
| Smoking status | ||
| Current | 12,862 (48.1) | 12,900 (48.3) |
| Former | 13,860 (51.9) | 13,832 (51.7) |
CT denotes computed tomography.
Patients in this age range were ineligible for inclusion in the screening trial but were enrolled and were included in all analyses.
Race or ethnic group was self-reported.
Adherence to Screening
The rate of adherence to the screening protocol across the three rounds was high: 95% in the low-dose CT group and 93% in the radiography group. Among LSS participants in the radiography group, the average annual rate of helical CT screening outside the NLST during the screening phase of the trial was 4.3%, which was well below the 10.0% rate estimated in the trial power calculations.
Results of Screening
In all three rounds, there was a substantially higher rate of positive screening tests in the low-dose CT group than in the radiography group (T0, 27.3% vs. 9.2%; T1, 27.9% vs. 6.2%; and T2, 16.8% vs. 5.0%) (Table 2). The rate of positive tests in both groups was noticeably lower at T2 than at T0 or T1 because the NLST protocol allowed tests showing abnormalities at T2 that were suspicious for cancer but were stable across all three rounds to be categorized as negative with minor abnormalities. During the screening phase of the trial, 39.1% of the participants in the low-dose CT group and 16.0% of those in the radiography group had at least one positive screening result. The percentage of all screening tests that identified a clinically significant abnormality other than an abnormality suspicious for lung cancer was more than three times as high in the low-dose CT group as in the radiography group (7.5% vs. 2.1%).
Table 2. Results of Three Rounds of Screening*.
| Screening Round | Low-Dose CT | Chest Radiography | ||||||
|---|---|---|---|---|---|---|---|---|
| Total No. Screened | Positive Result | Clinically Significant Abnormality Not Suspicious for Lung Cancer | No or Minor Abnormality | Total No. Screened | Positive Result | Clinically Significant Abnormality Not Suspicious for Lung Cancer | No or Minor Abnormality | |
| no. (% of screened) | no. (% of screened) | |||||||
| T0 | 26,309 | 7191 (27.3) | 2695 (10.2) | 16,423 (62.4) | 26,035 | 2387 (9.2) | 785 (3.0) | 22,863 (87.8) |
| T1 | 24,715 | 6901 (27.9) | 1519 (6.1) | 16,295 (65.9) | 24,089 | 1482 (6.2) | 429 (1.8) | 22,178 (92.1) |
| T2 | 24,102 | 4054 (16.8) | 1408 (5.8) | 18,640 (77.3) | 23,346 | 1174 (5.0) | 361 (1.5) | 21,811 (93.4) |
The screenings were performed at 1-year intervals, with the first screening (T0) performed soon after the time of randomization. Results of screening tests that were technically inadequate (7 in the low-dose CT group and 26 in the radiography group, across the three screening rounds) are not included in this table. A screening test with low-dose CT was considered to be positive if it revealed a nodule at least 4 mm in any diameter or other abnormalities that were suspicious for lung cancer. A screening test with chest radiography was considered to be positive if it revealed a nodule or mass of any size or other abnormalities suspicious for lung cancer.
Follow-up of Positive Results
More than 90% of the positive screening tests in the first round of screening (T0) led to a diagnostic evaluation (Table 3). Lower rates of follow-up were seen at later rounds. The diagnostic evaluation most often consisted of further imaging, and invasive procedures were performed infrequently. Across the three rounds, 96.4% of the positive results in the low-dose CT group and 94.5% of those in the radiography group were false positive results. These percentages varied little by round. Of the total number of low-dose CT screening tests in the three rounds, 24.2% were classified as positive and 23.3% had false positive results; of the total number of radiographic screening tests in the three rounds, 6.9% were classified as positive and 6.5% had false positive results.
Table 3. Diagnostic Follow-up of Positive Screening Results in the Three Screening Rounds*.
| Variable | Low-Dose CT | Chest Radiography | ||||||
|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | Total | T0 | T1 | T2 | Total | |
| number (percent) | ||||||||
| Total positive tests | 7191 (100.0) | 6901 (100.0) | 4054 (100.0) | 18,146 (100.0) | 2387 (100.0) | 1482 (100.0) | 1174 (100.0) | 5043 (100.0) |
