Abstract
We developed a realistic simulation dataset for simultaneous respiratory and cardiac (R&C) gated SPECT/CT using the 4D NURBS-based Cardiac-Torso (NCAT) Phantom and Monte Carlo simulation methods, and evaluated it for a sample application study. The 4D NCAT phantom included realistic respiratory motion and beating heart motion based on respiratory gated CT and cardiac tagged MRI data of normal human subjects. To model the respiratory motion, a set of 24 separate 3D NCAT phantoms excluding the heart was generated over a respiratory cycle. The beating heart motion was modelled separately with 48 frames per cardiac cycle for each of the 24 respiratory phases. The resultant set of 24×48 3D NCAT phantoms provides a realistic model of a normal human subject at different phases of combined R&C motions. An almost noise-free SPECT projection dataset for each of the 1,152 3D NCAT phantoms was generated using Monte Carlo simulation techniques and the radioactivity uptake distribution of 99mTc sestamibi in different organs. By grouping and summing the separate projection datasets, separate or simultaneous R&C gated acquired data with different gating schemes could be simulated. In the initial evaluation, we combined the projection datasets into no gating, 6 respiratory-gates only, 8 cardiac-gates only, and combined 6 respiratory-gates & 8 cardiac-gates projection datasets. Each dataset was reconstructed using 3D OS-EM without and with attenuation correction using the averaged and respiratory-gated attenuation maps, and the resulting reconstructed images were compared. These results were used to demonstrate the effects of R&C motions and the reduction of image artifact due to R&C motions by gating and attenuation corrections. We concluded that the realistic 4D NCAT phantom and Monte Carlo simulated SPECT projection datasets with R&C motions are powerful tools in the study of the effects of R&C motions, as well as in the development of R&C gating schemes and motion correction methods for improved SPECT/CT imaging.
Keywords: dual gating, respiratory motion, Gated SPECT, attenuation correction, image reconstruction
1. Introduction
It is well known that respiratory and cardiac (R&C) motions are important sources of image degradation and artifacts in myocardial imaging in positron computed tomography (PET) (Ter-Pogossian et al., 1982; McQuaid, 2009) and single-photon emission computed tomography (SPECT) (Yang et al., 2009). Cardiac contraction causes image blurring in myocardial perfusion images (Galt et al., 1990; Taillefer et al., 1999; Slomka et al., 2004). This kind of cardiac motion due to respiration is mainly in the craniocaudal direction and, to a lesser degree, in the anterior–posterior and lateral directions (Livieratos et al., 2006; Wang et al., 1995). Despite early recognition of these problems, the issue initially received little attention due to the practical limitations of the methodology (Pretorius and King, 1999; Ter-Pogossian et al., 1982; Tsui et al., 2000).
The cardiac gating method has been developed and implemented in clinical PET and SPECT to reduce image blurring due to cardiac motion (Dawood et al., 2007; Achtert et al., 1998; Smanio et al., 1997; Freiberg et al., 2004) and to provide information about the movement of the beating heart for diagnostic purposes (Saab et al., 2003; Freiberg et al., 2004; Hickey et al., 2004; Nichols et al., 2001; Sharir et al., 2001; Travin et al., 2004; Emmett et al., 2002). Respiratory gating methods based on external monitoring devices (McNamara et al., 2009; Klein et al., 1998) and acquired data alone (Büther F et al., 2009; Bundschuh et al., 2008; Kesner et al., 2013; Chung et al., 2006; Lamareet al., 2007; Chen and Tsui, 2008) have also been developed, and the data from these methods have been used to reduce image blurring due to respiratory motion. (Schleyer et al., 2013). Recently, dual cardiac and respiratory gating methods have been developed (Klein et al., 1998; Reutter et al., 1997; Martinez-Moller et al., 2007; Cho et al., 1999) and simultaneous R&C motion compensation methods have been developed, resulting in significantly improved PET and SPECT imaging quality in terms of reduction of motion blurring and increase in signal-to-noise ratio in the motion compensated images (Lee et al., 2011; Lee et al., 2013; Blume et al., 2010).
The goal of this study was to develop a realistic simulation dataset for simultaneous R&C motion gated SPECT/CT, and to perform an initial evaluation of the dataset for a sample application study. The dataset allows for modeling of SPECT projection data obtained by separate or dual R&C gating schemes with various gating parameters. Therefore, the dataset is an important tool in studying the effects of R&C motions, optimal gating parameters, and R&C motion compensation methods in image reconstruction for improved image quality.
2. Methods
2.1. Generation of the Simultaneous Cardiac and Respiratory Phantom Dataset
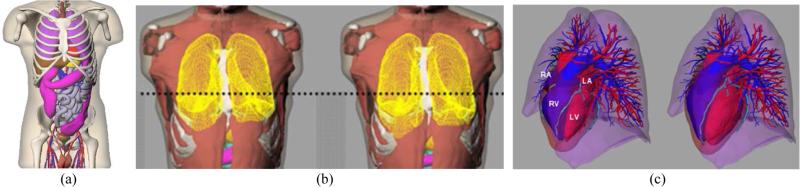
The four-dimensional (4D) NURBS-based CArdiac-Torso (NCAT) phantom was developed had been developed to realistically model the anatomical structures and cardiac and respiratory motion of an average, healthy human subject (Fig. 1 (a)) (Segars, May 2001). The 3D anatomical structures used in this study were based on imaging data of the Visible Human project from the National Library of Medicine (National Library of Medicine, 1997) and employ non-uniform rational B-splines (NURBS) for realistic and flexible modeling. Respiratory motion was based on a 4D respiratory gated CT image dataset (Fig. 1 (b)), and the beating heart model was based on a 4D cardiac gated tagged MR image dataset (Fig. 1 (c)) (Segars and Tsui, 2002; Segars et al., 2009). The 3D R&C motion vector fields were derived from the digitized 4D CT and tagged MR image datasets and were then interpolated and incorporated into the voxelized 4D NCAT phantom.
Figure 1.
(a) The anterior view of the 4D NCAT phantom. (b) The lung model at end-expiration (left) and end-inspiration (right) with respiratory motion. (c) The heart model at end-systole (left) and end-diastole (right) with cardiac motion.
