Abstract
Introduction
Current clinical trials utilize mesenchymal stromal cells (MSCs) expanded in culture, however these interventions carry considerable costs and concerns pertaining to culture-induced losses of potency. This study assessed the feasibility of new clinical-grade technology to obtain uncultured MSC isolates from three human intra-osseous tissue sources based on immunomagnetic selection for CD271-positive cells.
Materials and Methods
MSCs were isolated from bone marrow (BM) aspirates or surgical waste materials; enzymatically digested femoral heads (FHs) and reamer irrigator aspirator (RIA) waste fluids. Flow cytometry for the CD45−/lowCD73+CD271+ phenotype was used to evaluate uncultured MSCs before and after selection, and to measure MSC enrichment in parallel to colony forming-unit fibroblast assay. Trilineage differentiation assays and quantitative polymerase chain-reaction for key transcripts involved in bone regeneration was used to assess the functional utility of isolated cells for bone repair.
Results
Uncultured CD45−/lowCD271+ MSCs uniformly expressed CD73, CD90 and CD105 but showed variable expression of MSCA-1 and SUSD2 (BM>RIA>FH). MSCs were enriched over 150-fold from BM aspirates and RIA fluids, whereas the highest MSC purities were obtained from FH digests. Enriched fractions expressed increased levels of BMP-2, COL1A2, VEGFC, SPARC and CXCL12 transcripts (BM>RIA>FH), with the highest up-regulation detected for CXCL12 in BM (>1300-fold). Following culture expansion, CD271-selected MSCS were tri-potential and phenotypically identical to plastic adherence-selected MSCs.
Discussion
A CD271-based GMP-compliant immunomagnetic selection resulted in a substantial increase in MSC purity and elevated expression of transcripts involved in bone formation, vascularisation and chemo-attraction. Although this technology, particularly from RIA fluids, can be immediately applied by orthopaedic surgeons as autologous therapy, further improvements in MSC purities and pre-clinical testing of product safety would be required to develop this process for allogeneic applications.
Introduction
Culture expanded mesenchymal stromal cells (MSCs), also designated mesenchymal stem cells, have undergone trials as therapeutic agents to treat a range of conditions including osteogenesis imperfecta [1], cartilage defects [2], acute myocardial infarction [3] and steroid resistant graft versus host disease [4]. Thus far, expanded MSC therapy trials have resulted in variable clinical outcomes, even for the same disease. This may be related to the MSC source or route of administration or to differences in culture conditions and the degree of expansion employed [5–8]. Besides additional safety concerns associated with prolonged ex vivo cell cultivation including the risk of transformation [9–11], therapies utilising expanded cells must fulfil good manufacturing practice (GMP) conditions which makes the costs of therapies prohibitively high [12].
Conversely, uncultured cells, including CD34 selected or CD3, CD19 depleted cellular products following CliniMACS cell selection, have been used for haematopoietic stem cell transplantation for decades and have revolutionized the treatment of haematological diseases and saved the lives of thousands of patients [13]. There is therefore a strong impetus to develop similar clinical-grade procedures for the isolation of uncultured MSCs, especially given the potency and differentiation potentials that such MSCs may possess [14].
Although there have been attempts to increase MSC purity by physical means [15], positive selection based on a specific MSC marker offers an appealing alternative. Because MSCs are very rare in BM aspirates [16–19], the isolation of pure uncultured BM MSCs in the ‘research-scale’ settings is best achieved by cell sorting with a combination of positive and negative markers [17,20–23]. Amongst a number of positive markers proposed in the past [24–26], CD271 offers unique selectivity, defined as the least cross-reactivity with contaminating haematopoietic-lineage cells [7,17,23,27,28]. This is highly advantageous for the establishment of a single marker-based, immunomagnetic enrichment procedure.
Femoral Heads (FH) removed as part of total hip replacement surgery, represent a potential MSC source that is currently discarded but could be used in autologous settings to strengthen implant integration with the remaining bone [29,30]. We and others have previously demonstrated that enzymatic digestion of FH bone releases large numbers of MSCs that have a similar phenotype to MSCs in BM aspirates [31,32]. Reamer irrigator aspirator (RIA) waste fluid is another surgical waste by-product that is rich in MSCs, which is currently discarded [33–35]. It is a particularly attractive source of MSCs since unlike FHs, it does not require enzymatic digestion and can be used in autologous settings in complex bone reconstruction procedures.
This study therefore investigated the potential of clinical-grade anti-CD271 microbeads, in combination with the CliniMACS device, to isolate human uncultured MSCs from BM aspirates. Our secondary aim was to investigate whether this technology could be used to enrich MSCs from other intra-osseous sources, namely the FH digests and RIA waste fluid [32,36], both waste products of surgical interventions.
Materials and Methods
Ethics statement
All patients gave informed written consent and research was carried out in compliance with the Helsinki Declaration. Ethics committee approval for this study was obtained from the local National Health Service Research & Development Department, National Research Ethics Service, Leeds East and West Research Ethics Committees under permit numbers 06/Q1206/127 and 07/Q1205/27 respectively.
Patient cohorts
BM aspirates were obtained from patients undergoing elective orthopaedic surgery or were surplus BM donated by healthy donors, for haematopoietic stem cell transplantation (n = 23, median age 38, range: 4–72). FHs were collected from patients with osteoarthritis admitted for total hip arthroplasty (n = 6, median age 70, range: 58–81). RIA waste fluid (mean volume 867ml) were obtained from patients admitted for the treatment of fracture non-union involving autologous bone grafting (n = 6, median age 38, range: 28–63).
CD271 positive selection using QuadroMACS for functional characterisation
Mononuclear cells (MNCs) isolated from BM aspirates, FHs and RIA waste fluid using Lymphoprep reagent (Axis-Shield, Oslo, Norway) were labelled with clinical grade anti-CD271 microbeads (Miltenyi Biotec GmbH, Germany), according to the manufacturer’s instructions. These consist of murine anti-CD271 monoclonal antibodies (clone information confidential) conjugated to superparamagnetic iron dextran particles. Once labelled, cells were separated into positive and negative fractions using the QuadroMACS system (Miltenyi Biotec). Cells from the positive fraction were seeded into a T25 flask (Corning, NY, USA) and cultured in GMP compliant MSC expansion medium MSCGM Bulletkit (Lonza, Berkshire, UK). Three days later, culture medium was replaced and subsequently changed twice weekly. Once the cells (now denoted CD271-MSC) reached 80% confluence, the adherent cells were harvested with 0.25% trypsin/1mM ethylenediaminetetraacetic acid (EDTA) solution (Sigma-Aldrich, Dorset, UK) and passaged at the seeding density of 4x103/cm2 for further expansion. Control plastic adherent MSCs (PA-MSC) were initiated according to standard protocols [36] and passaged using the same seeding density as CD271-MSCs. At each passage cell population doubling (PD) time was calculated.