| Lung cancer confirmed | 270 (3.8) | 168 (2.4) | 211 (5.2) | 649 (3.6) | 136 (5.7) | 65 (4.4) | 78 (6.6) | 279 (5.5) |
| Lung cancer not confirmed† | 6921 (96.2) | 6733 (97.6) | 3843 (94.8) | 17,497 (96.4) | 2251 (94.3) | 1417 (95.6) | 1096 (93.4) | 4764 (94.5) |
| Positive screening results with complete diagnostic follow-up information | 7049 (100.0) | 6740 (100.0) | 3913 (100.0) | 17,702 (100.0) | 2348 (100.0) | 1456 (100.0) | 1149 (100.0) | 4953 (100.0) |
| Any diagnostic follow-up | 6369 (90.4) | 3866 (57.4) | 2522 (64.5) | 12,757 (72.1) | 2176 (92.7) | 1078 (74.0) | 957 (83.3) | 4211 (85.0) |
| Clinical procedure | 5089 (72.2) | 3190 (47.3) | 2151 (55.0) | 10,430 (58.9) | 1414 (60.2) | 723 (49.7) | 658 (57.3) | 2795 (56.4) |
| Imaging examination | 5717 (81.1) | 2520 (37.4) | 2009 (51.3) | 10,246 (57.9) | 2010 (85.6) | 968 (66.5) | 906 (78.9) | 3884 (78.4) |
| Chest radiography | 1284 (18.2) | 613 (9.1) | 650 (16.6) | 2,547 (14.4) | 867 (36.9) | 381 (26.2) | 365 (31.8) | 1613 (32.6) |
| Chest CT | 5153 (73.1) | 2046 (30.4) | 1608 (41.1) | 8,807 (49.8) | 1546 (65.8) | 745 (51.2) | 712 (62.0) | 3003 (60.6) |
| FDG PET or FDG PET-CT | 728 (10.3) | 350 (5.2) | 393 (10.0) | 1,471 (8.3) | 179 (7.6) | 105 (7.2) | 113 (9.8) | 397 (8.0) |
| Percutaneous cytologic examination or biopsy | 155 (2.2) | 74 (1.1) | 93 (2.4) | 322 (1.8) | 83 (3.5) | 37 (2.5) | 52 (4.5) | 172 (3.5) |
| Transthoracic | 120 (1.7) | 60 (0.9) | 74 (1.9) | 254 (1.4) | 67 (2.9) | 31 (2.1) | 43 (3.7) | 141 (2.8) |
| Extra thoracic | 39 (0.6) | 17 (0.3) | 24 (0.6) | 80 (0.5) | 20 (0.9) | 6 (0.4) | 13 (1.1) | 39 (0.8) |
| Bronchoscopy | 306 (4.3) | 178 (2.6) | 187 (4.8) | 671 (3.8) | 107 (4.6) | 56 (3.8) | 62 (5.4) | 225 (4.5) |
| With neither biopsy nor cytologic testing | 126 (1.8) | 95 (1.4) | 99 (2.5) | 320 (1.8) | 45 (1.9) | 19 (1.3) | 32 (2.8) | 96 (1.9) |
| With biopsy or cytologic testing | 194 (2.8) | 95 (1.4) | 102 (2.6) | 391 (2.2) | 74 (3.2) | 40 (2.7) | 36 (3.1) | 150 (3.0) |
| Surgical procedure | 297 (4.2) | 197 (2.9) | 219 (5.6) | 713 (4.0) | 121 (5.2) | 51 (3.5) | 67 (5.8) | 239 (4.8) |
| Mediastinoscopy or mediastinotomy | 60 (0.9) | 32 (0.5) | 25 (0.6) | 117 (0.7) | 22 (0.9) | 12 (0.8) | 21 (1.8) | 55 (1.1) |
| Thoracoscopy | 82 (1.2) | 56 (0.8) | 96 (2.5) | 234 (1.3) | 22 (0.9) | 11 (0.8) | 20 (1.7) | 53 (1.1) |
| Thoracotomy | 197 (2.8) | 148 (2.2) | 164 (4.2) | 509 (2.9) | 96 (4.1) | 44 (3.0) | 44 (3.8) | 184 (3.7) |
| Other procedures | 168 (2.4) | 96 (1.4) | 63 (1.6) | 327 (1.8) | 55 (2.3) | 33 (2.3) | 34 (3.0) | 122 (2.5) |
The screenings were performed at 1-year intervals, with the first screening (T0) performed soon after the time of randomization. FDG PET denotes 18F-fluorodeoxyglucose positron-emission tomography.
Positive tests with incomplete information on diagnostic follow-up are included in this category (142 at T0, 161 at Tl, and 141 at T2 in the low-dose CT group and 39 at T0, 26 at Tl, and 25 at T2 in the radiography group).
Adverse Events
Adverse events from the actual screening examinations were few and minor. The rates of complications after a diagnostic evaluation procedure for a positive screening test (listed by category in Table 1 in the Supplementary Appendix) were low; the rate of at least one complication was 1.4% in the low-dose CT group and 1.6% in the radiography group (Table 4). A total of 0.06% of the positive screening tests in the low-dose CT group that did not result in a diagnosis of lung cancer and 11.2% of those that did result in a diagnosis of lung cancer were associated with a major complication after an invasive procedure; the corresponding percentages in the radiography group were 0.02% and 8.2%. The frequency of major complications varied according to the type of invasive procedure. A total of 16 participants in the low-dose CT group (10 of whom had lung cancer) and 10 in the radiography group (all of whom had lung cancer) died within 60 days after an invasive diagnostic procedure. Although it is not known whether the complications from the diagnostic procedure caused the deaths, the low frequency of death within 60 days after the procedure suggests that death as a result of the diagnostic evaluation of positive screening tests is a rare occurrence.
Table 4. Complications after the Most Invasive Screening-Related Diagnostic Evaluation Procedure, According to Lung-Cancer Status*.