To model R&C motions, we generated a series of 3D NCAT phantoms that represent different phases of the R&C cycles for use in PET and SPECT imaging. The voxelized 3D NCAT phantoms with a voxel size of 1.5625 mm in the x, y, and z dimensions were used. Respiratory motion of the body was modeled without the heart in a total of 24 separate phantoms over a respiratory cycle of 5 seconds, the average period of respiratory motion. The beating heart was created separately, with 48 frames over a cardiac cycle with an average period of 1 second for each of the 24 respiratory phases. The heart fits inside the pericardial sac which doesn't change much over the cardiac cycle. It provides a free bounding area with which to insert the heart at any phase without any probability of overlap with the body remainder excluding the heart. We also checked the 4D phantom at each combination of respiratory and cardiac phase to ensure that there was no unrealistic protrusion or overlap of organs into each other.
The set of 1,152 (24 × 48) 3D NCAT phantoms represents different phases of the combined R&C motions. This master dataset can later be grouped and summed into datasets for use with different R&C gating schemes. Table 1 shows a brief summary of the phantom datasets. The phantom with the matrix size of 256 × 256 × 278 and a voxel size of 1.5625 mm was collapsed into 128 × 128 × 139 with a voxel size of 3.125 mm for SimSET+ARF simulation.
Table 1.
3D NCAT phantom datasets for use with different simultaneous cardiac and respiratory gating schemes
| Cardiac Datasets | Respiratory Datasets | |
|---|---|---|
| 3D NCAT phantoms | Heart only | Body except the heart |
| Frames/(average cycle time) | 48/(1 second) | 24/(5 seconds) |
| Possible gating schemes | 8, 16, 24 equally spaced time gates/cycle | 3, 4, 6 equally spaced time gates/cycle |
2.2. Generation of Gated Myocardial Perfusion SPECT Projection Datasets Using SimSET+ARF
In addition to the realistic sets of 3D NCAT phantoms that realistically model a normal human subject with R&C motions, an accurate and realistic simulation of the imaging process is also important for the generation of projection data in a simulation study. In this work, we used the SimSET (Simulation System for Emission Tomography) Monte Carlo code (Lewellen et al., 1998) to generate SPECT projection data from the 4D phantom datasets modeling the organ uptake distribution of Tc-99m labeled sestamibi, a myocardial perfusion (MP) agent used in SPECT imaging. The SimSET code provides accurate and efficient simulations of the photon transports through the phantoms. To provide such effective simulations of the collimator and detector response characteristics, we employed a pre-calculated table of the angular response functions (ARFs). The combined SimSET+ARF simulation method has been previously validated through several studies for various scintillation camera systems and a variety of radionuclides (Song et al., 2005; Du et al., 2002; Wang et al., 2002; He et al., 2005; Song et al., 2011). These studies showed that the results obtained by using the SimSET+ARF method were in good agreement with those acquired by full Monte Carlo simulations and physical phantom measurements. Note that because the SimSET was only used to simulate photon propagation inside phantom, the collimator and detector were not modeled. Therefore we did not use any importance sampling techniques such as the stratification and the forced detection. The coherent scatter was modeled during simulation.
By using the SimSET Monte Carlo code in combination with the ARF model and a computer cluster in our laboratory, we generated almost noise-free SPECT projection datasets from the set of 1,152 (24 × 48) 3D NCAT phantoms representing different phases of combined R&C motions. A pair of low-energy high-resolution (LEHR) collimators and a dual-head SPECT system with two large field-of-view cameras consisting of 9.5 mm thick NaI(Tl) crystals with perfect energy resolutions were modeled in the simulation. The effects of septal penetration, Pb X-rays, and scatter from the primary 140 keV photons of Tc-99m were included. The ARF tables were generated for 1 keV-wide energy windows from 115 keV to 145 keV. These 1 keV-wide projections were appropriately weighted and summed to produce SPECT data that could give information about any desired energy resolutions, in any desired energy windows. The projection data corresponding to the detected primary photons and scattered photons were saved separately to allow for the evaluation of the effects of scatter on image quality.. The projection data were generated with a 0.3125 cm bin size at 64 views over 180° using a non-circular body-contouring orbit. The separate projection datasets generated from the body without the heart and from the heart alone were then combined to represent the complete dataset of the body with the heart. In this study, noise-free projection dataset was used to evaluate the dataset in terms of the R&C motion without additional confounding factor, such as noise.
2.3. Image Reconstruction
The projection dataset of the 3D NCAT phantoms representing combinations of different R&C motions was reconstructed using 3D OS-EM SPECT image reconstruction methods without any correction and with attenuation corrections using two different sets of attenuation maps; a single attenuation map with an averaged respiratory motion, and a set of respiratory gated attenuation maps representing different respiratory phases.
2.4. Image Evaluation
We performed a preliminary evaluation study to assess the 4D dual gating phantom and SPECT projection datasets and their potential applications. We studied the effects of R&C gating in image quality and attenuation correction in SPECT. The 1,152 projection datasets were grouped and summed into 4 different gating schemes, including (1) no respiratory gating and no cardiac gating, (2) cardiac gating with 8 gated frames and no respiratory gating, (3) respiratory gating with 6 gated frames and no cardiac gating, and (4) simultaneous respiratory gating with 6 gated frames and cardiac gating with 8 gated frames.
The effects of R&C motions in 4D myocardial perfusion (MP) SPECT images were assessed by using a polar map, or ‘bull's-eye map’ display (Svane et al., 1989; Faber et al., 1995). In the myocardial polar map, the maximal-count circumferential profiles of well-defined short- and long-axis planes are extracted and combined in the polar map showing the 3D distribution of the MP tracer onto a two-dimensional polar representation. The map provides information on the perfusion of the whole left ventricle from apex to base, which permits the assessment of perfusion of individual wall segments and analysis of the extent of perfusion defects (De Puey et al., 2011). Interpretation of the polar map is useful in studying the reversibility and severity of ischemic heart diseases. Since a normal heart shows uniform radioactivity uptake throughout the left ventricular wall, deviation from the uniform activity uptake in the polar map can be used to study the effects of R&C motions, the R&C gated schemes and the attenuation maps.
In addition, regional mean values of the four major regions of the heart defined by the standard four-quadrant method (Eisner et al., 1988a; Eisner and Malko, 1988; Eisner et al., 1988b), i.e., septal, anterior, lateral, and inferior, were also derived from the cardiac polar map and used in the evaluation study.
