Abstract
Adult hippocampal neurogenesis has been implicated in hippocampus-dependent learning and memory. Furthermore, the decline of neurogenesis accompanying aging could be involved in age-related cognitive deficits. It is believed that the neural stem cell niche comprises a specialized microenvironment regulating stem cell activation and maintenance. However, little is known about the significance of the extracellular matrix in controlling adult stem cells. Reelin is a large glycoprotein of the extracelluar matrix known to be of crucial importance for neuronal migration. Here, we examined the local interrelation between Reelin expressing interneurons and putative hippocampal stem cells and investigated the effects of Reelin deficiency on stem cell and progenitor cell proliferation. Reelin-positive cells are found in close vicinity to putative stem cell processes, which would allow for stem cell regulation by Reelin. We investigated the proliferation of stem cells in the Reelin-deficient reeler hippocampus by Ki67 labeling and found a strong reduction of mitotic cells. A detailed analysis of dividing Type 1, type 2 and type 3 cells indicated that once a stem cell is recruited for proliferation, the progression to the next progenitor stage as well as the number of mitotic cycles is not altered in reeler. Our data point to a role for Reelin in either regulating stem cell quiescence or maintenance.
Introduction
Adult hippocampal neurogenesis plays an important role for hippocampus-dependent learning and memory [1, 2]. Further, evidence is accumulating that underlines a crucial role of adult neurogenesis in aging-related cognitive deficits and in depression [3, 4, 5, 6]. Conceptually, the stem cell niche controls maintenance and activation of the residing stem cells. The proximity of the niche to vasculature, glial cells and neurons is believed to keep stem cells under control by emitted vascular factors, paracrine or membrane-tethered factors and transmitters [7, 8, 9, 10, 11]. The extracellular matrix composed of large, often highly glycosylated molecules could play a role in regulating stem cells by either anchoring signaling factors thus leading to their accumulation or modulated stability and additionally by activating signaling pathways. Reelin is an extracellular matrix molecule whose importance for migration and synaptic integrity is well discerned. The lack of Reelin in the reeler mutant leads to severe defects, including a principally inversed cortical layering, dispersion of hippocampal granule cells, cerebellar hypoplasia and synaptic impairment [12, 13, 14, 15, 16, 17, 18]. Importantly, Reelin signaling has been found to be essential for the development of a normal hippocampal radial glial scaffold [19]. Radial glia are neurogenic during development and are thought to constitute the radial stem cells of the adult hippocampus [20, 21, 22]. Moreover, Reelin has previously been implicated in the regulation of adult hippocampal neurogenesis [23, 24]. The reeler mutant exhibited a decreased number of newly generated, Doublecortin-positive (DCX+) hippocampal neurons [25]. The underlying mechanisms remain to be elucidated. We aimed to find out whether Reelin regulates proliferation or differentiation of neuronal stem cells and progenitor cells. We therefore analyzed Ki67-expressing (Ki67+), dividing cells along the putative neuroprogenitor lineage and examined the morphological relation between Reelin-positive (Reelin+) cells and stem cells. Our analysis did not provide evidence for effects of Reelin on stem cells that have already entered the cell cycle. The data rather suggest a role for Reelin in controling stem cell quiescence or, alternatively, in the proper organization of the stem cell niche during development.
Material and Methods
Animals
Reeler mutant mice (B6C3Fe strain) were identified by their well-known morphological malformations in the cortex and hippocampus. The genotypes of the mutants were confirmed by PCR analysis of genomic DNA, as described previously [26]. Only males, aged 3 weeks were included into the study. Mice were housed in groups under standard conditions (23 ± 1°C, 40–50% humidity, food and water ad libitum) with an inverted 12:12 light:dark cycle (light off at 07:00 AM). All experiments were performed in accordance with the guidelines of the European Communities Council Directive of 22 September 2010 (2010/63/EU) and approved by the regional council approved by the Institutional Animal Care and Use Committee Center for Experimental Models and Transgenic Services-Freiburg (CEMT-FR) as well as the regional council of Freiburg.