| Complication | Lung Cancer Confirmed | Lung Cancer Not Confirmed | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Thoracotomy, Thoracoscopy, or Mediastinoscopy |
Bron- choscopy |
Needle Biopsy |
No Invasive Procedure |
Total | Thoracotomy, Thoracoscopy, or Mediastinoscopy |
Bronchoscopy | Needle Biopsy |
No Invasive Procedure |
Total | |
| number (percent) | number (percent) | |||||||||
| Low-dose CT group | ||||||||||
| Positive screening results for which diagnostic information was complete | 509 (100.0) | 76 (100.0) | 33 (100.0) | 31 (100.0) | 649 (100.0) | 164 (100.0) | 227 (100.0) | 66 (100.0) | 16,596 (100.0) | 17,053 (100.0) |
| No complication | 344 (67.6) | 69 (90.8) | 26 (78.8) | 26 (83.9) | 465 (71.6) | 138 (84.1) | 216 (95.2) | 59 (89.4) | 16,579 (99.9) | 16,992 (99.6) |
| At least one complication | 165 (32.4) | 7 (9.2) | 7 (21.2) | 5 (16.1) | 184 (28.4) | 26 (15.9) | 11 (4.8) | 7 (10.6) | 17 (0.1) | 61 (0.4) |
| Most severe complication classified as major | 71 (13.9) | 2 (2.6) | 0 | 2 (6.5) | 75 (11.6) | 9 (5.5) | 2 (0.9) | 0 | 1 (<0.1) | 12 (0.1) |
| Most severe complication classified as intermediate | 81 (15.9) | 5 (6.6) | 7 (21.2) | 2 (6.5) | 95 (14.6) | 13 (7.9) | 9 (4.0) | 6 (9.1) | 16 (0.1) | 44 (0.3) |
| Most severe complication classified as minor | 13 (2.6) | 0 | 0 | 1 (3.2) | 14 (2.2) | 4 (2.4) | 0 | 1 (1.5) | 0 | 5 (<0.1) |
| Death within 60 days after most invasive diagnostic procedure† | 5 (1.0) | 4 (5.3) | 1 (3.0) | 0 | 10 (1.5) | 2 (1.2) | 4 (1.8) | 0 | 5 (<0.1) | 11 (0.1) |
| Radiography group | ||||||||||
| Positive screening results for which diagnostic information was complete | 189 (100.0) | 46 (100.0) | 29 (100.0) | 15 (100.0) | 279 (100.0) | 45 (100.0) | 46 (100.0) | 24 (100.0) | 4,559 (100.0) | 4,674 (100.0) |
| No complication | 130 (68.8) | 42 (91.3) | 28 (96.6) | 14 (93.3) | 214 (76.7) | 38 (84.4) | 46 (100.0) | 23 (95.8) | 4,551 (99.8) | 4,658 (99.7) |
| At least one complication | 59 (31.2) | 4 (8.7) | 1 (3.4) | 1 (6.7) | 65 (23.3) | 7 (15.6) | 0 | 1 (4.2) | 8 (0.2) | 16 (0.3) |
| Most severe complication classified as major | 22 (11.6) | 1 (2.2) | 0 | 1 (6.7) | 24 (8.6) | 1 (2.2) | 0 | 0 | 3 (0.1) | 4 (0.1) |
| Most severe complication classified as intermediate | 32 (16.9) | 2 (4.3) | 1 (3.4) | 0 | 35 (12.5) | 6 (13.3) | 0 | 1 (4.2) | 2 (<0.1) | 9 (0.2) |
| Most severe complication classified as minor | 5 (2.6) | 1 (2.2) | 0 | 0 | 6 (2.2) | 0 | 0 | 0 | 3 (0.1) | 3 (0.1) |
| Death within 60 days after most invasive diagnostic procedure† | 4 (2.1) | 5 (10.9) | 1 (3.4) | 1 (6.7) | 11 (3.9) | 0 | 0 | 0 | 3 (0.1) | 3 (0.1) |
In the case of multiple evaluation procedures of the same type, the earliest is included. Complications that occurred before the most invasive procedure are not included. Participants could have up to three positive screening tests and therefore may be included up to three times in any row. Columns of procedures are arranged in decreasing order of invasiveness. In the case of the first procedure column, thoracotomy was considered to be more invasive than thoracoscopy, which was considered to be more invasive than mediastinoscopy.
For patients who did not undergo an invasive procedure, deaths were included if they occurred within 60 days after the positive screening result.
Incidence, Characteristics, and Treatment of Lung Cancers
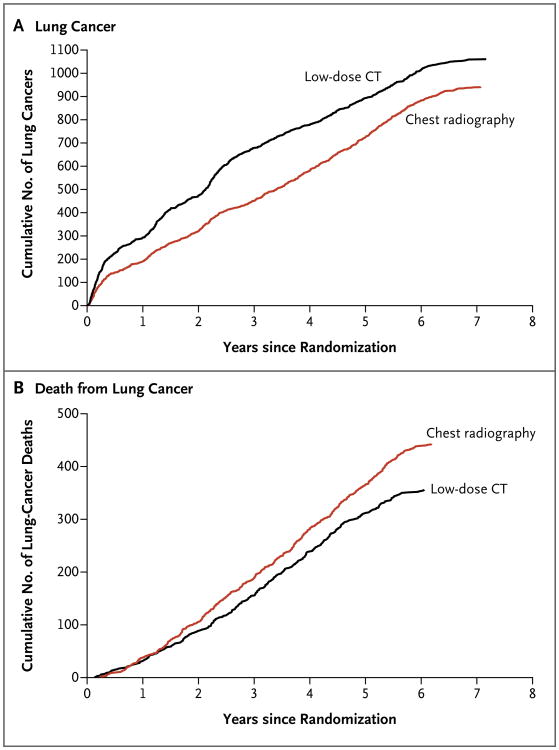
A total of 1060 lung cancers (645 per 100,000 person-years) were diagnosed in the low-dose CT group, as compared with 941 (572 per 100,000 person-years) in the radiography group (rate ratio, 1.13; 95% confidence interval [CI], 1.03 to 1.23). In the low-dose CT group, 649 cancers were diagnosed after a positive screening test, 44 after a negative screening test, and 367 among participants who either missed the screening or received the diagnosis after their trial screening phase was over (Table 5). In the radiography group, 279 cancers were diagnosed after a positive screening test, 137 after a negative screening test, and 525 among participants who either missed the screening or received the diagnosis after their trial screening phase was over. Figure 1A shows the cumulative number of lung cancers through December 31, 2009, according to the screening group. Detailed calculations of sensitivity, specificity, positive predictive value, and negative predictive value are not reported here.