3. Results and Discussions
3.1. Generation of Simultaneous Cardiac and Respiratory Phantom Dataset
Figure 2 shows the (a) transverse and (b) coronal slice through the static 4D NCAT phantom showing the activity distributions in (from left to right) the body background and the heart at the end-diastolic (ED) phase, and the averaged attenuation map of the same phantom slices. Figure 2 (c) shows sample coronal slices of the 4D NCAT phantom at four different phases of the respiratory-cardiac motions, i.e., end-diastolic (ED) cardiac phase and end-inhalation (EI) respiratory phase (EI-ED), end-systolic (ES) cardiac phase and EI respiratory phase (ES-ED), ED cardiac phase and end-exhalation (EX) respiratory phase (EX-ED), and ES cardiac phase and EX respiratory phase (EX-ES). These slices show the changes of the myocardial wall thickness and the positions of the heart and liver with respect to the static frame. The amplitude of the respiratory motion was set at 20 mm to more clearly demonstrate the effect.
Figure 2.
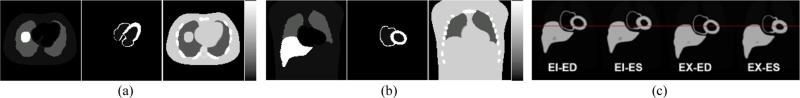
Sample (a) transverse and (b) coronal slice through the static 4D NCAT phantom showing the activity distributions in (from left to right) the body background and the heart at the end-diastolic (ED) phase, and the averaged attenuation map of the same phantom slices. (c) Sample coronal slices through the 4D NCAT phantom at various respiratory-cardiac phases (EI : end-inspiration, EX : end-expiration, ED : end-diastole, ES : end-systole). They show the changes of the myocardial wall thickness and the positions of the liver and the heart at the four different respiratory and cardiac phases with respect to a reference line in the static frame.
3.2. Generation of the Gated Myocardial Perfusion SPECT Projection Dataset
The entire 4D Monte Carlo simulated projection dataset obtained at different R&C phases of the 4D NCAT phantom effectively modeled the simultaneous dual R&C motion. The dataset can be represented by a 24 × 48 matrix, in which a matrix element represents a projection dataset from a specific phase of the R&C cycle as shown in figure 3 (a) through (d).
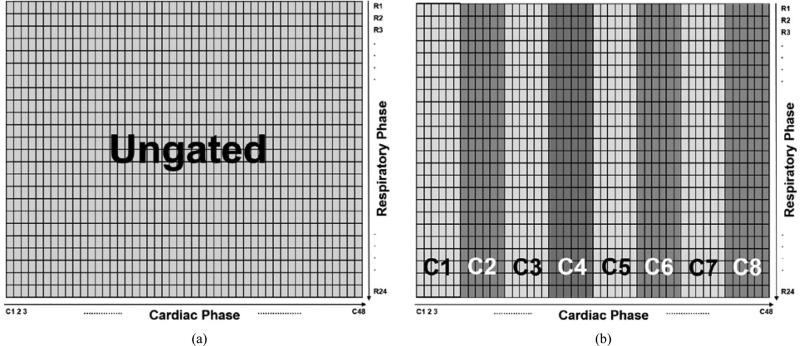
Figure 3.
Various R&C gating schemes that can be generated from the master 4D dual gating dataset shown in figure 4. Each different colored block represents one gated frame. (a) Ungated, (b) 8 cardiac gated frames with no respiratory gating, (c) 6 respiratory gated frames with no cardiac gating, and (d) simultaneous 6 respiratory gated and 8 cardiac gated frames.
Various R&C gating schemes can be generated with different combinations of R&C gated frames. For example, when all the elements of the projection dataset matrix were summed, the ‘ungated’ projection image was generated as shown in figure 3 (a). When the cardiac phases were divided into 8 cardiac gated frames and all the respiratory phases were summed for each cardiac frame, the projection dataset of ‘8 cardiac gates with no respiratory gating’ was generated as illustrated in figure 3 (b). Similarly, when the respiratory cycles were divided into 6 frames and all the cardiac phases were summed for each respiratory frame, the projection dataset of ‘6 respiratory gates with no cardiac gating’ was generated as shown in figure 3 (c). To generate a dual gating projection dataset that includes simultaneous R&C gating, the elements of the projection dataset matrix shown in figure 4 can be summed appropriately to simulate given numbers of R&C gated frames. Figure 3 (d) shows an example of a simultaneous dual R&C gated dataset of the ‘6 respiratory and 8 cardiac gating’ scheme. Each different colored box consisting of 24 matrix elements represents one R&C gated frame in the dual gating scheme.
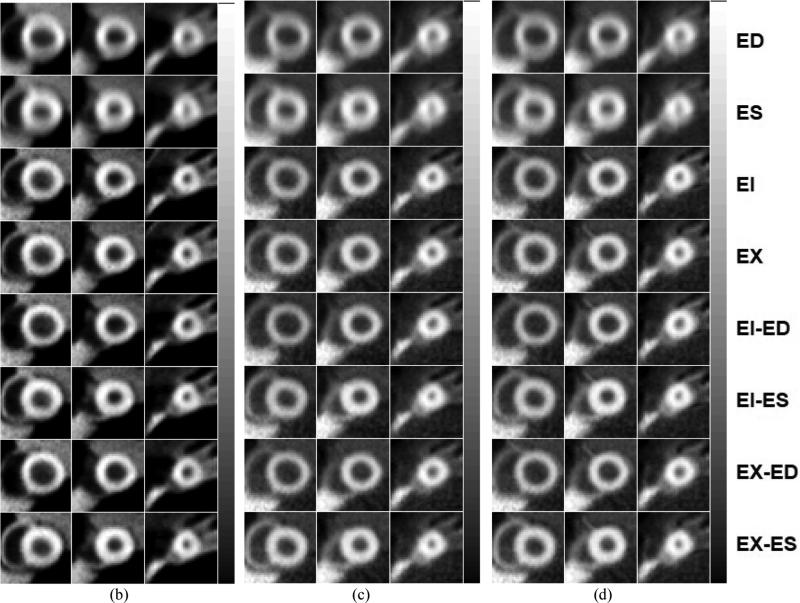
Figure 4.