Immunohistochemistry
Tissue preparation. Three-weeks old mice were deeply anesthetized with Narkodorm-n (Pentobarbital; 180 mg/kg, i.p.; Alvetra, Neumünster, Germany). For light microscopic immunohistochemistry animals were perfused transcardially with a fixative solution containing 4% paraformaldehyde (Merck, Darmstadt, Germany) in 0.1 M phosphate buffer (PB, pH 7.2) and postfixed for 2h. For electron microscopic immunohistochemistry, the fixative additionally contained 15% (v/v) saturated picric acid and 0.05% glutaraldehyde (Polyscience). Brains were sectioned on a vibratome at 50 μm in the coronal plane.
Light microscopy
Sections were blocked in 10% donkey serum in PB and incubated with antibodies detecting glial fibrillary acidic protein (GFAP, rabbit polyclonal, 1:1000, DAKO, Glostrup, Denmark or mouse monoclonal, mGFAP, 1:1000, Sigma-Aldrich, Taufkirchen, Germany), Ki67 (rabbit monoclonal, 1:1000, Novocastra, Wetzlar, Germany), Nestin (chicken polyclonal, 1:500, Abcam, Cambridge, UK) and Doublecortin (DCX, goat polyclonal, 1:1000, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). For visualization of immunostaining, Alexa-conjugated fluorescent secondary antibodies (1:500, Dianova, Hamburg, Germany) were used. Sections were coverslipped with fluorescent mounting medium (DAKO, Glostrup, Denmark).
Immunoelectron microscopy
For double immunoelectron microscopy, sections were cryoprotected and freeze-thawed. After blocking in 20% NGS in 50 mM TBS, sections were incubated with primary antibodies for GFAP (rabbit, polyclonal, DAKO) and Reelin (mouse-monoclonal, clone G10, Millipore, Billerica, MA, USA) in 50 mM TBS containing 3% NGS (Vector Laboratories, Burlingame, CA) for 24h at 4°C. After washes in TBS, the sections were incubated with biotinylated goat anti-mouse IgG antibody (1:100; Vector Laboratories) and goat anti-rabbit (Fab fragment, 1:100) coupled to 1.4 nm gold (Nanoprobes, Stony Brook, NY). Subsequently, sections were processed for silver enhancement of the gold particles with an HQ Silver kit (Nanoprobes) and incubated with avidin-biotin peroxidase complex (ABC kit; Vector Laboratories) that was visualized with 3,3′-diaminobenzidine tetrahydrochloride (0.05%) as a chromogen and 0.01% H2O2 as substrate. Sections were then treated with 1% osmium tetroxide and uranyl acetate, dehydrated and flat-embedded in epoxy resin (Durcupan ACM Fluka; Sigma-Aldrich). Ultrathin sections were cut at 60–70 nm on an ultramicrotome (Reichert Ultracut E; Leica), and viewed on a Philips CM100 electron microscope. Images were taken with a CCD camera (Orius SC600; GATAN) and analyzed using GATAN imaging software.
Quantitative analysis of immunostainings
For quantitative analysis of fluorescently labelled sections, images were captured using a confocal laser-scanning microscope (LSM 510, Carl Zeiss, Jena, Germany), equipped with an Argon laser (excitation lines: 458, 488 and 514) and a He-Ne laser (excitation lines: 543 and 635 nm). To image the area of the dentate gyrus, a 10x objective was used; for visualisation of immunostained cells a Plan-Neofluar 40xI 1.3 oil objective was used. The coordinates corresponded to Bregma −1.2 to −2.2. Due to the granule cell dispersion found in the reeler hippocampus, a subgranular zone (SGZ) cannot be defined. Therefore, the entire dentate gyrus was included into the analysis in control and reeler animals. For the evaluation of densities of Ki67+ cells, all Ki67+ cells within the dentate gyrus per image were counted; the area of the dentate gyrus was measured by tracing the dentate gyrus using ImageJ, and the ratio of Ki67+ cells per area calculated. The mean value per animal was acquired from all section images. For evaluating the proportions of type1, type 2, and type 3 cells among all Ki67+ cells, the percentage of each type per counted total of Ki67+ cell was calculated per animal.