Table 5. Stage and Histologic Type of Lung Cancers in the Two Screening Groups, According to the Result of Screening*.
| Stage and Histologic Type | Low-Dose CT | Chest Radiography | ||||||
|---|---|---|---|---|---|---|---|---|
| Positive Screening Test (N=649) | Negative Screening Test (N = 44)† | No Screening Test (N=367)‡ | Total (N = 1060) | Positive Screening Test (N = 279) | Negative Screening Test (N = 137)† | No Screening Test (N = 525)‡ | Total (N=941) | |
| number/total umber (percent) | ||||||||
| Stage | ||||||||
| IA | 329/635 (51.8) | 5/44 (11.4) | 82/361 (22.7) | 416/1040 (40.0) | 90/275 (32.7) | 16/135 (11.9) | 90/519 (17.3) | 196/929 (21.1) |
| IB | 71/635 (11.2) | 2/44 (4.5) | 31/361 (8.6) | 104/1040 (10.0) | 41/275 (14.9) | 6/135 (4.4) | 46/519 (8.9) | 93/929 (10.0) |
| IIA | 26/635 (4.1) | 2/44 (4.5) | 7/361 (1.9) | 35/1040 (3.4) | 14/275 (5.1) | 2/135 (1.5) | 16/519 (3.1) | 32/929 (3.4) |
| IIB | 20/635 (3.1) | 3/44 (6.8) | 15/361 (4.2) | 38/1040 (3.7) | 11/275 (4.0) | 6/135 (4.4) | 25/519 (4.8) | 42/929 (4.5) |
| IIIA | 59/635 (9.3) | 3/44 (6.8) | 37/361 (10.2) | 99/1040 (9.5) | 35/275 (12.7) | 21/135 (15.6) | 53/519 (10.2) | 109/929 (11.7) |
| IIIB | 49/635 (7.7) | 15/44 (34.1) | 58/361 (16.1) | 122/1040 (11.7) | 27/275 (9.8) | 24/135 (17.8) | 71/519 (13.7) | 122/929 (13.1) |
| IV | 81/635 (12.8) | 14/44 (31.8) | 131/361 (36.3) | 226/1040 (21.7) | 57/275 (20.7) | 60/135 (44.4) | 218/519 (42.0) | 335/929 (36.1) |
| Histologic type | ||||||||
| Bronchioloalveolar carcinoma | 95/646 (14.7) | 1/44 (2.3) | 14/358 (3.9) | 110/1048 (10.5) | 13/276 (4.7) | 1/135 (0.7) | 21/520 (4.0) | 35/931 (3.8) |
| Adenocarcinoma | 258/646 (39.9) | 8/44 (18.2) | 114/358 (31.8) | 380/1048 (36.3) | 112/276 (40.6) | 37/135 (27.4) | 179/520 (34.4) | 328/931 (35.2) |
| Squamous-cell carcinoma | 136/646 (21.1) | 13/44 (29.5) | 94/358 (26.3) | 243/1048 (23.2) | 70/276 (25.4) | 24/135 (17.8) | 112/520 (21.5) | 206/931 (22.1) |
| Large-cell carcinoma | 28/646 (4.3) | 3/44 (6.8) | 10/358 (2.8) | 41/1048 (3.9) | 12/276 (4.3) | 10/135 (7.4) | 21/520 (4.0) | 43/931 (4.6) |
| Non-small-cell carcinoma orother§ | 75/646 (11.6) | 4/44 (9.1) | 52/358 (14.5) | 131/1048 (12.5) | 40/276 (14.5) | 30/135 (22.2) | 88/520 (16.9) | 158/931 (17.0) |
| Small-cell carcinoma | 49/646 (7.6) | 15/44 (34.1) | 73/358 (20.4) | 137/1048 (13.1) | 28/276 (10.1) | 32/135 (23.7) | 99/520 (19.0) | 159/931 (17.1) |
| Carcinoid | 5/646 (0.8) | 0 | 1/358 (0.3) | 6/1048 (0.6) | 1/276 (0.4) | 1/135 (0.7) | 0 | 2/931 (0.2) |
The denominators represent only cancers with a known stage or known histologic type. The stage was not known in the case of 14 cancers after a positive screening test and 6 after no screening in the low-dose CT group and in the case of 4 cancers after a positive screening test, 2 after a negative screening test, and 6 after no screening in the radiography group. The histologic type was not known for 3 cancers after a positive screening test and 9 after no screening in the low-dose CT group and for 3 cancers after a positive screening test, 2 after a negative screening test, and 5 after no screening in the radiography group.
Negative screening tests included tests that revealed either minor or clinically significant abnormalities that were not suspicious for lung cancer.
The 892 lung cancers in participants with no screening test included 35 in participants who were never screened, 802 that were diagnosed during the post-screening period, and 55 in participants who were due for a screening test.
The 289 lung cancers in this category (in the two groups combined) included 28 adenosquamous carcinomas, 6 sarcomatoid carcinomas, 55 unclassified carcinomas, 1 anaplastic-type carcinoma, 1 carcinosarcoma, and 198 coded only as “non-small-cell carcinoma.”
Figure 1. Cumulative Numbers of Lung Cancers and of Deaths from Lung Cancer.
The number of lung cancers (Panel A) includes lung cancers that were diagnosed from the date of randomization through December 31, 2009. The number of deaths from lung cancer (Panel B) includes deaths that occurred from the date of randomization through January 15, 2009.
In each group, the percentage of stage IA and stage IB lung cancers was highest among cancers that were diagnosed after a positive screening test (Table 5). Fewer stage IV cancers were seen in the low-dose CT group than in the radiography group at the second and third screening rounds (Table 2 in the Supplementary Appendix). Low-dose CT screening identified a preponderance of adenocarcinomas, including bronchioloalveolar carcinomas. Although the use of the term bronchioloalveolar carcinoma is no longer recommended,23 while the NLST was ongoing, the term was used to denote in situ, minimally invasive, or invasive adenocarcinoma, lepidic predominant (i.e., neo-plastic cell growth restricted to preexisting alveolar structure). In both groups, many adenocarcinomas and squamous-cell carcinomas were detected at either stage I or stage II, although the stage distribution was more favorable in the low-dose CT group than in the radiography group (Table 6). Small-cell lung cancers were, in general, not detected at early stages by either low-dose CT or radiography. A total of 92.5% of stage IA and stage IB cancers in the low-dose CT group and 87.5% of those in the radiography group were treated with surgery alone or surgery combined with chemotherapy, radiation therapy, or both (Table 3 in the Supplementary Appendix).