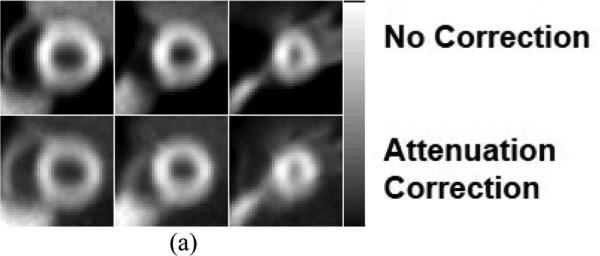
Sample simulated short-axis MP SPECT images in (from left to right of each row of images) basal, mid, and near apex area of the heart and from (a) ungated (top row) without and with (bottom row) attenuation correction, and cardiac gated images at various R&C motion phases with 3 different gating schemes: (b) (from top to bottom rows) cardiac-only gating at ED and ES, respiratory-only gating at EI and EX, and dual gating at EI-ED, EI-ES, EX-ED and EX-ES gating with no attenuation correction (c) similar to (b) except with attenuation correction applied using an averaged attenuation map, and (d) similar to (b) except with respiratory gated attenuation maps.
3.3. Application of 4D R&C Gated Myocardial Perfusion SPECT Datasets
To demonstrate and evaluate the application of the 4D R&C myocardial perfusion (MP) SPECT projection datasets, we reconstructed those datasets from the 4 different gating schemes shown in figure 3. We used 3D OS-EM-based SPECT image reconstruction methods without and with attenuation corrections and with 8 subsets and 20 iterations. In addition, two different attenuation maps – one averaged over the respiratory motion and the other consisting of a subset of 6 respiratory gated attenuation maps – were used. Figure 4 shows sample reconstructed images from the four R&C gating schemes at various R&C motion phases with and without attenuation correction applied. The results clearly demonstrate how the amount of motion blur artifacts in the reconstructed images was significantly reduced with the use of gating.
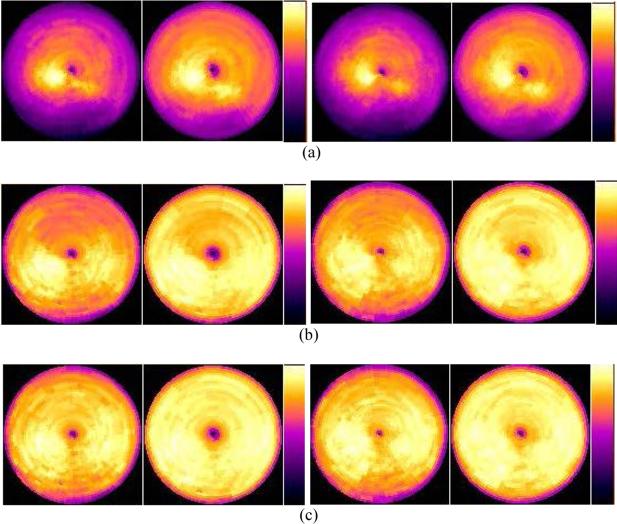
The image artifacts of the SPECT images obtained from the various gating schemes were also compared using the Bull's-eye plot display of the myocardium, as shown in figures 5 through 8. Compared to reconstructed images, cardiac polar maps represent the differences more effectively between the application or the lack of application of attenuation correction. Figure 5 shows clear differences in the level of artifacts without and with attenuation correction from the ungated MP SPECT images.
Figure 5.
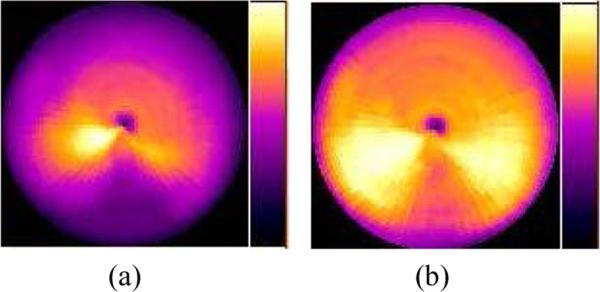
Bull's-eye plots of the myocardium from the ungated MP SPECT images with (a) no attenuation correction, and (b) with attenuation correction using an averaged attenuation map.
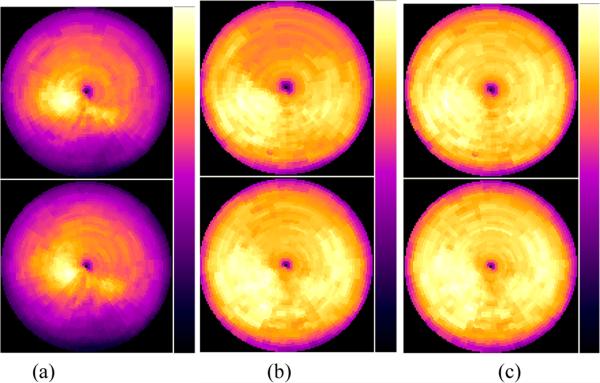
Figure 8.
Bull's-eye plots of the myocardium from the dual 6-frame respiratory and 8-frames cardiac gating scheme at (from left to right) EI-ED, EI-ES, EX-ED, and EX-ES, and obtained with (a) no attenuation correction, and with attenuation correction using (b) the corresponding attenuation maps at the same point of the respiratory cycle.
Figure 6 also shows the effect of attenuation correction in the 8 cardiac gated frames with no respiratory gating. The amount of artifacts was clearly reduced by the attenuation correction, though the difference between the use of an averaged and respiratory gated attenuation map is less significant in the cardiac-only gating. This was verified in the calculation of regional mean values as shown in figure 9 (a).
Figure 6.
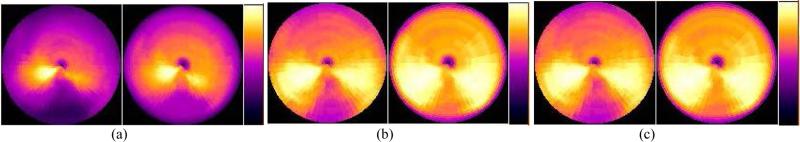
Bull's-eye plots of the myocardium from the 8-frame cardiac-only gated MP SPECT images at (left) ED and (right) ES obtained (a) with no attenuation correction, and with attenuation correction using (b) the averaged attenuation map, and (c) the corresponding attenuation maps at the same phase of respiratory cycle.