Quantitative analysis was performed using ImageJ (http://rsbweb.nih.gov/ij/index.html). Sections containing the dorsal hippocampus from 3 reeler mice, 3 heterozygous and 3 wild-type animals were used for acquisition of Ki67+ cell numbers. Sections for the analysis of type 1, 2, 3 cells derived from 3 reeler mutants and 2 control animals. Using the marker combination Ki67/Nestin/GFAP, a total of 144 control and 310 reeler Ki67+ cells were analyzed. For the combination Ki67/Nestin/DCX a total of 274 control and 202 reeler cells were used.
Statistical analysis
Statistical analysis was done using GraphPad InStat 3.0 (GraphPad Software, Inc., San Diego, CA). Student’s t-test and ANOVA were employed. Statistical significance was assumed with p ≤ 0.05. Values are expressed as mean ± SEM.
Results
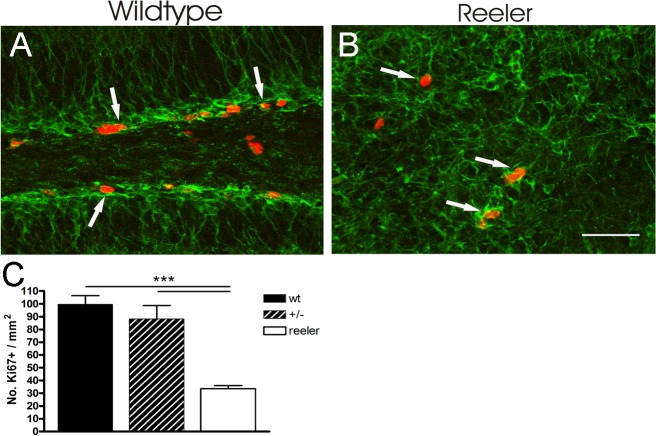
To find out whether Reelin plays a role in regulating stem cell proliferation we analyzed the number of dividing cells positive for Ki67, a protein present during all active phases of the cell cycle, in the dentate gyrus of Reelin—deficient reeler mice in comparison to control animals (Fig. 1). Whereas in control animals 99.6 ± 6.8 cells/mm2 underwent cell cycling, in reeler animals only 33.5 ± 2.5/mm2 Ki67—positive (Ki67+) cells were found. The results were statistically significant (ANOVA, p ≤ 0.0001, F = 22.8, post-hoc Tukey's Multiple Comparison Test, p ≤ 0.001). With 88.2 ± 10.5/mm2 dividing cells in heterozygous animals, numbers were comparable to wild-type mice.
Fig 1. Reduced proliferation in reeler hippocampus.
A, B show an example of Ki67+ mitotic cells (red, arrows) of the hippocampal dentate gyrus in a wild-type mouse (A) and a reeler mouse (B). Additional labeling of young DCX+ (green) neurons reveals the structural disorganization in reeler. C Quantification of Ki67+ cells. There are significantly reduced numbers of mitotic cells in the reeler dentate gyrus. Mean values + SEM are given, *** p < 0.001. Scale bar: 50 μm. wt: wild-type, GCL: granule cell layer, SGZ: subgranular zone, H: hilus.