To investigate MSC chondrogenic, osteogenic and adipogenic potentials, cells were centrifuged in chondrogenic pellets (2x105cells/pellet) or seeded into 6-well plates (osteogenic and adipogenic seeding density of 2x104/well and 2x105/well, respectively). Cells were cultured in either StemMACS ChondroDiff Media, StemMACS OsteoDiff Media or StemMACS AdipoDiff Media (all Miltenyi Biotec) with twice-weekly media changes. After 21 days of differentiation, chondrogenic pellets were fixed in 10% formalin, embedded in paraffin and 5μm sections were stained with 0.1% Alcian Blue to detect proteoglycan deposition. Osteogenic cultures were first stained with alkaline phosphatase substrate solution followed by a 1-hour treatment with 3% silver nitrate to detect mineralization. Adipogenic cultures were fixed in 60% isopropanol before being stained with Oil Red O solution. Images were captured using a Zeiss Axio Imager II (Carl Zeiss) and processed using NIS Elements Imaging Software version 4.0 (Nikon).
Cell phenotyping
Phenotypic analysis of cells from BM, FH and RIA was carried out using flow cytometry. Samples were incubated with antibodies or isotype controls in FACS buffer (PBS pH7.4, 1% bovine serum albumin) according to the manufacturer’s instructions. In each case 4,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich) or aqua dead cell stain (Life technologies, Paisley, UK) was added prior to acquisition to exclude dead cells.
Previously selected, expanded PA-MSCs and CD271-MSCs from each passage were probed for expression of CD73, CD105, CD90, CD34, CD45, CD19, CD14 and HLA-DR (Table 1) [37]; this data was acquired using a BD FACSCanto II flow cytometer (BD). All data was analysed using FlowJo v7.6.5 software (Treestar, Oregon, USA).
Table 1. Antibodies used.
| Specificity | Conjugate | Clone | Manufacturer |
|---|---|---|---|
| Cultured MSC phenotype | |||
| CD14 | FITC | MφP9 | BD |
| CD19 | FITC | HIB19 | BD |
| CD34 | FITC | 581 | BD |
| CD45 | FITC | 2D1 | BD |
| CD73 | PE | AD2 | BD |
| CD90 | PerCP Cy5.5 | 5E10 | BD |
| CD105 | APC | 266 | BD |
| HLA-DR | APC-H7 | L243 | BD |
| Extended phenotyping | |||
| CD3 | V450 | UCHT1 | BD |
| CD14 | PE | M5E2 | BD |
| CD19 | Alexa fluor 700 | HIB19 | BD |
| CD31 | BV610 | WM59 | Biolegend |
| CD34 | APC | 8G12 | BD |
| CD45 | PEcy7 | HI30 | BD |
| CD45 | BV780 | H130 | Biolegend |
| CD73 | PerCP Cy5.5 | AD2 | Mitenyi Biotec |
| CD90 | PECy7 | 5E10 | BD |
| CD105 | FITC | 43A4E1 | Mitenyi Biotec |
| CD146 | FITC | 3A6 | BD |
| CD271 | APC | ME20.4-1H4 | Mitenyi Biotec |
| CD271 | PE Vio770 | ME20.4-1H4 | Mitenyi Biotec |
| CD235a | PE | GA-R2 | BD |
| MSCA-1 | PE | W8B2 | Mitenyi Biotec |
| SUSD2 | APC | W5C5 | Biolegend |
| Assessment of MSC content | |||
| CD45 | PEcy7 | HI30 | BD |
| CD73 | PE | AD2 | BD |
| CD271 | APC | ME20.4-1H4 | Mitenyi Biotec |
All antibodies used for phenotypic and enrichment analysis. All antibodies listed were monoclonal and raised in mouse.
MSCs identified by negative to low expression of CD45 and positive expression of CD271 (CD45−/lowCD271+) [16,32,34], as well as cells with positive expression of CD45 and low expression of CD271 (CD45+CD271low) were examined in detail. Expression of a range of cell surface markers was examined by incubation with monoclonal antibodies; CD3, CD14, CD19, CD31, CD34, CD73, CD90, CD105, CD146, CD235a, mesenchymal stem cell antigen 1 (MSCA-1) and sushi domain containing 2 (SUSD2) [23,38,39] (Table 1). Antigen expression on these populations was detected using a BD biosciences LSR II flow cytometer (BD Biosciences, Oxford, UK).
Processing of BM aspirates, FH specimens and RIA fluids prior to CliniMACS procedures
BM aspirates were harvested as previously described [16], with a minor modification in order to ensure maximal MSC yields [40]. In brief, 5ml of BM was harvested from the first needle insertion site, the needle was subsequently repositioned and a further 5ml was harvested; this was repeated until a total volume of 20ml had been collected. A small volume, 1ml pre-enrichment fraction (Pre) was retained for further analysis. A subset of these samples were taken forward for CliniMACS enrichment, in which case the remaining sample was transferred into a sterile 600ml silicone transfer bag (Miltenyi Biotec). Samples not selected for enrichment were used for direct MSC enumeration by flow cytometry using a CD45/CD271/CD73 antibody combination (Table 1), as previously described [16].
Once removed, FHs were bisected by the operating surgeon and stored immersed in sterile saline overnight at 4°C. The following morning, FHs were removed from saline, broken into small fragments (<1g) using a sterile Noviomagus bone mill (De Puy, Leeds, UK) and digested with 600U/ml collagenase (Worthington, Lakewood, US) for 4 hours at 37°C. The liquid fraction was passed through a 70μm filter (BD) to remove any remaining bone fragments and loaded into a 600ml transfer bag. The RIA waste fluid was directly transferred from the RIA device’s collection bag into two 600ml transfer bags.
Clinical-grade MSC selection using CD271 microbeads and the CliniMACS system
Prior to separation, cells were washed (400xg for 20 minutes) with GMP-grade PBS/EDTA (Miltenyi Biotec) containing GMP-grade 5% w/v human serum albumin (HSA, Bio Products Laboratory, Elstree, UK) and the supernatant was subsequently discarded using a closed system, in which all sample or waste bags were connected using a TSCDI sterile tube welder and detached using a sterile tube fuser (both from Terumo).
The volume in the transfer bag was next adjusted to 95ml by addition of PBS/EDTA/HSA buffer as above, to which the contents of one vial of anti-CD271 microbeads was added. The cell/bead mixture was incubated for 30 minutes on a tilting shaker at room temperature (RT). Subsequently, a second wash was performed as above followed by volume adjustment to 95ml. The transfer bag was next loaded onto the CliniMACS device and attached to a CliniMACS tubing set. An automated protocol [41] was initiated resulting in separation into a positive (Post) and a negative fractions. The Post fraction was eluted in 40ml of PBS/EDTA/HSA buffer and concentrated by centrifugation to 1ml for further analysis of purity by flow cytometry and CFU-F assay.
CFU-F assays were performed in duplicate as previously described [36]. Pre and Post fractions were seeded at 1x105 and 1x104 nucleated cells/dish, respectively.
Flow cytometry to assess MSC content before and after selection
For the staining, 50μl of the Pre and Post enrichment fractions originating from BM aspirates, enzymatically digested FH or RIA waste fluid were incubated with CD45, CD271, and CD73 (Table 1) for 15 minutes at RT at manufacturer’s recommended concentrations. Erythrocytes were lysed by the addition of 2ml ammonium chloride solution (168mM NH2Cl, 10mM KHCO3, 1mM EDTA, pH8.0) containing 0.5μg/ml DAPI (Sigma) and incubation at RT for 10 minutes. MSCs were defined as CD45-/lowCD271+CD73+ cells as described previously [16,24,27].