Table 6. Histologic Type of Lung Cancers in the Two Screening Groups, According to Tumor Stage*.
| Histologic Type | Total No. of Cancers | Stage of Cancer | ||||||
|---|---|---|---|---|---|---|---|---|
| IA | IB | IIA | IIB | IIIA | IIIB | IV | ||
| number/total number (percent) | ||||||||
| Low-dose CT group | ||||||||
| Branch ioloalveolar carcinoma | 110 | 83/110 (75.5) | 6/110 (5.5) | 3/110 (2.7) | 1/110 (0.9) | 1/110 (0.9) | 8/110 (7.3) | 8/110 (7.3) |
| Adenocarcinoma | 380 | 173/376 (46.0) | 48/376 (12.8) | 17/376 (4.5) | 10/376 (2.7) | 31/376 (8.2) | 33/376 (8.8) | 64/376 (17.0) |
| Squamous-cell carcinoma | 243 | 90/239 (37.7) | 35/239 (14.6) | 9/239 (3.8) | 16/239 (6.7) | 26/239 (10.9) | 32/239 (13.4) | 31/239 (13.0) |
| Large-cell carcinoma | 41 | 17/41 (41.5) | 4/41 (9.8) | 0/41 | 3/41 (7.3) | 7/41 (17.1) | 5/41 (12.2) | 5/41 (12.2) |
| Non-small-cell carcinoma, other† | 131 | 38/127 (29.9) | 10/127 (7.9) | 1/127 (0.8) | 5/127 (3.9) | 16/127 (12.6) | 17/127 (13.4) | 40/127 (31.5) |
| Small-cell carcinoma | 137 | 8/133 (6.0) | 1/133 (0.8) | 5/133 (3.8) | 3/133 (2.3) | 17/133 (12.8) | 27/133 (20.3) | 72/133 (54.1) |
| Carcinoid | 6 | 2/2 (100.0) | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 |
| Unknown | 12 | 5/12 (41.7) | 0/12 | 0/12 | 0/12 | 1/12 (8.3) | 0/12 | 6/12 (50.0) |
| Total | 1060 | 416/1040 (40.0) | 104/1040 (10.0) | 35/1040 (3.4) | 38/1040 (3.7) | 99/1040 (9.5) | 122/1040 (11.7) | 226/1040 (21.7) |
| Radiography group | ||||||||
| Branch ioloalveolar carcinoma | 35 | 17/35 (48.6) | 1/35 (2.9) | 1/35 (2.9) | 2/35 (5.7) | 3/35 (8.6) | 5/35 (14.3) | 6/35 (17.1) |
| Adenocarcinoma | 328 | 83/326 (25.5) | 42/326 (12.9) | 17/326 (5.2) | 12/326 (3.7) | 29/326 (8.9) | 29/326 (8.9) | 114/326 (35.0) |
| Squamous-cell carcinoma | 206 | 51/205 (24.9) | 29/205 (14.1) | 6/205 (2.9) | 17/205 (8.3) | 24/205 (11.7) | 28/205 (13.7) | 50/205 (24.4) |
| Large-cell carcinoma | 43 | 9/42 (21.4) | 5/42 (11.9) | 1/42 (2.4) | 1/42 (2.4) | 10/42 (23.8) | 7/42 (16.7) | 9/42 (21.4) |
| Non-small-cell carcinoma or other† | 158 | 20/155 (12.9) | 9/155 (5.8) | 3/155 (1.9) | 5/155 (3.2) | 24/155 (15.5) | 24/155 (15.5) | 70/155 (45.2) |
| Small-cell carcinoma | 159 | 11/157 (7.0) | 6/157 (3.8) | 4/157 (2.5) | 5/157 (3.2) | 18/157 (11.5) | 28/157 (17.8) | 85/157 (54.1) |
| Carcinoid | 2 | 2/2 (100.0) | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 |
| Unknown | 10 | 3/7 (42.9) | 1/7 (14.3) | 0/7 | 0/7 | 1/7 (14.3) | 1/7 (14.3) | 1/7 (14.3) |
| Total | 941 | 196/929 (21.1) | 93/929 (10.0) | 32/929 (3.4) | 42/929 (4.5) | 109/929 (11.7) | 122/929 (13.1) | 335/929 (36.1) |
The denominators represent only cancers for which the stage was known.
The 289 lung cancers in this category (in the two groups combined) included 28 adenosquamous carcinomas, 6 sarcomatoid carcinomas, 55 unclassified carcinomas, 1 anaplastic-type carcinoma, 1 carcinosarcoma, and 198 coded only as “non-small-cell carcinoma.”
Lung-Cancer–Specific Mortality
After the accrual of 144,103 person-years in the low-dose CT group and 143,368 person-years in the radiography group, 356 and 443 deaths from lung cancer in the two groups, respectively, had occurred, corresponding to rates of death from lung cancer of 247 and 309 deaths per 100,000 person-years, respectively, and a relative reduction in the rate of death from lung cancer with low-dose CT screening of 20.0% (95% CI, 6.8 to 26.7; P = 0.004). Figure 1B shows the cumulative number of deaths from lung cancer in the two screening groups through January 15, 2009. When only participants who underwent at least one screening test were included, there were 346 deaths from lung cancer among 26,455 participants in the low-dose CT group and 425 deaths among 26,232 participants in the radiography group. The number needed to screen with low-dose CT to prevent one death from lung cancer was 320.
Overall Mortality
There were 1877 deaths in the low-dose CT group, as compared with 2000 deaths in the radiography group, representing a significant reduction with low-dose CT screening of 6.7% (95% CI, 1.2 to 13.6) in the rate of death from any cause (P = 0.02). We were unable to obtain the death certificates for two of the participants in the radiography group who died, but the occurrence of death was confirmed through a review by the end-point verification team. Although lung cancer accounted for 24.1% of all the deaths in the trial, 60.3% of the excess deaths in the radiography group were due to lung cancer (Table 7). When deaths from lung cancer were excluded from the comparison, the reduction in overall mortality with the use of low-dose CT dropped to 3.2% and was not significant (P = 0.28).
