Abstract
In our previous study, we demonstrated that the use of the autoluminescent Mycobacterium tuberculosis as a reporter strain had the potential to drastically reduce the time, effort, animals and costs consumed in evaluation of the activities of drugs and vaccines in live mice. However, the strains were relatively unstable and lost reporter with time without selection. The kanamycin selection marker used wasn’t the best choice as it provides resistance to amino glycosides which are an important class of second line drugs used in tuberculosis treatment. In addition, the marker could limit utility of the strains for screening of new potential drugs or evaluating drug combinations for tuberculosis treatment. Limited selection marker genes for mycobacterial genetic manipulation is a major drawback for such a marker-containing strain in many research fields. Therefore, selectable marker-free, more stable autoluminescent mycobacteria are highly needed. After trying several strategies, we created such mycobacterial strains successfully by using an integrative vector and removing both the resistance maker and integrase genes by Xer site-specific recombination in one step. The corresponding plasmid vectors developed in this study could be very convenient in constructing other selectable marker-free, more stable reporter mycobacteria with diverse applications.
Introduction
Many severe bacterial diseases, such as tuberculosis (TB), leprosy and Buruli ulcers are caused by mycobacteria. For example, TB, an infectious disease caused by Mycobacterium tuberculosis (MTB), is one of the greatest single infectious diseases causing morbidity and death in the world. The only TB vaccine in use for over 90 years, Mycobacterium bovis BCG (BCG), has very limited protection efficacy in older children and adults. The 9.0 million incident cases of TB, 1.5 million deaths from TB patients in 2013 alone [1], and the appearance of multi drug-resistant (MDR) [2,3], extensively drug-resistant (XDR) [2,3] and even totally drug-resistant (TDR) TB [4] presents a striking reminder of the magnitude of destruction caused by TB. All these indicate that new, more effective drugs and vaccines are urgently needed.
Routine drug susceptibility testing for MTB depends on a positive culture for diagnosis after which a drug susceptibility test is performed which usually takes 3–6 weeks [5]. The slow diagnosis and in some cases inaccurate or false negative phenotypic results [6], is a major contributor to the current drug resistant epidemic and hindrance to mycobacterial research. The Buruli ulcers causing pathogen, Mycobacterium ulcerans, grows even much slower as 3 months are needed for counting the visible colonies after plating. The necessity to work under stringent biosafety level 3-containment also makes studies of MTB very expensive, especially for long-term use facilities. Therefore, the lack of an effective, rapid, reliable and inexpensive reporter strain in TB research, especially for in vivo studies, is a major drawback.
In our previous studies [7,8], we constructed autoluminescent MTB and Mycobacterium ulcerans as reporter strains which expressed the luxCDABE operon from Photorhabdus luminescens [9]. The operon encodes enzymes for both light production and for recycling reaction substrates. Therefore, use of the autoluminescent reporter strains for testing drugs does not need the addition of an exogenous substrate. The same samples can be monitored in real time, and the colony forming units (CFU) and the light intensity (relative light unit, RLUs) correlate very well. Use of this system demonstrates the potential to drastically reduce the time, effort, animals and costs consumed in evaluation of the activities of drugs and vaccines in live mice as it only takes 3 seconds to detect light in live mouse using an inexpensive device [7,8]. The autoluminescent strains created have been proved to be essentially as virulent as their wild-type parent strains and the drug susceptibilities including for aminoglycosides such as streptomycin are not affected except for kanamycin (KAN) which was used as a selection marker. These properties make the reporter strains appealing for testing drug activity both in vitro and in vivo as only very small amount of samples, a few mice and short time are needed to infer the activity of a compound with very good reproducibility and no addition of exogenous substrate. However, the strains are relatively unstable probably as a result of excision of the luxCDABE operon by the L5 mycobacteriophage integrase at a very low rate [10,7]. This assumption was recently approved in a similar study describing the construction of a recombinant MTB expressing firefly luciferase gene in an integrative plasmid with the integrase gene removed [11]. In addition, the selection marker used could have been inappropriate for molecular genetic manipulation [12], screening of potential drug combinations and testing therapeutic regimens containing KAN in vivo due to possible cross drug resistance. The limited antibiotic resistance markers for mycobacterial genetic manipulation pose a serious challenge, and therefore, development of selectable marker-free, more stable, autoluminescent mycobacteria is highly needful.
The strategies for construction of selectable marker-free mycobacterial strains are summarized in our recently published report [12]. Herein, we tried 2 main strategies for constructing selectable marker-free mycobacteria. The antibiotic resistance cassette flanked by two short DNA sequences in direct orientation could possibly be recognized and removed either by the exogenous resolvase or the endogenous mycobacterial recombinases XerCD. The integrase gene also needed to be removed to make the autoluminescent mycobacteria more stable. We finally succeeded using an integrative plasmid expressing the natural luxCDABE operon from Photorhabdus luminescens [9] at the downstream of Hsp60 promoter [13]. The L5 integrase gene (int) and hygromycin (HYG)-resistant gene in the same cassette were resolved by the endogenous XerC and XerD recombinases [14] using our recently published system [12]. The selectable marker-free strains were proved to be more stable than the previously reported ones and could be widely used in anti-mycobacterial drug screening and evaluation. Additionally, their derivative strains could possibly be used widely in many research fields of mycobacteria.