Quantitative real time PCR
In order to examine the potential enrichment of key transcripts involved in bone regeneration as a result of CD271 selection, RNA was isolated from the total cellular component of BM, FH and RIA samples pre and post CD271 selection. Cells were lysed and RNA isolated using Norgen Total RNA Isolation Kit (Geneflow, Lichfield, UK). Following removal of genomic DNA, cDNA was produced using TaqMan MultiScribe reverse transcription reagents (Life Technologies). TaqMan gene expression assays were then used according to the manufacturer’s instructions to measure transcript expression of genes important for bone regeneration. These were bone morphogenic protein 2 (BMP-2) an essential mediator of fracture healing [42], vascular endothelial growth factor C (VEGFC) an important stimulator of angiogenesis [43], Collagen type I alpha 2 (COL1A2) and osteonectin (SPARC) two factors involved in matrix deposition and mineralisation [34] and Stromal derived factor 1 (CXCL12) a chemokine capable of influencing chemotaxis of a wide range of cell types including MSCs [44,45]. Transcript expression was measured relative to the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase (HPRT) in three matched samples, Pre and Post enrichment, from each tissue.
Statistics
The Mann-Whitney U test was used for comparison of CD271-MSCs and PA-MSC characteristics. The Spearman rank correlation coefficient was used to assess statistical dependence between CD271 and CD73 expression on uncultured BM MSCs. SPSS version 21 (IBM) was used to calculate all statistics, GraphPad Prism 6 (GraphPad Software) was used to generate all graphs. All bar charts show mean (bar height) and standard deviation.
Results
Enrichment of MSCs from BM aspirates using clinical-grade CD271 beads
Previous work investigating the functionality of CD271-enriched BM MSCs, in terms of morphology, immunophenotype and tri-lineage potential, has employed research-grade anti-CD271 microbeads [17,24,46]. Here, we assessed the suitability of clinical-grade CD271 microbeads to isolate functionally competent MSCs. CD271-MSC and PA-MSC exhibited similar spindle-shaped fibroblastoid morphology and growth kinetics over the first three passages and showed no significant difference in population doubling times (Fig. 1A). CD271-MSC and PA-MSC also displayed equal positivity for CD73, CD90 and CD105 while lacking expression of the HLA-DR as well as CD14, CD19, CD34 and CD45 (termed Lin) over the first three passages (Fig. 1B), conforming to the classical MSC phenotype [37]. Given that magnetic bead technology relies on binding of microbeads to surface CD271 molecules, there is a possibility that this could compromise further binding of ‘detection’ anti-CD271 antibodies. Furthermore, transient binding of microbeads could also result in internalisation of the CD271 molecule [47]; both factors could therefore affect MSC purity determination post-selection. To account for this, we assessed the value of another MSC-specific molecule: CD73 as an alternative marker for quantification of MSCs before and after CD271-based selections [16,19]. The frequencies of CD45−/lowCD73+ population (Fig. 1C) and the CD45−/lowCD271+ population (Fig. 1D) were measured in parallel in BM aspirates and the correlation between these two populations was examined (Fig. 1E). A remarkably high degree of correlation (R = 0.985, p<0.001) indicated the validity of measuring CD45−/lowCD73+ cells to assess the MSC purity after CD271 selection.
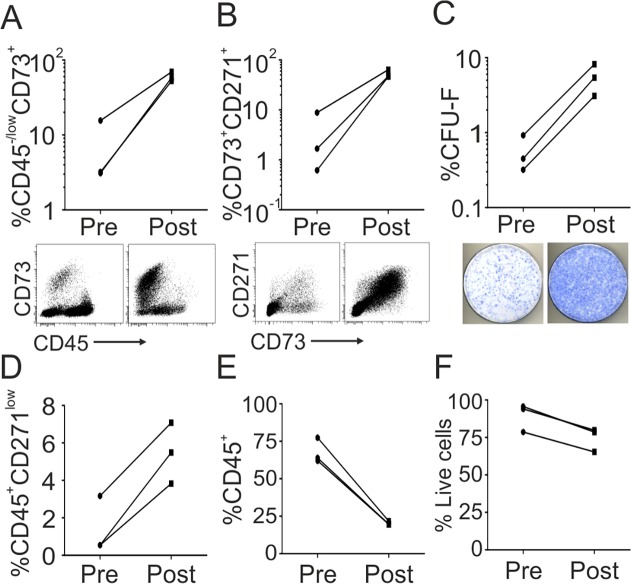
Fig 1. CD271-based enrichment of MSCs from BM aspirates.
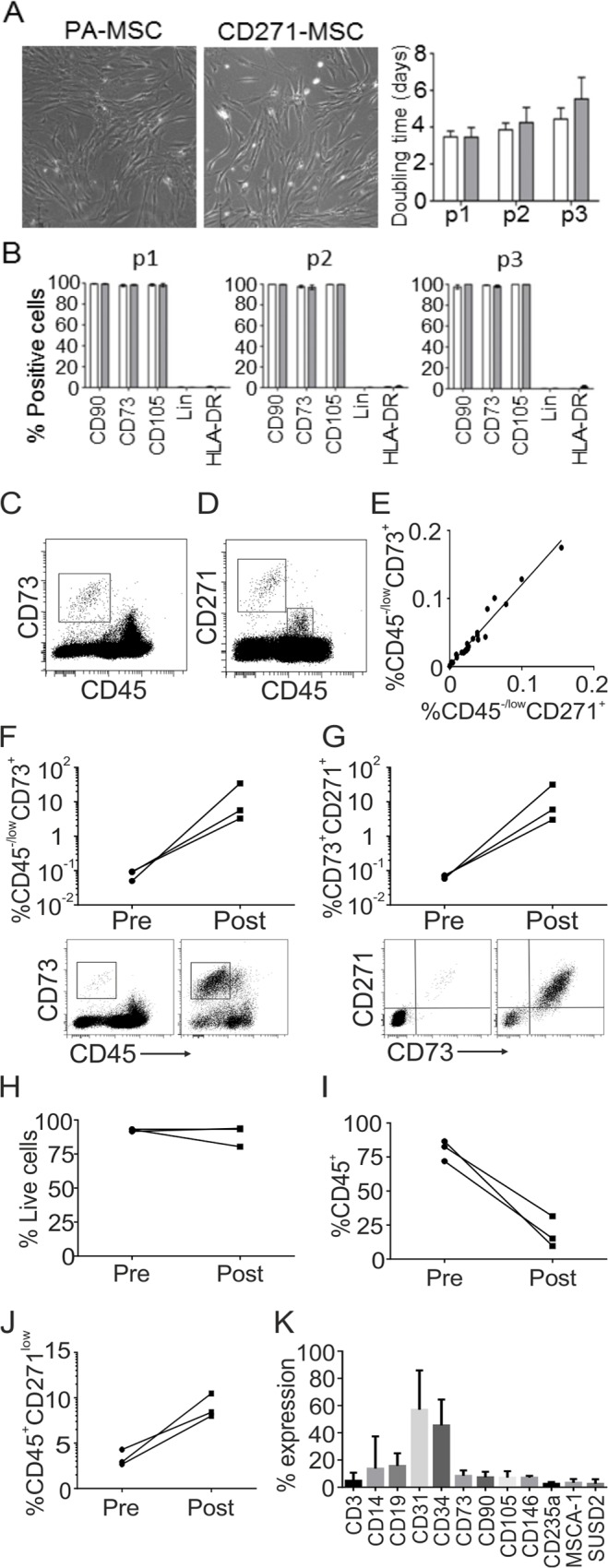
Comparison of the morphology (x10 magnification) and population doubling time between PA-MSC (open bars) and CD271-MSC isolated using the QuadraMACS system (closed bars, n = 5); Bar charts show means + SEM (A). Immunophenotypic analysis of PA-MSC (open bars n = 5) and CD271-MSC (closed bars) from passage 1 to passage 3. (Lin corresponds to a cocktail of CD14, CD34, CD45 and CD19) (B). Representative dot plots generated from a single BM aspirate showing the CD45−/lowCD73+ population (box) (C), the CD45−/lowCD271+ population (large box) and the CD45+CD271low population (small box) (D). The relationship between the proportion of CD45−/lowCD73+ and CD45−/lowCD271+ cells (23 separate BM aspirates collected from 12 donors) (E). The proportion of CD45−/lowCD73+ (F) and CD45−/lowCD73+CD271+ (G) cells, pre and post CliniMACS enrichment with representative dot plots below (n = 3). The proportion of total live cells (H) and CD45+ leukocytes (I) pre and post CliniMACS enrichment. The proportion of CD45+CD271low ‘passenger’ cells (J), pre and post enrichment and a detailed analysis of ‘passenger cell’ phenotype (K). (Data F-K, n = 3)