Table 7. Cause of Death on the Death Certificate, According to Screening Group*.
| Cause of Death | Low-Dose CT Group | Radiography Group | Total |
|---|---|---|---|
| number/total number (percent) | |||
| Neoplasm of bronchus and lung† | 427/1865 (22.9) | 503/1991 (25.3) | 930/3856 (24.1) |
| Other neoplasm | 416/1865 (22.3) | 442/1991 (22.2) | 858/3856 (22.3) |
| Cardiovascular illness | 486/1865 (26.1) | 470/1991 (23.6) | 956/3856 (24.8) |
| Respiratory illness | 175/1865 (9.4) | 226/1991 (11.4) | 401/3856 (10.4) |
| Complications of medical or surgical care | 12/1865 (0.6) | 7/1991 (0.4) | 19/3856 (0.5) |
| Other | 349/1865 (18.7) | 343/1991 (17.2) | 692/3856 (17.9) |
A total of 3875 death certificates were received (1877 for participants in the low-dose CT group and 1998 for those in the radiography group), but the cause of death was unknown for 12 participants in the low-dose CT group and 7 in the radiography group. The denominators represent only the deaths for which the cause was known. Causes of death were categorized according to the following codes in the International Classification of Diseases, 10th Revision (ICD-10): neoplasms of bronchus and lung, C33-C34; neoplasms other than bronchus and lung, C00-D48 (excluding C33 and C34); cardiovascular illness, I00-I99; respiratory illness, J00-J99; complications of medical or surgical care, S00-T17.8, T18-T99, and Y40-Y84; unknown, R96-R99 and death certificates without a coded cause of death; and other, all remaining codes.
The number of deaths from neoplasm of the bronchus and lung in this table is not equal to the number of lung-cancer deaths in the lung-cancer mortality analysis. The lung-cancer deaths included here are those that were determined from information on the death certificate only (without review by the end-point verification team) and include deaths that occurred through December 31, 2009.
Discussion
In the NLST, a 20.0% decrease in mortality from lung cancer was observed in the low-dose CT group as compared with the radiography group. The rate of positive results was higher with low-dose CT screening than with radiographic screening by a factor of more than 3, and low-dose CT screening was associated with a high rate of false positive results; however, the vast majority of false positive results were probably due to the presence of benign intrapulmonary lymph nodes or non-calcified granulomas, as confirmed noninvasively by the stability of the findings on follow-up CT scans. Complications from invasive diagnostic evaluation procedures were uncommon, with death or severe complications occurring only rarely, particularly among participants who did not have lung cancer. The decrease in the rate of death from any cause with the use of low-dose CT screening suggests that such screening is not, on the whole, deleterious.
A high rate of adherence to the screening, low rates of lung-cancer screening outside the NLST, and thorough ascertainment of lung cancers and deaths contributed to the success of the NLST. Moreover, because there was no mandated diagnostic evaluation algorithm, the follow-up of positive screening tests reflected the practice patterns at the participating medical centers. A multidisciplinary team ensured that all aspects of the NLST were conducted rigorously.
There are several limitations of the NLST. First, as is possible in any clinical study, the findings may be affected by the “healthy-volunteer” effect, which can bias results such that they are more favorable than those that will be observed when the intervention is implemented in the community.24 The role of this bias in our results cannot be ascertained at this time. Second, the scanners that are currently used are technologically more advanced than those that were used in the trial. This difference may mean that screening with today's scanners will result in a larger reduction in the rate of death from lung cancer than was observed in the NLST; however, the ability to detect more abnormalities may result only in higher rates of false positive results.25 Third, the NLST was conducted at a variety of medical institutions, many of which are recognized for their expertise in radiology and in the diagnosis and treatment of cancer. It is possible that community facilities will be less prepared to undertake screening programs and the medical care that must be associated with them. For example, one of the most important factors determining the success of screening will be the mortality associated with surgical resection, which was much lower in the NLST than has been reported previously in the general U.S. population (1% vs. 4%).26 Finally, the reduction in the rate of death from lung cancer associated with an ongoing low-dose CT screening program was not estimated in the NLST and may be larger than the 20% reduction observed with only three rounds of screening.
Radiographic screening rather than community care (care that a participant usually receives) was chosen as the comparator in the NLST because radiographic screening was being evaluated in the PLCO trial at the time the NLST was designed.11
The designers of the NLST reasoned that if the PLCO trial were to show a reduction in lung-cancer mortality with radiographic screening, a trial of low-dose CT screening in which a community-care group was the control would be of less value, since the standard of care would have become screening with chest radiography. Nevertheless, the choice of radiography precludes a direct comparison of low-dose CT with community care. Analysis of the subgroup of PLCO participants who met the NLST criteria for age and smoking history indicated that radiography, as compared with community care, does not reduce mortality from lung cancer.27 Therefore, a similar reduction in lung-cancer mortality would probably have been observed in the NLST if community care had been chosen instead for the control group.