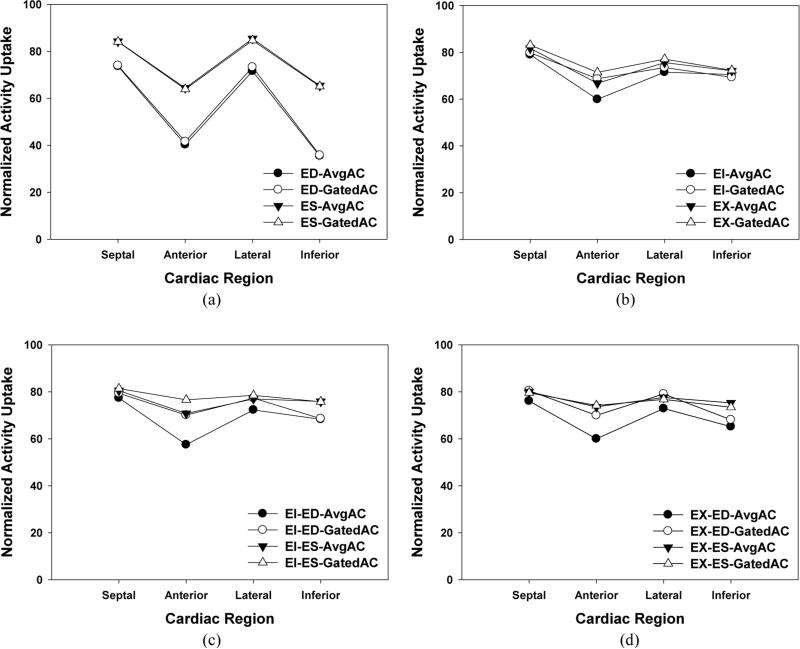
Figure 9.
Normalized mean of activity uptakes in the four quadrants of the Bull's-eye plots from the MP SPECT images obtained using (a) cardiac-only gating, (b) respiratory-only gating, and dual R&C gating at the (c) EI and (d) EX phases. At each gating scheme, results from ED, ES, EI, EX, AvgAC (attenuation correction using an averaged attenuation map), GatedAC (attenuation correction using corresponding attenuation map at the same point of respiratory cycle) are shown.
Figure 7 shows the effect of attenuation correction on the MP SPECT images with the 6 respiratory gated frames and no cardiac gating. Similar to the results shown in figure 6, the severity of image artifacts was clearly reduced by the attenuation correction. However, compared to the ungated or cardiac-only gating, the amount of the artifacts was significantly decreased, resulting in a relatively uniform activity distribution in the Bull's-eye plot. This was also verified in the calculation of regional mean values as shown in figure 9 (b), and demonstrates that the effect of respiratory gating was significantly greater than that of cardiac gating in terms of reducing motion artifacts.
Figure 7.
Bull's-eye plots of the myocardium from the 6-frame respiratory-only gating SPECT images at (top) EI and (bottom) EX obtained (a) with no attenuation correction, and with attenuation correction using (b) the averaged attenuation map, and (c) the corresponding attenuation maps at the same phase of respiratory cycle.
While use of the averaged attenuation map significantly reduced the motion artifacts with respiratory gating as shown in figure 7 (b), further reduction of the artifacts was shown when the attenuation map at the corresponding respiratory phase was used, as shown in figure 7 (c), especially in both anterior and inferior regions. This improvement was also confirmed in the calculation of regional mean values as shown in figure 9 (b). For each respiratory phase, the mean values calculated with respiratory gated attenuation maps showed less fluctuation across the four cardiac regions than did an averaged attenuation map.
Note that the artifactual blurring in the polar map is less in the End-Expiration (EX) phase than the End-Inspiration (EI) phase, as shown in figure 7 (b) and (c) (top and bottom). It is considered that this is due to the less motion in the EX phase than in the EI phase of respiratory gating.
Figure 8 shows the effect of attenuation correction using the simultaneous dual R&C gating scheme with 6 respiratory gated frames and 8 cardiac gated frames. Figure 8 (a) shows the Bull's-eye plots at various R&C motion phases without attenuation correction. Although they show less motion artifacts compared to those without attenuation correction from the respiratory-only and cardiac-only gating schemes, the dual gating alone shows limitation in the reduction of image artifacts without attenuation correction. However, with attenuation correction, the amount of the artifacts in the dual R&C gated images was significantly decreased in most cardiac regions, which resulted in the most uniform activity distribution in the Bull's-eye plots among the tested R&C gating schemes. This was verified in the calculation of regional mean values as shown in figure 9 (c) and (d), and shows that, among the test gating schemes used in this study, simultaneous dual gating with attenuation correction was the most effective way to minimize the motion artifacts.
Similar to the previous gating schemes, using an averaged attenuation map for attenuation correction significantly reduced the motion artifacts as displayed in figure 8 (b), and further reduction of the artifact was shown when the respiratory gated attenuation maps were used for the attenuation correction, as shown in figure 8 (c). Again, the improvement was shown in both anterior and inferior regions of the ED and ES phases, which was also confirmed in the calculation of regional mean values as shown in figure 9 (c) and (d). For both cardiac and respiratory phases, mean values calculated with respiratory gated attenuation maps showed less fluctuation across the four cardiac regions than those with an averaged attenuation map.
Figure 9 shows the normalized mean of activity uptakes in the septal, anterior, lateral, and inferior areas of the Bull's-eye plot. It shows that the respiratory-only gating method (figure 9(b)) was more effective in reducing the variation of activity uptake across the myocardial regions than the cardiac-only gating method (figure 9 (a)). The variation was further reduced by the simultaneous dual R&C gating method, as shown in figures 9 (c) and (d). Overall, attenuation correction using respiratory gated attenuation maps provided more uniform activity uptake across the myocardial regions than using an averaged attenuation map. Comparisons to the reconstructed images without attenuation correction were left out because they are obvious in the polar maps presented previously.
4. Discussion
In this paper we reported the development and initial evaluation of the application of a realistic simulation dataset for simultaneous respiratory and cardiac (R&C) gated SPECT/CT. The realistic dataset was based on the 4D NCAT phantom and was generated by using Monte Carlo simulation methods. The simultaneous dual R&C gating dataset included 24 equally-spaced time frames over a respiratory cycle, while the beating heart motion over a cardiac cycle was modeled as 48 equally-spaced time frames for each of the 24 respiratory phases. We evaluated the application of the master R&C gated dataset to SPECT/CT imaging by assessing the effects of R&C motions on the reconstructed images in various R&C gating schemes without and with attenuation correction. Results of the initial evaluation demonstrated the feasibility and potential utilization of the master dataset for further studies on the effect of R&C motion on SPECT/CT, the evaluation of R&C gating schemes, and 4D image reconstruction methods with R&C motion and attenuation compensation.