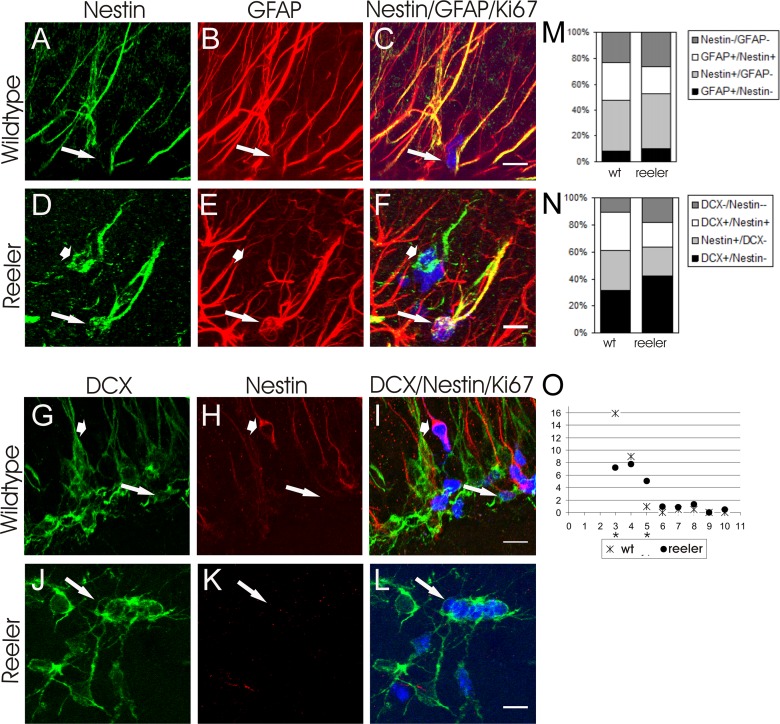
Within the hippocampal stem cell niche a heterogeneous population of stem- and progenitor cells reside. According to a classification proposed by Kempermann et al. [27] the Nestin− as well as GFAP− positive, putative type 1 stem cells can be discriminated from Nestin+/GFAP− type 2a and Nestin+/DCX+ type 2b cells. Type 3 cells finally exhibit DCX but have lost Nestin expression. Does the reduced proliferation observed in reeler mutants result from Reelin influencing proliferation or differentiation of a certain type of stem cell or progenitor cell? To analyze this, the numbers of stem cells and progenitor cells among Ki67+ dividing cells were determined using Ki67 labeling in combination with GFAP and Nestin or Ki67 in combination with Nestin and DCX (Fig. 2). In reeler animals 20.4 ± 4.0% of Ki67+ cells were Nestin+/GFAP+, therefore could be classified as type 1 cells. 42.9 ± 3.6% were Nestin+/GFAP− type 2, and 42.6 ± 7.4% DCX+/Nestin− type 3 cells. It should be noted that these values derive from two different labeling experiments. The number of type 1, type 2 and type 3 cells in control animals were 28.9 ± 1.9%, 39.2 ± 2.7 and 30.9 ± 0.5%, respectively, and did not differ significantly from reeler animals (Student’s t-test, p > 0.05).
Fig 2. Type 1, type 2 and type 3 cells undergoing mitosis.
A-C show an example of a Nestin+/GFAP+/Ki67+ type 1 cell in the control dentate gyrus (arrow: Ki67, blue), D-F in the reeler dentate gyrus (arrow: Ki67). In D-F additionally a Nestin+/GFAP−/Ki67+ is labelled (short arrow). G-I display a DCX+/Nestin−/Ki67+ type 3 cell (arrow) in a wild-type animal, J-L in reeler. In G-I an additional DCX-/Nestin+/Ki67+, presumptive type 1 cell is present (short arrow). M, N Quantified cell fractions among all mitotic cells in the dentate gyrus for the two triple-staining experiments performed. O Incidence of Ki67+ cluster size in control and reeler dentate gyrus. In control animals cell clusters often consisted of only three cells, whereas clusters in reeler contained significantly more, often five cells. Data are represented by mean values; * p < 0.05, *** p < 0.0001. Scale bars: 10 μm.