Immunomagnetic enrichment for MSCs using the CliniMACS system and clinical grade CD271-based microbeads was first assessed using BM aspirates (n = 3 donors). Due to volume-related limitations with the starting material (20ml of aspirate collected using an optimized technique), CFU-F assays were not performed in these experiments and MSC purity was assessed by analysis of the CD45-/lowCD73+ cells (Fig. 1F). The proportion of CD45−/lowCD73+ cells before separation (Pre) was 0.078%, consistent with previously published data [19]; it increased to 14.5% following enrichment, thus representing a 186-fold increase (Fig. 1F). To address our concern regarding the binding of ‘detection’ anti-CD271 antibodies to positively-selected cells, the CD271 antibody was also added to the cocktail and the double-positive (CD45−/lowCD73+CD271+) population was additionally measured. The proportion of double-positive cells increased from 0.066% to 13.5% representing a 204-fold increase (Fig. 1G). This indicated that microbead binding to the CD271 molecule on the surface of MSCs did not significantly affect binding of the anti-CD271 ‘detection’ antibodies.
Total cell viability was not substantially compromised by the enrichment process (Fig. 1H). CD45+ leucocytes [48] represent the majority of cells in BM aspirates. Before enrichment, they represented 80.3% of total cells and although they were substantially depleted following selection, there was still a considerable proportion remaining in the Post fraction (18.7%, Fig. 1I). BM contains a significant portion of cells that express CD271 at a low level as well as CD45 (CD45+CD271low) [23, 26], illustrated on Fig. 1D, small box. Contamination of the positive fraction with this ‘passenger’ cell type was anticipated. There was however, a relatively modest 15-fold increase in this population from a 0.48% to 7.10% (Fig. 1J). This was quite remarkable since these ‘passenger’ cells are approximately 3-fold more numerous than CD45−/lowCD271+ cells in BM [16]. An extended phenotypic analysis of these ‘passenger’ cells confirmed the lack of expression of classical positive markers of BM MSCs such as CD73, CD90 and CD105. ‘Passenger’ cells also lacked expression of MSCA-1 and SUSD2; whereas, CD34 and CD31 were expressed at variable levels (Fig. 1K). Other contaminating cells present following enrichment included low levels of T-cells (3.8%), B-Cells (3.7%), monocytes (4.5%) and endothelial cells (3.0%).
Enrichment of MSCs from intra-osseous surgical waste materials using clinical-grade CD271 beads
Since obtaining large volumes of high-quality aspirates is problematic, we next evaluated clinical-grade MSC isolation procedures using alternative sources of intra-osseous MSCs. Using cells enzymatically-released from FHs as a starting material, we observed that CD45−/lowCD73+ cells increased by of 8.2-fold from 7.3% in the Pre fraction to 60.0% in the Post fraction (Fig. 2A). The same trends were observed for the double-positive CD45−/lowCD73+CD271+ population (14-fold increase, from 3.7% to 52.5%, Fig. 2B). Additional confirmation was provided by CFU-F assay that showed a 9.9-fold increase in the proportion of colony-forming cells (Fig. 2C), giving a mean total 2.4x105 CFU-F in the Post fraction. Similar to our experiments with BM aspirates, a smaller 3.8-fold increase was found in the proportion of CD45+CD271low ‘passenger’ cells (from 1.4% to 5.5%, Fig. 2D). Other contaminating cells included T-cells (3.0%), B-cells (5.1%), monocytes (0.7%) and endothelial cells (2.7%). CD45+ leucocytes were depleted by 3.3-fold from 67.8% to 20.3% (Fig. 2E). Finally, a slight decrease in cell viability was observed from 89.4% to 74.6% (Fig. 2F), possibly as a result of increased storage, exposure to the enzyme and corresponding processing time.
Fig 2. CD271-based enrichment of MSCs from discarded femoral heads.
The proportion of CD45−/lowCD73+ (A) and CD45−/lowCD73+CD271+ (B) cells, from enzymatically digested femoral heads pre and post CliniMACS enrichment with representative dot plots below. The proportion of CFU-Fs pre and post CliniMACS enrichment with representative images of CFU-F dishes below (C). The proportion of CD45+CD271low ‘passenger’ cells (D), CD45+ leukocytes (E) and total live cells (F) pre and post CliniMACS enrichment. (All data n = 3).
Enrichment was also performed using cells collected in RIA waste fluid, surgical procedure for obtaining RIA waste fluid is shown on Fig. 3A. Following CliniMACS enrichment, CD45−/lowCD73+ cells increased by 163-fold from 0.25% to 40.8% (Fig. 3B); this was supported by measurements of double-positive cells, which increased by 304-fold, from 0.11% to 35.0% (Fig. 3C). CFU-F assay confirmed significant MSC enrichment and showed a 173-fold increase in the proportion of colony-forming cells (Fig. 3D), giving a total of 1.2x104 CFU-Fs in the Post fraction. The number of contaminating cells was low, including T-cells (2.2%), B-cells (4.3%), monocytes (1.5%) and endothelial cells (1.8%). The proportion of CD45+CD271low ‘passenger’ cells increased 76-fold, from 0.2% to 18.2% (Fig. 3E) and CD45+ leucocytes were depleted by 2.5-fold (Fig. 3F). There was no substantial decrease in cell viability (Fig. 3G).
Fig 3. CD271-based enrichment of MSCs from RIA waste fluid.