The assessment of attenuation correction methods in SPECT/CT with respiratory motion has recently been of particular interest. According to the studies of image artifact reduction in reconstructed images, attenuation correction methods using CT-based attenuation maps with various respiratory gating schemes may require undesirable additional radiation dose to the patient (Nehmeh et al., 2004a; Nehmeh et al., 2004b). We demonstrated the use of the master dual R&C gated dataset in investigating the trade-off between potential improvements in image quality provided by respiratory gated attenuation maps and ungated or an averaged attenuation map with additional patient radiation dose. Results of our study demonstrated that respiratory gating can more effectively reduce motion artifacts or blurring across the cardiac regions than can the cardiac-only gating method. Although attenuation correction using respiratory gated attenuation maps provided better compensation than using an averaged attenuation map (especially in the anterior region of the myocardium), the difference between them was trivial compared to that between the use or lack of use of attenuation correction. This result suggests that if patient radiation dose is a concern, attenuation correction using an average attenuation map is effective in MP SPECT and incurs a minimum amount of image artifacts.
The clinical impact of the non-uniformities in polar maps due to the R&C motions depends on the specific application (Le Meunier et al., 2006). When the intensity value from one of the cardiac sectors is compared to that from a normal database, it might approach the abnormal limit due to large respiratory motion amplitude. The clinical significance of this effect depends on the relative magnitudes of the respiratory motion effect and the normal database variability. Normal database variability in turn may depend on the pharmaceutical, the cardiac sector, and the details of how the database was implemented.
The 4D dual R&C gating master dataset can also be useful for performing rigorous and comprehensive research on the application and evaluation of the SPECT/CT imaging instrumentation, data acquisition, and 4D image reconstruction and compensation methods in the context of MP SPECT imaging with various R&C gating schemes with and without attenuation correction. An example is a study of the application of the dataset to investigate the effects of R&C motion compensation methods for dual R&C gated MP SPECT (Lee et al., 2013).
5. Conclusions
We developed a simulated R&C gated MP SPECT projection dataset which includes simultaneous dual R&C motions using the 4D NCAT phantom that provides realistic models of the anatomical structure, R&C motions, and activity distribution of a MP SPECT agent. Also, we performed an initial application of the dataset to investigate the effects of various R&C gating schemes with and without attenuation correction using a respiratory gated and an average attenuation map. The effects of the different R&C gating schemes and the attenuation correction methods were evaluated in terms of artifacts in the reconstructed images. The results of our initial application study demonstrated that simultaneous R&C gating with attenuation correction using the corresponding attenuation map at the same point of the respiratory cycle provided the best SPECT/CT image quality in terms of minimal image artifacts caused by R&C motions and attenuation for potential improvements in diagnostic accuracy. We concluded that the realistic simulated SPECT projection dataset with simultaneous dual R&C gating provided a powerful tool for the study of effects of R&C motions, the development of optimal dual R&C gating schemes, and 4D image reconstruction methods with and without attenuation corrections for much improved SPECT/CT image quality and potential for improvements in diagnostic accuracy.
ACKNOWLEDGMENT
This work was supported by the Public Health Service grant R01 HL68075 and R01 EB 168.
References
- Achtert AD, King MA, Dahlberg ST, Pretorius PH, LaCroix KJ, Tsui BM. An investigation of the estimation of ejection fractions and cardiac volumes by a quantitative gated SPECT software package in simulated gated SPECT images. J. Nucl. Cardiol. 1998;5:144–52. doi: 10.1016/s1071-3581(98)90197-0. [DOI] [PubMed] [Google Scholar]
- Blume M, Martinez-Moller A, Keil A, Navab N, Rafecas M. Joint reconstruction of image and motion in gated positron emission tomography. IEEE Trans. Med. Imaging. 2010;29:1892–906. doi: 10.1109/TMI.2010.2053212. [DOI] [PubMed] [Google Scholar]
- Bundschuh RA, Martinez-Moller A, Essler M, Nekolla SG, Ziegler SI, Schwaiger M. Local motion correction for lung tumours in PET/CT--first results. Eur. J. Nucl. Med. Mol. 2008;35:1981–8. doi: 10.1007/s00259-008-0868-0. [DOI] [PubMed] [Google Scholar]
- Büther F, Dawood M, Stegger L, Wübbeling F, Schäfers M, Schober O, P SK. List mode-driven cardiac and respiratory gating in PET. J. Nucl. Med. 2009;50:674–81. doi: 10.2967/jnumed.108.059204. [DOI] [PubMed] [Google Scholar]
- Chen S, Tsui BMW. Accuracy Analysis of Image Registration Based Respiratory Motion Compensation in Respiratory-Gated FDG Oncologcial PET Reconstruction. Proc. IEEE Medical Imaging Conf. (Dresden, Germany, Oct. 2008) 2008:M06–417. [Google Scholar]
- Cho K, Kumiata S, Kumazaki T. Development of a respiratory gated myocardial SPECT system. J. Nucl. Cardiol. 1999;6:20–8. doi: 10.1016/s1071-3581(99)90061-2. [DOI] [PubMed] [Google Scholar]
- Chung A, Camici P, Yang G-Z. List-Mode Affine Rebinning for Respiratory Motion Correction in PET Cardiac Imaging. In: Yang G-Z, et al., editors. Medical Imaging and Augmented Reality. Springer Berlin; Heidelberg: 2006. pp. 293–300. [Google Scholar]