In addition to the high rate of false positive results, two other potentially harmful effects of low-dose CT screening must be mentioned. Over-diagnosis, a major source of controversy surrounding low-dose CT lung-cancer screening, results from the detection of cancers that never would have become symptomatic.28 Although additional follow-up would be necessary to measure the magnitude of overdiagnosis in the NLST, a comparison of the number of cancers diagnosed in the two trial groups suggests that the magnitude of overdiagnosis with low-dose CT as compared with radiographic screening is not large. The other harmful effect, the association of low-dose CT with the development of radiation-induced cancers, could not be measured directly, is a long-term phenomenon, and must be assessed in future analyses.29
A number of smaller, randomized trials of low-dose CT screening are under way in Europe. 30-36 Because none of these trials have sufficient statistical power to detect a reduction in lung-cancer mortality of the magnitude seen in the NLST, it is expected that meta-analyses of the findings from these trials will be performed. The European studies are gathering types of data that were not collected by the NLST and will be able to address additional questions about low-dose CT screening, including the best strategies for the management of nodules observed with screening.37
The observation that low-dose CT screening can reduce the rate of death from lung cancer has generated many questions. Will populations with risk profiles that are different from those of the NLST participants benefit? Are less frequent screening regimens equally effective? For how long should screening continue? Would the use of different criteria for a positive screening result, such as a larger nodule diameter, still result in a benefit? It is unlikely that large, definitive, randomized trials will be undertaken to answer these questions, but modeling and microsimulation can be used to address them. Although some agencies and organizations are contemplating the establishment of lung-cancer screening recommendations on the basis of the findings of the NLST, the current NLST data alone are, in our opinion, insufficient to fully inform such important decisions.
Before public policy recommendations are crafted, the cost-effectiveness of low-dose CT screening must be rigorously analyzed. The reduction in lung-cancer mortality must be weighed against the harms from positive screening results and overdiagnosis, as well as the costs. The cost component of low-dose CT screening includes not only the screening examination itself but also the diagnostic follow-up and treatment. The benefits, harms, and costs of screening will all depend on the way in which low-dose CT screening is implemented, specifically in regard to the eligibility criteria, screening frequency, interpretation threshold, diagnostic follow-up, and treatment. For example, although there are currently only about 7 million persons in the United States who would meet the eligibility criteria for the NLST, there are 94 million current or former smokers6 and many more with secondhand exposure to smoke or other risk factors. The cost-effectiveness of low-dose CT screening must also be considered in the context of competing interventions, particularly smoking cessation. NLST investigators are currently analyzing the quality-of-life effects, costs, and cost-effectiveness of screening in the NLST and are planning collaborations with the Cancer Intervention and Surveillance Modeling Network to investigate the potential effect of low-dose CT screening in a wide range of scenarios.
Other strategies for early detection of lung cancer — in particular, molecular markers in blood, sputum, and urine, which can be studied in specimens that were obtained as part of ACRIN's NLST activities and are available to the research community — may one day help select persons who are best suited for low-dose CT screening or identify persons with positive low-dose CT screening tests who should undergo more rigorous diagnostic evaluation.
Supplementary Material
Acknowledgments
The American College of Radiology Imaging Network component of the National Lung Screening Trial (NLST) was funded through grants (U01-CA-80098 and U01-CA-79778) under a cooperative agreement with the Cancer Imaging Program, Division of Cancer Treatment and Diagnosis. The Lung Screening Study sites of the NLST were funded through contracts with the Early Detection Research Group and Biometry Research Group, Division of Cancer Prevention: University of Colorado Denver (N01-CN-25514), Georgetown University (N01-CN-25522), Pacific Health Research and Education Institute (N01-CN-25515), Henry Ford Health System (N01-CN-25512), University of Minnesota (N01-CN-25513), Washington University in St. Louis (N01-CN-25516), University of Pittsburgh (N01-CN-25511), University of Utah (N01-CN-25524), Marshfield Clinic Research Foundation (N01-CN-25518), University of Alabama at Birmingham (N01-CN-75022), Westat (N01-CN-25476), and Information Management Services (N02-CN-63300).
We thank the trial participants for their contributions in making this trial possible.
Appendix
The members of the writing team of the National Lung Screening Trial Research Team are as follows: Denise R. Aberle, M.D., University of California at Los Angeles, Los Angeles; Amanda M. Adams, M.P.H., American College of Radiology Imaging Network (ACRIN) Biostatistics Center, Brown University, Providence, RI; Christine D. Berg, M.D., Division of Cancer Prevention, National Cancer Institute, Bethesda, MD; William C. Black, M.D., Dartmouth–Hitchcock Medical Center, Lebanon, NH; Jonathan D. Clapp, B.S., Information Management Services, Rockville, MD; Richard M. Fagerstrom, Ph.D., Division of Cancer Prevention, National Cancer Institute, Bethesda, MD; Ilana F. Gareen, Ph.D., ACRIN Biostatistics Center, Brown University, Providence, RI; Constantine Gatsonis, Ph.D., ACRIN Biostatistics Center, Brown University, Providence, RI; Pamela M. Marcus, Ph.D., Division of Cancer Prevention, National Cancer Institute, Bethesda, MD; and JoRean D. Sicks, M.S., ACRIN Biostatistics Center, Brown University, Providence, RI.
Footnotes
Mr. Clapp reports holding a financial interest in Human Genome Sciences; and Dr. Gatsonis, receiving consulting fees from Wilex, MELA Sciences, and Endocyte, lecture fees from Bayer HealthCare, and support from the Radiological Society of North America for developing educational presentations. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–75. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders HR, Albitar M. Somatic mutations of signaling genes in non-small-cell lung cancer. Cancer Genet Cytogenet. 2010;203:7–15. doi: 10.1016/j.cancergencyto.2010.07.134. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. Erratum, CA Cancer J Clin 2011;61:133-4. [DOI] [PubMed] [Google Scholar]
- 4.Cancer of the lung and bronchus (invasive): trends in SEER incidence and U.S. mortality using the Joinpoint Regression Program, 1975-2007 with up to four Join-points, 1992-2007 with up to two Join-points, both sexes by race/ethnicity. http://seer.cancer.gov/csr/1975_2007/results_merged/sect_15_lung_bronchus.pdf.