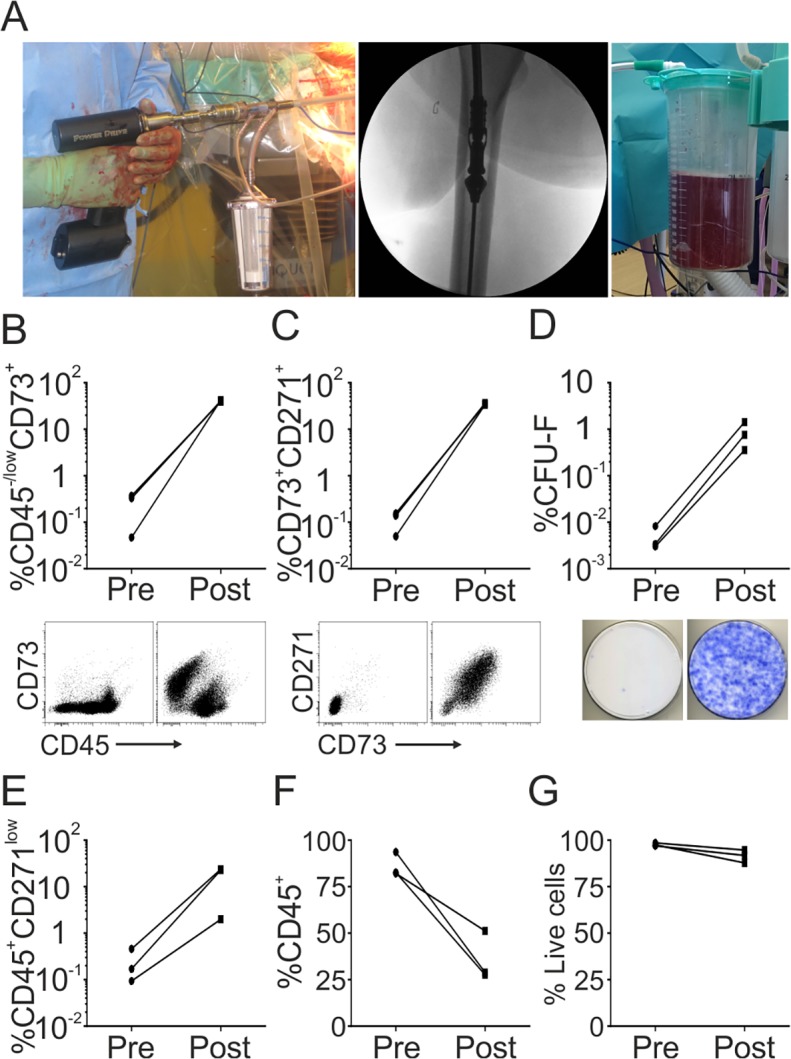
Inter-operative images of the RIA device showing the main body of the device (left panel), the reamer head passing through the intramedullary canal (middle panel) and the waste fluid collection bag (right panel) (A). The proportions of CD45−/lowCD73+ (B) and CD45−/lowCD73+CD271+ cells (C) in RIA waste fluid pre and post CliniMACS enrichment, with representative dot plots shown below. The proportion of CFU-Fs pre and post CliniMACS enrichment and representative CFU-F dishes below (D). The proportion of CD45+CD271low ‘passenger’ cells (E), CD45+ leukocytes (F) and total live cells (G) pre and post CliniMACS enrichment. (All data n = 3).
Altogether these data showed that the best MSC purities and yields were achieved using FH as a starting material, where the initial proportions of MSCs were the highest. RIA waste fluid, on the other hand, gave better cell viability but lower degrees of MSC purity and total MSC number.
Phenotypic and functional characteristics of CD271 enriched MSCs from BM, FH and RIA
The functional and phenotypic characteristics of CD271 selected cells from BM, FH and RIA were compared next. In every case CD271 selection resulted in the isolation of cells capable of osteogenic, adipogenic and chondrogenic differentiation (Fig. 4A-C). Differentiation capacity was comparable to MSCs selected by plastic adherence as assessed by positive staining using alkaline phosphatase/von Kossa, Oil Red O and Alcian Blue respectively.
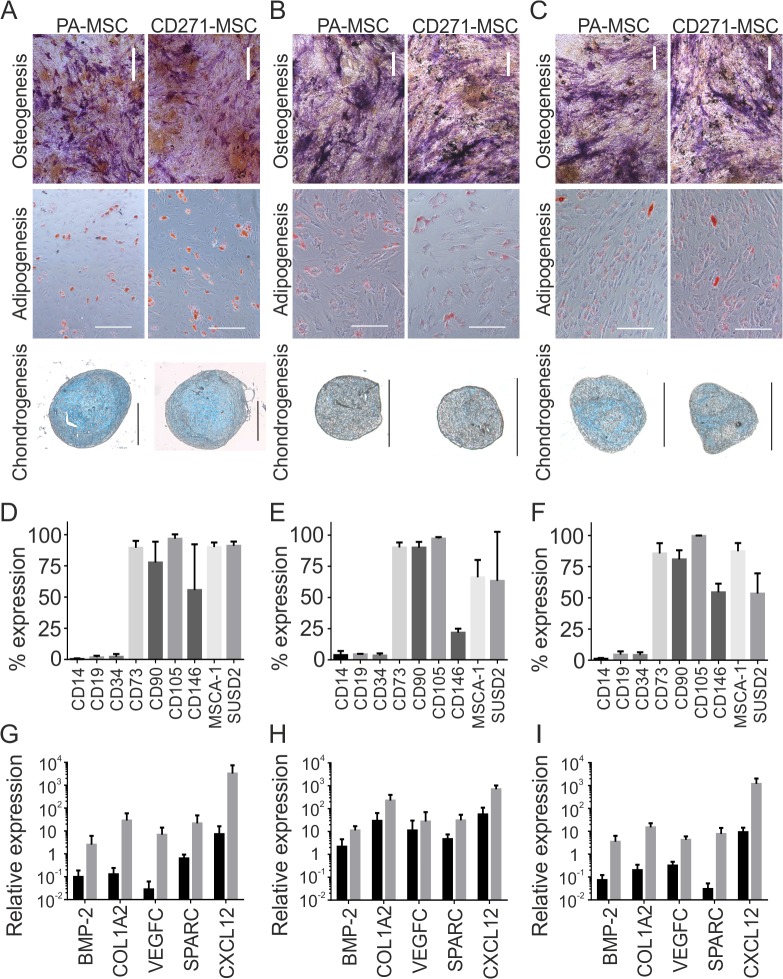
Fig 4. Functional analysis, phenotypic profile and transcripts expression of CD271 selected MSCs from BM, FH and RIA.
Differentiation potential of PA-MSCs and CD271-MSCs. Osteogenesis, adipogenesis and chondrogenesis was measured on day-21 post-induction by staining with alkaline phosphatase/von Kossa, Oil Red O and Alcian Blue, respectively in cells selected from BM (A), FH (B) and RIA (C), all size bars represent 500μm. Phenotypic analysis of the CD45−/lowCD271+ population observed in BM (D), FH (E) and RIA (F). Analysis of the total expression of BMP-2, COL1A2, VEGFC, SPARC and CXCL12 transcripts relative to HPRT in BM (G), FH (H) and RIA (I) pre (black bars) and post (grey bars) CD271 enrichment. (All data n = 3).