Conspicuously, in the reeler mutant Ki67+ proliferating cells more often were found in larger cell clusters (Fig. 2). By analyzing the occurrence of Ki67+ cells in relation to cell cluster size we indeed found enhanced attachment, respectively a shift to higher cell numbers per cluster in Reelin deficient animals. Whereas in control animals 15.90 ± 4.0% Ki67+ cells were found in small clusters consisting of only 3 cells, 7.18 ± 1.3% Ki67+ cells were located in clusters of 3 in reeler animals (Student’s t-test, p ≤ 0.05). To the contrary, only 0.90 ± 0.5% of Ki67+ cells were found in larger clusters of 5 in control animals compared to 5.05 ± 0.2% in Reelin deficient mice (Student’s t-test, p ≤ 0.0001).
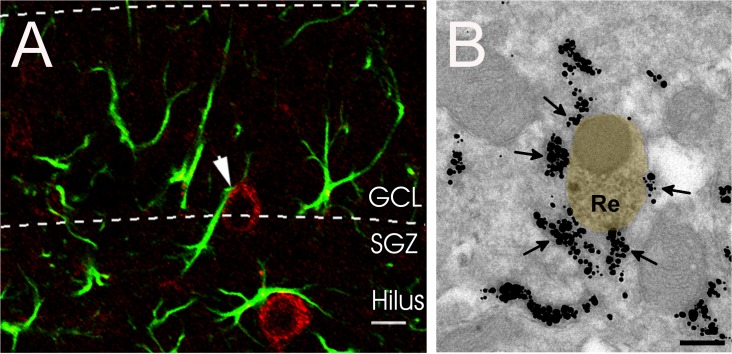
The major source of Reelin in the adult brain are Parvalbumin-expressing interneurons [28,29]. We further aimed to elucidate the morphological relationship of Reelin-expressing interneurons and GFAP+ stem cells of the SGZ. Using immunohistochemistry we could detect Reelin+ interneurons in close apposition to GFAP+ radial-oriented putative stem cells of the SGZ (Fig. 3A). Furthermore, in electron microscopic analysis GFAP+ and Reelin+ processes could be found in close apposition in the SGZ which is supporting a possible regulation of postnatal stem cells by Reelin secreted from interneurons (Fig. 3B). Measuring the extent of Reelin+ membrane covered by GFAP+ profiles resulted in 32.4 ± 3.2% coverage (from a total of nine examples).
Fig 3. A Close proximity between a Reelin+ interneuron (red) and a GFAP+ putative stem cell (green) in the SGZ of a wild-type mice.
B Example of close apposition of GFAP+ glial processes and a Reelin+ dendrite as revealed by electron microscopic double immunolabeling. Reelin labeling is visualized by DAB precipitate (yellow overlay), GFAP by gold particles (arrows). Scale bar in A: 10 μm, in B: 0.2 μm. GCL: granule cell layer, SGZ: subgranular zone.
Discussion
In this study we aimed at gaining insight into a regulatory role of Reelin in stem cell or neuronal progenitor proliferation and differentiation. Analysis of proliferating Ki67+ cells in reeler mutants, heterozygous animals, and wild-type mice revealed a dramatic reduction of proliferation in the dentate gyrus of Reelin-deficient reeler mice in comparison to both, heterozygous and wild-type animals. We are confident that these findings reflect an effect of Reelin on the stem cell niche although no detailed stereological analysis was performed. Our results confirm and extend previous studies [25] and suggest that altered proliferation is the cause of the reduced numbers of newly generated DCX+ cells 15 days after the first BrdU injection, as reported [25]. In heterozygous animals intermediate levels of Reelin expression were found [30]. Apparently in contrast to a previous study [31], in our experimental paradigm this lower amount of Reelin in heterozygous animals was sufficient to maintain a normal stem cell niche. Similarly, neuronal layering of the cortex, hippocampus and dentate gyrus and the vertical arrangement of the dentate radial glial scaffold, which are distorted in reeler, develop normally in heterozygous animals [19,32].