- Dawood M, Buther F, Lang N, Schober O, Schafers KP. Respiratory gating in positron emission tomography: A quantitative comparison of different gating schemes. Med. Phys. 2007;34:3067–76. doi: 10.1118/1.2748104. [DOI] [PubMed] [Google Scholar]
- De Puey EG, Garcia EV, Berman DS. Cardiac SPECT Imaging. 2nd ed. Lippincott Willians & Wilkins; Philadelphia: 2011. [Google Scholar]
- Du Y, Frey EC, Wang WT, Tocharoenchai C, Baird WH, Tsui BMW. Combination of MCNP and SimSET for Monte Carlo simulation of SPECT with medium- and high-energy photons. IEEE Trans. Nucl. Sci. 2002;49:669–74. [Google Scholar]
- Eisner R, Churchwell A, Noever T, Nowak D, Cloninger K, Dunn D, Carlson W, Oates J, Jones J, Morris D, et al. Quantitative analysis of the tomographic thallium-201 myocardial bullseye display: critical role of correcting for patient motion. J. Nucl. Med. 1988a;29:91–7. [PubMed] [Google Scholar]
- Eisner RL, Malko JA. More on bulls-eye thallium display. J. Nucl. Med. 1988;29:1466. [PubMed] [Google Scholar]
- Eisner RL, Tamas MJ, Cloninger K, Shonkoff D, Oates JA, Gober AM, Dunn DW, Malko JA, Churchwell AL, Patterson RE. Normal SPECT thallium-201 bull's-eye display: gender differences. J. Nucl. Med. 1988b;29:1901–9. [PubMed] [Google Scholar]
- Emmett L, Iwanochko RM, Freeman MR, Barolet A, Lee DS, Husain M. Reversible regional wall motion abnormalities on exercise technetium-99m-gated cardiac single photon emission computed tomography predict high-grade angiographic stenoses. J. Am. Coll. Cardiol. 2002;39:991–8. doi: 10.1016/s0735-1097(02)01707-2. [DOI] [PubMed] [Google Scholar]
- Faber TL, Cooke CD, Peifer JW, Pettigrew RI, Vansant JP, Leyendecker JR, Garcia EV. Three-dimensional displays of left ventricular epicardial surface from standard cardiac SPECT perfusion quantification techniques. J. Nucl. Med. 1995;36:697–703. [PubMed] [Google Scholar]
- Freiberg J, Hove JD, Kofoed KF, Fritz-Hansen T, Holm S, Larsson HB, Kelbaek H. Absolute quantitation of left ventricular wall and cavity parameters using ECG-gated PET. J. Nucl. Med. 2004;11:38–46. doi: 10.1016/j.nuclcard.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Galt JR, Garcia EV, Robbins WL. Effects of myocardial wall thickness on SPECT quantitation. IEEE Trans. Med. Imaging. 1990;MI-9:144–50. doi: 10.1109/42.56338. [DOI] [PubMed] [Google Scholar]
- Ghaly M, Du Y, Fung GS, Tsui BM, Links JM, Frey E. Design of a digital phantom population for myocardial perfusion SPECT imaging research. Phys. Med. Biol. 2014;59:2935–53. doi: 10.1088/0031-9155/59/12/2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Du Y, Song XY, Segars WP, Frey EC. A Monte Carlo and physical phantom evaluation of quantitative In-111SPECT. Phys. Med. Biol. 2005;50:4169–85. doi: 10.1088/0031-9155/50/17/018. [DOI] [PubMed] [Google Scholar]
- Hickey KT, Sciacca RR, Bokhari S, Rodriguez O, Chou RL, Faber TL, Cooke CD, Garcia EV, Nichols K, Bergmann SR. Assessment of cardiac wall motion and ejection fraction with gated PET using N-13 ammonia. Clin. Nucl. Med. 2004;29:243–8. doi: 10.1097/01.rlu.0000118001.14457.c3. [DOI] [PubMed] [Google Scholar]
- Kesner AL, Abourbeh G, Mishani E, Chisin R, Tshori S, Freedman N. Gating, enhanced gating, and beyond: information utilization strategies for motion management, applied to preclinical PET. Eur. J. Nucl. Med. Mol. Imaging Research. 2013;3:29. doi: 10.1186/2191-219X-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein GJ, Reutter BW, Ho MH, Reed JH, Huesman RH. Real-time system for respiratory-cardiac gating in positron tomography. IEEE Trans. Nucl. Sci. 1998;45:2139–43. [Google Scholar]
- Lamare F, Ledesma Carbayo MJ, Cresson T, Kontaxakis G, Santos A, Le Rest CC, Reader AJ, Visvikis D. List-mode-based reconstruction for respiratory motion correction in PET using non-rigid body transformations. Phys Med Biol. 2007;52:5187–204. doi: 10.1088/0031-9155/52/17/006. [DOI] [PubMed] [Google Scholar]
- Le Meunier L, Maass-Moreno R, Carrasquillo JA, Dieckmann W, Bacharach SL. PET/CT imaging: effect of respiratory motion on apparent myocardial uptake. J. Nucl. Cardiol. 2006;13:821–30. doi: 10.1016/j.nuclcard.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T-S, Feng T, Tsui BMW. Cardiac and Respiratory Motion Compensation for Dual Gated SPECT. Proc. Society of Nuclear Medicine and Molecular Imaging Conference (Vancouver, BC, Canada, Jun. 2013) 2013;54:2146. [Google Scholar]
- Lee T-S, Park MJ, Tsui BMW. A Simulation Study of the Effect of Phase-Shift on Dual Gated Myocardial Perfusion ECT; Proc. IEEE Medical Imaging Conf. (Valencia, Spain, Oct. 2011); 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewellen TK, Harrison RL, Vannoy S. The SimSET program. Institute of Physics Publishing; Bristol: 1998. pp. 77–92. [Google Scholar]
- Livieratos L, Rajappan K, Stegger L, Schafers K, Bailey DL, Camici PG. Respiratory gating of cardiac PET data in list-mode acquisition. Eur. J. Nucl. Med. Mol. Imaging. 2006;33:584–8. doi: 10.1007/s00259-005-0031-0. [DOI] [PubMed] [Google Scholar]
- Martinez-Moller A, Zikic D, Botnar RM, Bundschuh RA, Howe W, Ziegler SI, Navab N, Schwaiger M, Nekolla SG. Dual cardiac-respiratory gated PET: implementation and results from a feasibility study. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:1447–54. doi: 10.1007/s00259-007-0374-9. [DOI] [PubMed] [Google Scholar]
- McNamara JE, Pretorius PH, Johnson K, Mukherjee JM, Dey J, Gennert MA, King MA. A flexible multicamera visual-tracking system for detecting and correcting motion-induced artifacts in cardiac SPECT slices. Med. Phys. 2009;36:1913–23. doi: 10.1118/1.3117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaid SJ. Characterisation and correction of respiratory-motion artefacts in cardiac PET-CT. University College; London: 2009. [Google Scholar]
- National Library of Medicine Visible Human Project. 1997.