Analysis of the extended phenotype of CD45−lowCD271+ MSCs revealed a high degree of similarity with regard to the expression of the classical phenotypic markers of MSCs. CD45−low CD271+ cells in all tissues did not express CD14, CD19 and CD34 and had high expression of CD73, CD90 and CD105 (Fig. 4D-E). Two proposed alternative markers for MSC enrichment MSCA-1 and SUSD2 were highly expressed on CD45−low CD271+ cells from the BM (mean of 90% and 91% respectively, Fig. 4D). However, in FH the proportion of cells expressing MSCA-1 and SUSD2 fell to 66.1 and 63.3, respectively (Fig. 4E). In RIA fluid MSCA-1 was highly expressed on CD45−low CD271+ cells (87.4%), but this was not the case for SUSD2 (63.25%, Fig. 4F).
Altogether, these data confirmed that the selection of MSCs using clinical-grade CD271 microbeads resulted in the isolation of native MSCs with classical MSC functional and phenotypic characteristics.
CD271 selected fractions express elevated levels of transcripts important in bone regeneration
Relative expression of BMP-2 transcript was increased (mean of 311-fold) by CD271 enrichment from BM (Fig. 4G). For VEGFC, an even higher level of enrichment (733-fold) was observed. COL1A2 and SPARC were also highly enriched (687- and 42-fold respectively) (Fig. 4G). Finally, the relative expression of CXCL12 was increased by the greatest degree following CD271 enrichment from BM (1381-fold, Fig. 4G). The expression of these transcripts were uniformly increased in FH although to a lesser degree (Fig. 4H), possibly reflecting much higher initial MSC content in this tissue, and therefore higher initial relative expression. Transcript levels in RIA fluid pre and post enrichment closely resembled those in BM (Fig. 4I).
In summary, CD271 selection led to substantially increased expression levels of BMP-2, VEGFC, COL1A2, SPARC and CXCL12 transcripts.
Discussion
This study is the first assessment of a CD271-based GMP compliant immunomagnetic enrichment procedure to isolate uncultured MSCs for their use in bone repair applications. In every case the selection procedure resulted in a substantial increase in MSC purity and was accompanied by an increased expression of transcripts directly involved in bone formation and vascularisation. Overall, our data demonstrated the feasibility to successfully enrich functionally competent MSCs using an appropriate marker, appropriate device and appropriate procedure for clinical use. We also provided the first evidence supporting the possibility and feasibility to acquire clinically-relevant numbers of uncultured and functionally viable MSCs from discarded FHs or RIA fluids for their future autologous use.
We found that cultures initiated with CD271-selected cells were similar to plastic adherence selected cultures from the same donors, consistent with observations by Poloni et al [49] and extended these observations to CD271-selected MSCs from FHs and RIA fluids. Both plastic adherence and CD271 selected cultures possessed classical MSC phenotype [37] and were capable of tri-lineage differentiation in vitro. In vivo bone forming capacity of CD271 derived cultures has been demonstrated in previous studies [23,50].
Although we observed that cells expressing the CD45-/lowCD271+ phenotype in all three tissues shared very similar expression levels of standard MSC markers such as CD73, CD105 and CD90 [37], we were aware that tissue residence (i.e. within trabecular or cortical bone) could have an additional impact on their in vivo composition [50–52]. To assess potential differences in MSCs selected from BM aspirates, FHs and RIA, we investigated the relative expression of two additional markers that have been suggested to offer highly specific selection for MSCs in BM, MSCA-1 and SUSD2 [38,39]. We confirmed the findings by the Buhring group that in BM aspirates MSCA-1 and SUSD2 were both uniformly and specifically expressed on CD45−/lowCD271+ cells (i.e. were not expressed outside of the CD45−/lowCD271+ population). However, in FH and RIA preparations, a significant proportion of CD45−/lowCD271+ cells lacked expression of either MSCA-1 or SUSD2. It is therefore possible that in BM aspirates these markers may offer superior selectivity for MSCs by avoiding collection of the ‘passenger cell’ population however we believe that such MSCA-1 or SUSD2-based selection could in fact miss out some MSCs, if FHs or RIA fluids are used as a starting material. Regarding the enrichment of CD45+CD271low ‘passenger’ cells in general, in all cases these were enriched to a lesser degree than CD45−/lowCD73+ MSCs or CFU-Fs, suggesting that clinical-grade CD271 microbeads preferentially selected for MSCs, which express higher levels of CD271 [16,23]. The presence of other contaminating cells, which could potentially elicit an immune response, indicated that further improvements in this technology would be required to consider the use of such isolates in allogeneic settings.
In terms of autologous applications, it is the dose and volumetric concentration of MSCs, rather than their purity, appears to be critical. Hernigou et al showed that successful treatment of fracture non-union, by percutaneous injection of concentrated BM, was associated with an average total dose of 30,000 MSCs, measured by CFU-F assay at a concentration of ≥1000 CFU-Fs/ml [53]. The advantage of MSCs enriched using the CliniMACS system in this context is that the Post-fraction can be re-suspended in any final volume making concentrations of over 1000 CFU-Fs/ml easily attainable. Up to now the direct engraftment, proliferation and differentiation of MSCs injected into the fracture site has not been demonstrated however it is very plausible that injected cells exert their regenerative by the release of trophic factors [54,55], rather than extensive in situ expansion and differentiation. A possibility underlined by our findings showing a substantial increase in VEGF and SDF-1 post CD271 enrichment.
With over 71,000 total hip replacements performed annually in England and Wales alone [56], FHs offer an abundant readily available alternative to BM. However, the osteoarthritic environment in which these MSC reside may affect their functionality [57,58] and be a factor limiting their clinical allogeneic use. In comparison, reaming waste fluid represents a more attractive MSC source for future applications; it does not require enzymatic digestion for MSC extraction [33], and offers large volumes of easily obtainable, MSC-rich material that is currently discarded but could be used immediately or banked for the manufacture of high-purity MSC isolates. It must be noted that any MSC preparation is subject to patient related factors known to affect MSC potency such as donor age [8] and these should be taken into account when considering RIA-fluids for MSC therapy. Although the total MSC content following enrichment from RIA fluids was lower than anticipated (an average of 12,000 MSCs measured by CFU-F assay), we believe it can be significantly improved by further optimising fluid collection and tissue handling, including more rigorous anti-clotting measures.
In summary, the utility and commercial viability of MSC-based therapies for bone regeneration and in broader therapeutic settings, with or without culture expansion, are dependent on several factors the most salient of these are: safety, purity, cost and time required for processing (Table 2). The clinical uptake of novel therapeutic interventions based on culture-expanded MSCs is currently impeded by high regulatory demands and cost implications. CD271-based MSC selection from RIA waste fluids would significantly increase both MSC numbers and their purities and as such represents the most viable route for the clinical use of uncultured MSCs in the autologous settings.