The adult hippocampal stem cell niche consists of a heterogeneous group of putative stem- and progenitor cells that at least in part can be identified by application of different marker combinations. If and how these stem cell/progenitor types are specifically regulated is not known. In order to find out about a potential role of Reelin in influencing proliferation or differentiation of type 1, type 2 or type 3 stem cells, we determined the numbers of Ki67+/Nestin+/GFAP+ type 1 cells, Ki67+/Nestin+/GFAP− type 2 cells and Ki67+/Nestin−/DCX+ type 3 cells. Since the lack of Reelin has been described to influence cell polarity, the orientation of cells could not reliably be used as acriterion. We regard it as a main finding of the present study that the ratios of type 1, type 2, and type 3 cells among all proliferating cells were not significantly different between genotypes. However, in view of the low number of animals available to us, and relatively high standard deviations we cannot exclude that a type II error may have occurred and that we missed significant differences. We can conclude at least that Reelin deficiency did not alter cell cycling or the differentiation of specific stem cell/progenitor cell types in a range that would reflect the 66% difference in the density of Ki67+ cells between wild-type mice and reeler mutants. Since cell death has been reported previously to be not enhanced in the reeler SGZ [25], the observed reduction of proliferating cells to one-third in reeler may likely be due to either i) reduced numbers of quiescent stem cells being recruited for proliferation or ii) a developmentally disrupted stem cell niche not sufficiently differentiated to maintain normal stem cell numbers. Which of these alternatives holds true, needs to be addressed in future experiments by analyzing inducible knock-out mice. One finding in support of a potential function of Reelin in controlling the stem cell niche is the close proximity of Reelin+ and GFAP+ cells and processes in the SGZ.
Finally, we found a higher number of proliferating cells in reeler that formed clusters of 5 or more cells. Reelin has been previously discussed as a detachment signal for migrating neurons [33], and our result would support such a hypothesis. Furthermore, feedback signaling from adult generated neurons to progenitor stages has been reported [34, 35]. The reduced ability of newly generated cells to leave their niche could also influence stem cell behavior and may have contributed to the reduction in proliferation we observed in reeler.
In summary then, our data indicate a role for Reelin either in developing, maintaining or regulating type 1 stem cell activation. Reelin deficiency, in turn, results in reduced adult-generated neurons in the reeler mutant [25]. Furthermore, Reelin signaling has been shown to be crucial for normal dendritic maturation of adult-generated neurons [36]. The importance of adult hippocampal neurogenesis for learning and memory processes has been demonstrated [1,2], and impaired adult neurogenesis might play a role in deficits accompanying aging as well as depression [3, 4, 5, 6]. Along this line, Reelin signaling was found altered in several neurological disorders including depression, bipolar disorder, schizophrenia and autism [37, 38, 39,40]. We hypothesize Reelin to be an essential player in normal brain function by controlling adult neurogenesis via recruiting quiescent stem cells for proliferation, thereby contributing to an adaptation to environmental needs.
Acknowledgments
We are grateful to Akos Kulik for discussion and Natalie Callies for excellent technical help.