- Nehmeh SA, Erdi YE, Pan T, Pevsner A, Rosenzweig KE, Yorke E, Mageras GS, Schoder H, Vernon P, Squire O, Mostafavi H, Larson SM, Humm JL. Four-dimensional (4D) PET/CT imaging of the thorax. Med. Phys. 2004a;31:3179–86. doi: 10.1118/1.1809778. [DOI] [PubMed] [Google Scholar]
- Nehmeh SA, Erdi YE, Pan T, Yorke E, Mageras GS, Rosenzweig KE, Schoder H, Mostafavi H, Squire O, Pevsner A, Larson SM, Humm JL. Quantitation of respiratory motion during 4D-PET/CT acquisition. Med. Phys. 2004b;31:1333–8. doi: 10.1118/1.1739671. [DOI] [PubMed] [Google Scholar]
- Nichols K, Yao SS, Kamran M, Faber TL, Cooke CD, DePuey EG. Clinical impact of arrhythmias on gated SPECT cardiac myocardial perfusion and function assessment. J. Nucl. Cardiol. 2001;8:19–30. doi: 10.1067/mnc.2001.111087. [DOI] [PubMed] [Google Scholar]
- Pretorius PH, King MA. A Study of Possible Causes of Artifactual Decreases in the Left Ventricular Apex with SPECT Cardiac Perfusion Imaging. IEEE Trans. Nucl. Sci. 1999;46:1016–23. [Google Scholar]
- Reutter BW, Klein GJ, Huesman RH. Automated 3-D segmentation of respiratory-gated PET transmission images. IEEE Trans. Nucl. Sci. 1997;44:2473–6. [Google Scholar]
- Saab G, Dekemp RA, Ukkonen H, Ruddy TD, Germano G, Beanlands RS. Gated fluorine 18 fluorodeoxyglucose positron emission tomography: determination of global and regional left ventricular function and myocardial tissue characterization. J. Nucl. Cardiol. 2003;10:297–303. doi: 10.1016/s1071-3581(02)43240-0. [DOI] [PubMed] [Google Scholar]
- Schleyer PJ, O'Doherty MJ, Barrington SF, Morton G, Marsden PK. Comparing approaches to correct for respiratory motion in NH3 PET-CT cardiac perfusion imaging. Nucl. Med. Commun. 2013;34:1174–84. doi: 10.1097/MNM.0b013e328365bb27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segars W, Lalush D, Frey E, Manocha D, King M, Tsui B. Improved Dynamic Cardiac Phantom Based on 4D NURBS and Tagged MRI. IEEE. Trans. Nucl. Sci. 2009;56:2728–38. doi: 10.1109/TNS.2009.2016196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segars WP. PhD dissertation. The University of North Carolina; May, 2001. Development of a new dynamic NURBS-based cardiac-torso (NCAT) phantom. [Google Scholar]
- Segars WP, Tsui BMW. Study of the efficacy of respiratory gating in myocardial SPECT using the new 4-D NCAT phantom. IEEE Trans. Nucl. Sci. 2002;49:675–9. [Google Scholar]
- Sharir T, Berman DS, Waechter PB, Areeda J, Kavanagh PB, Gerlach J, Kang X, Germano G. Quantitative analysis of regional motion and thickening by gated myocardial perfusion SPECT: normal heterogeneity and criteria for abnormality. J. Nucl. Med. 2001;42:1630–8. [PubMed] [Google Scholar]
- Slomka PJ, Nishina H, Berman DS, Kang X, Akincioglu C, Friedman JD, Hayes SW, Aladl UE, Germano G. “Motion-frozen” display and quantification of myocardial perfusion. J. Nucl. Med. 2004;45:1128–34. [PubMed] [Google Scholar]
- Smanio PE, Watson DD, Segalla DL, Vinson EL, Smith WH, Beller GA. Value of gating of technetium-99m sestamibi single-photon emission computed tomographic imaging. J. Am. Coll. Cardiol. 1997;30:1687–92. doi: 10.1016/s0735-1097(97)00363-x. [DOI] [PubMed] [Google Scholar]
- Song N, Du Y, He B, Frey EC. Development and evaluation of a model-based downscatter compensation method for quantitative I-131 SPECT. Med. Phys. 2011;38:3193–204. doi: 10.1118/1.3590382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Segars WP, Du Y, Tsui BM, Frey EC. Fast modelling of the collimator-detector response in Monte Carlo simulation of SPECT imaging using the angular response function. Phys. Med. Biol. 2005;50:1791–804. doi: 10.1088/0031-9155/50/8/011. [DOI] [PubMed] [Google Scholar]
- Svane B, Bone D, Holmgren A, Landou C. Polar presentation of coronary angiography and thallium-201 single photon emission computed tomography. A method for comparing anatomic and pathologic findings in coronary angiography with isotope distribution in thallium-201 myocardial SPECT. Acta. Radiol. 1989;30:561–74. [PubMed] [Google Scholar]
- Taillefer R, DePuey EG, Udelson JE, Beller GA, Benjamin C, Gagnon A. Comparison between the end-diastolic images and the summed images of gated 99mTc-sestamibi SPECT perfusion study in detection of coronary artery disease in women. J. Nucl. Cardiol. 1999;6:169–76. doi: 10.1016/s1071-3581(99)90077-6. [DOI] [PubMed] [Google Scholar]
- Ter-Pogossian MM, Bergmann SR, Sobel BE. Influence of cardiac and respiratory motion on tomographic reconstructions of the heart: Implications for quantitative nuclear cardiology. J. Comput. Assist. Tomogr. 1982;6:1148–54. doi: 10.1097/00004728-198212000-00016. [DOI] [PubMed] [Google Scholar]
- Travin MI, Heller GV, Johnson LL, Katten D, Ahlberg AW, Isasi CR, Kaplan RC, Taub CC, Demus D. The prognostic value of ECG-gated SPECT imaging in patients undergoing stress Tc-99m sestamibi myocardial perfusion imaging. J. Nucl. Cardiol. 2004;11:253–62. doi: 10.1016/j.nuclcard.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Tsui BMW, Segars WP, Lalush DS. Effects of Upward Creep and Respiratory Motion in Myocardial SPECT. IEEE Trans. Nucl. Sci. 2000;47:1192–5. [Google Scholar]
- Wang WT, Frey EC, Tsui BMW, Tocharoenchai C, Baird WH. Parameterization of Pb X-ray contamination in simultaneous Tl-201 and Tc-99m dual-isotope imaging. IEEE Trans. Nucl. Sci. 2002;49:680–92. [Google Scholar]
- Wang Y, Riederer S, Ehman R. Respiratory motion of the heart: Kinematics and the implications for the spatial resolution in coronary imaging. Magn. Reson. Med. 1995;33:713–9. doi: 10.1002/mrm.1910330517. [DOI] [PubMed] [Google Scholar]
- Yang YW, Chen JC, He X, Wang SJ, Tsui BMW. Evaluation of Respiratory Motion Effect on Defect Detection in Myocardial Perfusion SPECT: A Simulation Study. IEEE Trans. Nucl. Sci. 2009;56:671–6. doi: 10.1109/TNS.2009.2015446. [DOI] [PMC free article] [PubMed] [Google Scholar]