Table 2. Summary of the advantages and disadvantages of uncultured MSCs for clinical applications.
| Factor | Advantage | Disadvantage |
|---|---|---|
| Safety | Potential of spontaneous transformation and transfer of animal pathogens [9–11] is limited by avoiding in vitro culture. | Potential carry-over of undesirable phenotypic characteristics in case of FH [57,58]. |
| Purity | Cells are not subject to in vitro aging associated with culture expansion [8,59], therefore no or minimal loss of native phenotypic [21,60] and functional characteristics. | Currently-achieved purity levels are unlikely to be sufficient for allogeneic use. |
| Cost | Reduced cost due to lower regulatory burden [12] and reduced use of GMP-grade culture facilities and reagents. | Total cost is dependent on the application and the numbers of MSCs required; these may be limited by the source material. |
| Time | Cells are ready for use within hours and do not require a lengthy expansion period. | For autologous use extraction/enrichment procedure must fit with intraoperative time frame. |
Acknowledgments
We gratefully acknowledge the help of Mr David Macdonald, Tarek Roshdy and George Cox for help with sample collection, as well as Sarah Churchman for support with transcript assays. We also thank Jason Jones and Kathrin Godhardt at Miltenyi Biotec for all their support as well as for the provision of CliniMACS consumables. Thanks to all the staff at Leeds General Infirmary Trauma unit and the NHS blood and Transplant, Leeds Blood Centre, Leeds, for all their help and for the use of their facilities.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was part-funded by the NIHR/LMBRU and WELMEC, a Centre of Excellence in Medical Engineering funded by the Wellcome Trust and EPSRC, under grant number WT 088908/Z/09/Z. Additional funding came from Arthritis Research UK Award 19429 and CellEurope project, FP7-People-2012-ITN, No. 315963. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, et al. (2002) Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A 99: 8932–8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haleem AM, Singergy AA, Sabry D, Atta HM, Rashed LA, et al. (2010) The Clinical Use of Human Culture-Expanded Autologous Bone Marrow Mesenchymal Stem Cells Transplanted on Platelet-Rich Fibrin Glue in the Treatment of Articular Cartilage Defects: A Pilot Study and Preliminary Results. Cartilage 1: 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, et al. (2009) A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 54: 2277–2286. 10.1016/j.jacc.2009.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, et al. (2008) Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371: 1579–1586. 10.1016/S0140-6736(08)60690-X [DOI] [PubMed] [Google Scholar]
- 5. Bianco P, Barker R, Brustle O, Cattaneo E, Clevers H, et al. (2013) Regulation of stem cell therapies under attack in Europe: for whom the bell tolls. EMBO J 32: 1489–1495. 10.1038/emboj.2013.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galipeau J (2013) The mesenchymal stromal cells dilemma—does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy 15: 2–8. 10.1016/j.jcyt.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 7. Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, et al. (2013) The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 19: 35–42. 10.1038/nm.3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wagner W, Bork S, Horn P, Krunic D, Walenda T, et al. (2009) Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS One 4: e5846 10.1371/journal.pone.0005846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prockop DJ, Brenner M, Fibbe WE, Horwitz E, Le Blanc K, et al. (2010) Defining the risks of mesenchymal stromal cell therapy. Cytotherapy 12: 576–578. 10.3109/14653249.2010.507330 [DOI] [PubMed] [Google Scholar]
- 10. Sensebe L, Bourin P, Tarte K (2011) Good manufacturing practices production of mesenchymal stem/stromal cells. Hum Gene Ther 22: 19–26. 10.1089/hum.2010.197 [DOI] [PubMed] [Google Scholar]
- 11. Sensebe L, Tarte K, Galipeau J, Krampera M, Martin I, et al. (2012) Limited acquisition of chromosomal aberrations in human adult mesenchymal stromal cells. Cell Stem Cell 10: 9–10; author reply 10–11. 10.1016/j.stem.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 12. Couto DS, Perez-Breva L, Cooney CL (2012) Regenerative medicine: learning from past examples. Tissue Eng Part A 18: 2386–2393. 10.1089/ten.TEA.2011.0639 [DOI] [PubMed] [Google Scholar]
- 13. Appelbaum FR (2007) Hematopoietic-cell transplantation at 50. N Engl J Med 357: 1472–1475. [DOI] [PubMed] [Google Scholar]
- 14. Aslan H, Zilberman Y, Kandel L, Liebergall M, Oskouian RJ, et al. (2006) Osteogenic differentiation of noncultured immunoisolated bone marrow-derived CD105+ cells. Stem Cells 24: 1728–1737. [DOI] [PubMed] [Google Scholar]
- 15. Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, et al. (2002) Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells 20: 249–258. [DOI] [PubMed] [Google Scholar]
- 16. Cuthbert R, Boxall SA, Tan HB, Giannoudis PV, McGonagle D, et al. (2012) Single-platform quality control assay to quantify multipotential stromal cells in bone marrow aspirates prior to bulk manufacture or direct therapeutic use. Cytotherapy 14: 431–440. 10.3109/14653249.2011.651533 [DOI] [PubMed] [Google Scholar]
- 17. Jones EA, English A, Kinsey SE, Straszynski L, Emery P, et al. (2006) Optimization of a flow cytometry-based protocol for detection and phenotypic characterization of multipotent mesenchymal stromal cells from human bone marrow. Cytometry B Clin Cytom 70: 391–399. [DOI] [PubMed] [Google Scholar]
- 18. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147. [DOI] [PubMed] [Google Scholar]
- 19. Veyrat-Masson R, Boiret-Dupre N, Rapatel C, Descamps S, Guillouard L, et al. (2007) Mesenchymal content of fresh bone marrow: a proposed quality control method for cell therapy. Br J Haematol 139: 312–320. [DOI] [PubMed] [Google Scholar]
- 20. Delorme B, Ringe J, Gallay N, Le Vern Y, Kerboeuf D, et al. (2008) Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood 111: 2631–2635. [DOI] [PubMed] [Google Scholar]
- 21. Churchman SM, Ponchel F, Boxall SA, Cuthbert R, Kouroupis D, et al. (2012) Transcriptional profile of native CD271+ multipotential stromal cells: evidence for multiple fates, with prominent osteogenic and Wnt pathway signaling activity. Arthritis Rheum 64: 2632–2643. 10.1002/art.34434 [DOI] [PubMed] [Google Scholar]
- 22. Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, et al. (2003) Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci 116: 1827–1835. [DOI] [PubMed] [Google Scholar]
- 23. Tormin A, Li O, Brune JC, Walsh S, Schutz B, et al. (2011) CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood 117: 5067–5077. 10.1182/blood-2010-08-304287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, et al. (2002) Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol 30: 783–791. [DOI] [PubMed] [Google Scholar]
- 25. Mabuchi Y, Houlihan DD, Akazawa C, Okano H, Matsuzaki Y (2013) Prospective isolation of murine and human bone marrow mesenchymal stem cells based on surface markers. Stem Cells Int 2013: 507301 10.1155/2013/507301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harichandan A, Buhring HJ (2011) Prospective isolation of human MSC. Best Pract Res Clin Haematol 24: 25–36. 10.1016/j.beha.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 27. Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, et al. (2002) Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum 46: 3349–3360. [DOI] [PubMed] [Google Scholar]