Our work was supported by DFG (SFB/TR3, FR 620/12-1). MF is Senior Research Professor of the Hertie Foundation.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors' work was supported by Deutsche Forschungsgemeinschaft (DFG) SFB/TR3 and FR 620/12-1. MF is a Senior Research Professor of the Hertie Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, et al. (2009) Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell 139: 814–827. 10.1016/j.cell.2009.10.020 [DOI] [PubMed] [Google Scholar]
- 2. Deng W, Aimone JB, Gage FH (2010) New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11: 339–350. 10.1038/nrn2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000) Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20: 9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–809. [DOI] [PubMed] [Google Scholar]
- 5. Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA (2011) Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476: 458–461. 10.1038/nature10287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miranda CJ, Braun L, Jiang Y, Hester ME, Zhang L, Riolo M, et al. (2012) Aging Brain Microenvironment Decreases Hippocampal Neurogenesis Through Wnt-Mediated Survivin Signaling. Aging Cell. [DOI] [PMC free article] [PubMed]
- 7. Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, et al. (2003) Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron 39: 937–950. [DOI] [PubMed] [Google Scholar]
- 8. Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, et al. (2010) Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell 7: 78–89. 10.1016/j.stem.2010.04.016 [DOI] [PubMed] [Google Scholar]
- 9. Oliveira SL, Pillat MM, Cheffer A, Lameu C, Schwindt TT, Ulrich H (2013) Functions of neurotrophins and growth factors in neurogenesis and brain repair. Cytometry A 83: 76–89. 10.1002/cyto.a.22161 [DOI] [PubMed] [Google Scholar]
- 10. Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P (2007) Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci U S A 104: 20558–20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ehm O, Goritz C, Covic M, Schaffner I, Schwarz TJ, Karaca E, et al. (2010) RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J Neurosci 30: 13794–13807. 10.1523/JNEUROSCI.1567-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caviness VS Jr., Sidman RL (1973) Time of origin or corresponding cell classes in the cerebral cortex of normal and reeler mutant mice: an autoradiographic analysis. J Comp Neurol 148: 141–151. [DOI] [PubMed] [Google Scholar]
- 13. Caviness VS Jr. (1976) Patterns of cell and fiber distribution in the neocortex of the reeler mutant mouse. J Comp Neurol 170: 435–447. [DOI] [PubMed] [Google Scholar]
- 14. Rice DS, Curran T (2001) Role of the reelin signaling pathway in central nervous system development. Annu Rev Neurosci 24: 1005–1039. [DOI] [PubMed] [Google Scholar]
- 15. Del Rio JA, Heimrich B, Borrell V, Förster E, Drakew A, Alcantara S, et al. (1997) A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature 385: 70–74. [DOI] [PubMed] [Google Scholar]
- 16. Drakew A, Deller T, Heimrich B, Gebhardt C, Del TD, Tielsch A, et al. (2002) Dentate granule cells in reeler mutants and VLDLR and ApoER2 knockout mice. Exp Neurol 176: 12–24. [DOI] [PubMed] [Google Scholar]
- 17. Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, et al. (2005) Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron 47: 567–579. [DOI] [PubMed] [Google Scholar]
- 18. Hack I, Hellwig S, Junghans D, Brunne B, Bock HH, Zhao S, et al. (2007) Divergent roles of ApoER2 and Vldlr in the migration of cortical neurons. Development 134: 3883–3891. [DOI] [PubMed] [Google Scholar]
- 19. Weiss KH, Johanssen C, Tielsch A, Herz J, Deller T, Frotscher M, et al. (2003) Malformation of the radial glial scaffold in the dentate gyrus of reeler mice, scrambler mice, and ApoER2/VLDLR-deficient mice. J Comp Neurol 460: 56–65. [DOI] [PubMed] [Google Scholar]
- 20. Malatesta P, Hartfuss E, Gotz M (2000) Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 127: 5253–5263. [DOI] [PubMed] [Google Scholar]