- 5.Pierce JP, Messer K, White MM, Cowling DW, Thomas DP. Prevalence of heavy smoking in California and the United States, 1965-2007. JAMA. 2011;305:1106–12. doi: 10.1001/jama.2011.334. [DOI] [PubMed] [Google Scholar]
- 6.Cigarette smoking among adults and trends in smoking cessation — United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1227–32. [PubMed] [Google Scholar]
- 7.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 8.Doria-Rose VP, Szabo E. Screening and prevention of lung cancer. In: Kern-stine KH, Reckamp KL, editors. Lung cancer: a multidisciplinary approach to diagnosis and management. New York: Demos Medical Publishing; 2010. pp. 53–72. [Google Scholar]
- 9.Naidich DP, Marshall CH, Gribbin C, Arams RS, McCauley DI. Low-dose CT of the lungs: preliminary observations. Radiology. 1990;175:729–31. doi: 10.1148/radiology.175.3.2343122. [DOI] [PubMed] [Google Scholar]
- 10.The National Lung Screening Trial Research Team. The National Lung Screening Trial: overview and study design. Radiology. 2011;258:243–53. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Church TR National Lung Screening Trial Executive Committee. Chest radiography as the comparison for spiral CT in the National Lung Screening Trial. Acad Radiol. 2003;10:713–5. doi: 10.1016/s1076-6332(03)80095-8. [DOI] [PubMed] [Google Scholar]
- 12.The National Lung Screening Trial Research Team. Baseline characteristics of participants in the randomized National Lung Screening Trial. J Natl Cancer Inst. 2010;102:1771–9. doi: 10.1093/jnci/djq434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cagnon CH, Cody DD, McNitt-Gray MF, Seibert JA, Judy PF, Aberle DR. Description and implementation of a quality control program in an imaging-based clinical trial. Acad Radiol. 2006;13:1431–41. doi: 10.1016/j.acra.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Gierada DS, Garg K, Nath H, Strollo DC, Fagerstrom RM, Ford MB. CT quality assurance in the lung screening study component of the National Lung Screening Trial: implications for multicenter imaging trials. AJR Am J Roentgenol. 2009;193:419–24. doi: 10.2214/AJR.08.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fritz A, Percy C, Jack A, et al., editors. International classification of diseases for oncology. 3rd. Geneva: World Health Organization; 2000. [Google Scholar]
- 16.Greene FL, Page DL, Fleming ID, et al. AJCC cancer staging manual. 6th. New York: Springer-Verlag; 2002. [Google Scholar]
- 17.Izmirlian G. Estimation of the relative risk following group sequential procedure based upon the weighted log-rank statistic arXiv. Cornell University Library; 2011. http://arxiv.org/abs/1102.5088. [Google Scholar]
- 18.SAS/STAT, version 9.2. Cary, NC: SAS Institute; 2004. [Google Scholar]
- 19.R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2009. [Google Scholar]
- 20.Jennison C, Turnbull BW. Group sequential methods with applications to clinical trials. Boca Raton, FL: Chapman & Hall/CRC; 2000. [Google Scholar]
- 21.Statement concerning the National Lung Screening Trial. 2010 Oct 28; http://www.cancer.gov/images/DSMB-NLST.pdf.
- 22.Department of Commerce, Census Bureau. National Cancer Institute and Centers for Disease Control and Prevention co-sponsored tobacco use special cessation supplement to the current population survey. 2003 http://riskfactor.cancer.gov/studies/tus-cps.
- 23.Travis WD, Branbilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinsky PF, Miller A, Kramer BS, et al. Evidence of a healthy volunteer effect in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Epidemiol. 2007;165:874–81. doi: 10.1093/aje/kwk075. [DOI] [PubMed] [Google Scholar]
- 25.Mahesh M, Hevezi JM. Slice wars vs dose wars in multiple-row detector CT. J Am Coll Radiol. 2009;6:201–2. doi: 10.1016/j.jacr.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 26.Bach PB, Cramer LD, Schrag D, Downey RJ, Gelfand SE, Begg CB. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345:181–8. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 27.NCI NLST press conference. NCI radio broadcasts. 2010 Nov; http://www.cancer.gov/newscenter/radio-broadcasts.
- 28.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–13. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 29.Berrington de González A, Kim KP, Berg CD. Low-dose lung computed tomography screening before age 55: estimates of the mortality reduction required to outweigh the radiation-induced cancer risk. J Med Screen. 2008;15:153–8. doi: 10.1258/jms.2008.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Iersel CA, de Koning HJ, Draisma G, et al. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON) Int J Cancer. 2007;120:868–74. doi: 10.1002/ijc.22134. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen JH, Ashraf H, Dirksen A, et al. The Danish Randomized lung cancer ct screening trial — overall design and results of the prevalence round. J Thorac Oncol. 2009;4:608–14. doi: 10.1097/JTO.0b013e3181a0d98f. [DOI] [PubMed] [Google Scholar]
- 32.Lopes Pegna A, Picozzi G, Mascalchi M, et al. Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low-dose CT. Lung Cancer. 2009;64:34–40. doi: 10.1016/j.lungcan.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Infante M, Cavuto S, Lutman FR, et al. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009;180:445–53. doi: 10.1164/rccm.200901-0076OC. [DOI] [PubMed] [Google Scholar]
- 34.Marchianò A, Calabrò E, Civelli E, et al. Pulmonary nodules: volume repeatability at multidetector CT lung cancer screening. Radiology. 2009;251:919–25. doi: 10.1148/radiol.2513081313. [DOI] [PubMed] [Google Scholar]
- 35.Becker N, Delorme S, Kauczor HU. LUSI: the German component of the European trial on the efficacy of multi-slice CT for the early detection of lung cancer. Onkologie. 2008;31(Suppl 1):PO320. abstract. [Google Scholar]
- 36.Baldwin DR, Duffy SW, Wald NJ, Page R, Hansell DM, Field JK. UK Lung Screen (UKLS) nodule management protocol: modelling of a single screen randomised controlled trial of low-dose CT screening for lung cancer. Thorax. 2011;66:308–13. doi: 10.1136/thx.2010.152066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med. 2009;361:2221–9. doi: 10.1056/NEJMoa0906085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.