- 28. Boxall SA, Jones E (2012) Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int 2012: 975871 10.1155/2012/975871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vulcano E, Murena L, Falvo DA, Baj A, Toniolo A, et al. (2013) Bone marrow aspirate and bone allograft to treat acetabular bone defects in revision total hip arthroplasty: preliminary report. Eur Rev Med Pharmacol Sci 17: 2240–2249. [PubMed] [Google Scholar]
- 30. Dozza B, Di Bella C, Lucarelli E, Giavaresi G, Fini M, et al. (2011) Mesenchymal stem cells and platelet lysate in fibrin or collagen scaffold promote non-cemented hip prosthesis integration. J Orthop Res 29: 961–968. 10.1002/jor.21333 [DOI] [PubMed] [Google Scholar]
- 31. Sakaguchi Y, Sekiya I, Yagishita K, Ichinose S, Shinomiya K, et al. (2004) Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Blood 104: 2728–2735. [DOI] [PubMed] [Google Scholar]
- 32. Jones E, English A, Churchman SM, Kouroupis D, Boxall SA, et al. (2010) Large-scale extraction and characterization of CD271+ multipotential stromal cells from trabecular bone in health and osteoarthritis: implications for bone regeneration strategies based on uncultured or minimally cultured multipotential stromal cells. Arthritis Rheum 62: 1944–1954. 10.1002/art.27451 [DOI] [PubMed] [Google Scholar]
- 33. Cox G, McGonagle D, Boxall SA, Buckley CT, Jones E, et al. (2011) The use of the reamer-irrigator-aspirator to harvest mesenchymal stem cells. J Bone Joint Surg Br 93: 517–524. 10.1302/0301-620X.93B4.25506 [DOI] [PubMed] [Google Scholar]
- 34. Churchman SM, Kouroupis D, Boxall SA, Roshdy T, Tan HB, et al. (2013) Yield optimisation and molecular characterisation of uncultured CD271+ mesenchymal stem cells in the Reamer Irrigator Aspirator waste bag. Eur Cell Mater 26: 252–262. [DOI] [PubMed] [Google Scholar]
- 35. Porter RM, Liu F, Pilapil C, Betz OB, Vrahas MS, et al. (2009) Osteogenic potential of reamer irrigator aspirator (RIA) aspirate collected from patients undergoing hip arthroplasty. J Orthop Res 27: 42–49. 10.1002/jor.20715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cox G, Boxall SA, Giannoudis PV, Buckley CT, Roshdy T, et al. (2012) High abundance of CD271(+) multipotential stromal cells (MSCs) in intramedullary cavities of long bones. Bone 50: 510–517. 10.1016/j.bone.2011.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317. [DOI] [PubMed] [Google Scholar]
- 38. Sobiesiak M, Sivasubramaniyan K, Hermann C, Tan C, Orgel M, et al. (2010) The mesenchymal stem cell antigen MSCA-1 is identical to tissue non-specific alkaline phosphatase. Stem Cells Dev 19: 669–677. 10.1089/scd.2009.0290 [DOI] [PubMed] [Google Scholar]
- 39. Sivasubramaniyan K, Harichandan A, Schumann S, Sobiesiak M, Lengerke C, et al. (2013) Prospective isolation of mesenchymal stem cells from human bone marrow using novel antibodies directed against Sushi domain containing 2. Stem Cells Dev 22: 1944–1954. 10.1089/scd.2012.0584 [DOI] [PubMed] [Google Scholar]
- 40. Hernigou P, Homma Y, Flouzat Lachaniette CH, Poignard A, Allain J, et al. (2013) Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop 37: 2279–2287. 10.1007/s00264-013-2017-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dvorak CC, Gilman AL, Horn B, Oon CY, Dunn EA, et al. (2013) Haploidentical related-donor hematopoietic cell transplantation in children using megadoses of CliniMACs-selected CD34(+) cells and a fixed CD3(+) dose. Bone Marrow Transplant 48: 508–513. 10.1038/bmt.2012.186 [DOI] [PubMed] [Google Scholar]
- 42. Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, et al. (2006) BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet 38: 1424–1429. [DOI] [PubMed] [Google Scholar]
- 43. Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, et al. (1999) VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med 5: 623–628. [DOI] [PubMed] [Google Scholar]
- 44. Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, et al. (2007) The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 25: 1737–1745. [DOI] [PubMed] [Google Scholar]
- 45. Miller RJ, Banisadr G, Bhattacharyya BJ (2008) CXCR4 signaling in the regulation of stem cell migration and development. J Neuroimmunol 198: 31–38. 10.1016/j.jneuroim.2008.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jarocha D, Lesko E, Ratajczak MZ, Majka M (2006) Comparison of different strategies of MSC isolation revels advantage to expand MSC directly from purified CD105(+) and CD271(+) cells. Blood 108: 725A–725A. [Google Scholar]
- 47. Carcenac M, Dorvillius M, Garambois V, Glaussel F, Larroque C, et al. (2001) Internalisation enhances photo-induced cytotoxicity of monoclonal antibody-phthalocyanine conjugates. Br J Cancer 85: 1787–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Terstappen LW, Loken MR (1988) Five-dimensional flow cytometry as a new approach for blood and bone marrow differentials. Cytometry 9: 548–556. [DOI] [PubMed] [Google Scholar]
- 49. Poloni A, Maurizi G, Rosini V, Mondini E, Mancini S, et al. (2009) Selection of CD271(+) cells and human AB serum allows a large expansion of mesenchymal stromal cells from human bone marrow. Cytotherapy 11: 153–162. 10.1080/14653240802582125 [DOI] [PubMed] [Google Scholar]
- 50. Li H, Ghazanfari R, Zacharaki D, Ditzel N, Isern J, et al. (2014) Low/Negative Expression of PDGFR-a Identifies the Candidate Primary Mesenchymal Stromal Cells in Adult Human Bone Marrow. Stem Cell Reports 3: 1–10. 10.1016/j.stemcr.2014.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, et al. (2006) Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone 38: 758–768. [DOI] [PubMed] [Google Scholar]
- 52. Watson JT, Foo T, Wu J, Moed BR, Thorpe M, et al. (2013) CD271 as a marker for mesenchymal stem cells in bone marrow versus umbilical cord blood. Cells Tissues Organs 197: 496–504. 10.1159/000348794 [DOI] [PubMed] [Google Scholar]
- 53. Hernigou P, Poignard A, Beaujean F, Rouard H (2005) Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am 87: 1430–1437. [DOI] [PubMed] [Google Scholar]
- 54. Sato T, Iso Y, Uyama T, Kawachi K, Wakabayashi K, et al. (2011) Coronary vein infusion of multipotent stromal cells from bone marrow preserves cardiac function in swine ischemic cardiomyopathy via enhanced neovascularization. Lab Invest 91: 553–564. 10.1038/labinvest.2010.202 [DOI] [PubMed] [Google Scholar]
- 55. Prockop DJ (2009) Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther 17: 939–946. 10.1038/mt.2009.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. National Joint Registry for E, Wales (2011) Annual report Didcot: NJR. [Google Scholar]
- 57. Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, et al. (2002) Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum 46: 704–713. [DOI] [PubMed] [Google Scholar]
- 58. Zhen G, Wen C, Jia X, Li Y, Crane JL, et al. (2013) Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med 19: 704–712. 10.1038/nm.3143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schellenberg A, Stiehl T, Horn P, Joussen S, Pallua N, et al. (2012) Population dynamics of mesenchymal stromal cells during culture expansion. Cytotherapy 14: 401–411. 10.3109/14653249.2011.640669 [DOI] [PubMed] [Google Scholar]
- 60. Qian H, Le Blanc K, Sigvardsson M (2012) Primary mesenchymal stem and progenitor cells from bone marrow lack expression of CD44 protein. J Biol Chem 287: 25795–25807. 10.1074/jbc.M112.339622 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.