- 21. Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR (2001) Neurons derived from radial glial cells establish radial units in neocortex. Nature 409: 714–720. [DOI] [PubMed] [Google Scholar]
- 22. Alvarez-Buylla A, Seri B, Doetsch F (2002) Identification of neural stem cells in the adult vertebrate brain. Brain Res Bull 57: 751–758. [DOI] [PubMed] [Google Scholar]
- 23. Won SJ, Kim SH, Xie L, Wang Y, Mao XO, Jin K, et al. (2006) Reelin-deficient mice show impaired neurogenesis and increased stroke size. Exp Neurol 198: 250–259. [DOI] [PubMed] [Google Scholar]
- 24. Massalini S, Pellegatta S, Pisati F, Finocchiaro G, Farace MG, Ciafre SA (2009) Reelin affects chain-migration and differentiation of neural precursor cells. Mol Cell Neurosci 42: 341–349. 10.1016/j.mcn.2009.08.006 [DOI] [PubMed] [Google Scholar]
- 25. Zhao S, Chai X, Frotscher M (2007) Balance between neurogenesis and gliogenesis in the adult hippocampus: role for reelin. Dev Neurosci 29: 84–90. [DOI] [PubMed] [Google Scholar]
- 26. Deller T, Drakew A, Frotscher M (1999) Different primary target cells are important for fiber lamination in the fascia dentata: a lesson from reeler mutant mice. Exp Neurol 156: 239–253. [DOI] [PubMed] [Google Scholar]
- 27. Kempermann G, Jessberger S, Steiner B, Kronenberg G (2004) Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27: 447–452. [DOI] [PubMed] [Google Scholar]
- 28. Alcantara S, Ruiz M, D'Arcangelo G, Ezan F, de LL, Curran T, et al. (1998) Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci 18: 7779–7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pesold C, Impagnatiello F, Pisu MG, Uzunov DP, Costa E, Guidotti A, et al. (1998) Reelin is preferentially expressed in neurons synthesizing gamma-aminobutyric acid in cortex and hippocampus of adult rats. Proc Natl Acad Sci U S A 95: 3221–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T (1995) A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374: 719–723. [DOI] [PubMed] [Google Scholar]
- 31. Kim HM, Qu T, Kriho V, Lacor P, Smalheiser N, Pappas GD, et al. (2002) Reelin function in neural stem cell biology. Proc Natl Acad Sci U S A 99: 4020–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stanfield BB, Cowan WM (1979) The morphology of the hippocampus and dentate gyrus in normal and reeler mice. J Comp Neurol 185: 393–422. [DOI] [PubMed] [Google Scholar]
- 33. Hack I, Bancila M, Loulier K, Carroll P, Cremer H (2002) Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis. Nat Neurosci 5: 939–945. [DOI] [PubMed] [Google Scholar]
- 34. Nguyen L, Malgrange B, Breuskin I, Bettendorff L, Moonen G, Belachew S, et al. (2003) Autocrine/paracrine activation of the GABA(A) receptor inhibits the proliferation of neurogenic polysialylated neural cell adhesion molecule-positive (PSA-NCAM+) precursor cells from postnatal striatum. J Neurosci 23: 3278–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu X, Wang Q, Haydar TF, Bordey A (2005) Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci 8: 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Teixeira CM, Kron MM, Masachs N, Zhang H, Lagace DC, Martinez A, et al. (2012) Cell-autonomous inactivation of the reelin pathway impairs adult neurogenesis in the hippocampus. J Neurosci 32: 12051–12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fatemi SH, Earle JA, McMenomy T (2000) Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry 5: 654–63, 571. [DOI] [PubMed] [Google Scholar]
- 38. Fatemi SH, Snow AV, Stary JM, raghi-Niknam M, Reutiman TJ, Lee S, et al. (2005) Reelin signaling is impaired in autism. Biol Psychiatry 57: 777–787. [DOI] [PubMed] [Google Scholar]
- 39. Fatemi SH, Kroll JL, Stary JM (2001) Altered levels of Reelin and its isoforms in schizophrenia and mood disorders. Neuroreport 12: 3209–3215. [DOI] [PubMed] [Google Scholar]
- 40. Teixeira CM, Martin ED, Sahun I, Masachs N, Pujadas L, Corvelo A, et al. (2011) Overexpression of Reelin prevents the manifestation of behavioral phenotypes related to schizophrenia and bipolar disorder. Neuropsychopharmacology 36: 2395–2405. 10.1038/npp.2011.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.