Materials and Methods
Bacterial strains (Table 1) and culture media
Table 1. Bacterial strains in this study.
| Strains | Relevant characteristic(s) | Source or reference |
|---|---|---|
| E. coli DH5α | General-purpose cloning strain; F- [φ80d lacZΔM15] ΔD(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 glnV44 thi-1 gyrA96 relA | [11] |
| M. smegmatis mc2155 | Highly transformable derivative of ATCC 607 | [16] |
| MSM-OHP | MSM cotransformed with pOHP and pInt | This study |
| MSM-OHP | MSM cotransformed with pOHP and pInt | This study |
| AlMSMT1 | MSM containing pOHIhd | This study |
| AlMSMT2 | MSM containing pOPHI | This study |
| UAlMSM | Selectable marker-free autoluminescent MSM | This study |
| M. tuberculosis H37Rv | Widely used virulent laboratory MTB strain, ATCC 27294 | [12] |
| AlRv | Autoluminescent MTB H37Rv resistant to KAN | [7] |
| AlRvT1 | MTB H37Rv::pOHIhd, MTB H37Rv containing pOHIhd | This study |
| AlRvT2 | MTB H37Rv::pOPHI MTB, H37Rv containing pOPHI | This study |
| UAlRv | Selectable marker-free autoluminescent MTB H37Rv | This study |
| M. tuberculosis H37Ra | Widely used avirulent laboratory MTB strain, ATCC25177 | [13] |
| AlRaT2 | MTB H37Ra::pOPHI MTB, H37Ra containing pOPHI | This study |
| UAlRa | Selectable marker-free autoluminescent MTB H37Ra | This study |
| M. bovis BCG Tice | The live attenuated TB vaccine | [15] |
| AlBCGT2 | BCG::pOPHI, BCG containing pOPHI | This study |
| UABCG | Selectable marker-free autoluminescent BCG | This study |
ATCC: The American Type Culture Collection.
Escherichia coli strain DH5α [15] and the corresponding transformants were grown at 37°C in Luria-Bertani (LB) broth or on agar containing KAN (Invitrogen), ampicillin (Sigma-Aldrich, USA) or HYG (Roche Diagnostics, Switzerland) at final concentrations (μg/ml) of 40, 100 and 200, respectively. MTB H37Rv [16], MTB H37Ra [17] and Mycobacterium bovis BCG Tice (BCG) [18,19] were grown at 37°C in Middlebrook 7H9 broth (Becton Dickinson, USA) supplemented with 10% oleic acid albumin dextrose catalase (OADC, Becton Dickinson, USA) and 0.05% Tween80 where indicated, or on 7H11 agar supplemented with OADC. M. smegmatis mc2155 (MSM) [20] was grown in LB broth or on LB agar or Middlebrook 7H11 agar (Difco) supplemented with albumin dextrose catalase at 37°C. KAN, HYG, carbenicillin and cycloheximide were added to agar when required to final concentrations (μg/ml) of 40, 50, 50 and 10 respectively for MTB and BCG, and the same concentrations for MSM except for HYG 150. The concentrations (μg/ml) in liquid broth were KAN 20 and HYG 100 for MSM and HYG 10 for MTB and BCG.
General DNA techniques
For polymerase chain reaction (PCR) amplification reactions were performed with pfu DNA polymerase (Takara) and 5% DMSO was added due to the high G+C content of the mycobacterial genomes. The PCR products were analyzed by electrophoresis in agarose gels and purified using a DNA gel extraction kit (Bioflux). Plasmids were also extracted and purified using kits from the same company. Purified PCR products, plasmids or plasmids transformed into E. coli strains were sequenced at BGI, Shenzhen, China. MSM was transformed as previously described [20], while MTB and BCG were transformed as previously described [21] with some modifications. The competent MTB and BCG cells were first incubated at 37°C for 10 min before electroporation, and transformation was performed at room temperature. The genomic mycobacterial DNA was extracted using the CTAB method as previously described [15].
Construction of marker-free autoluminescent mycobacteria using the endogenous Xer recombinase system
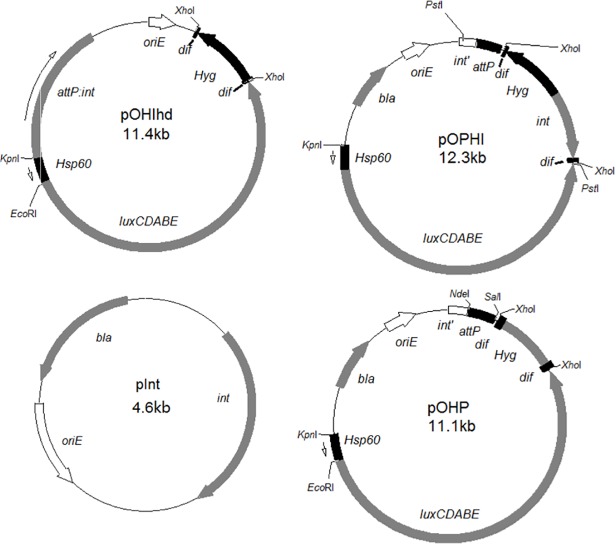
This strategy was designed to deliver the Hsp60-luxCDABE into the mycobacterial genome by an integrative plasmid. The Hyg gene could then either be removed by the endogenous Xer recombinase system to form selectable marker-free strains (plasmid pOHIhd, Table 2, Fig. 1), or alternatively, both Hyg+int genes could be removed together by the same system to form selectable marker-free and more stable strains (plasmid pOPHI, Table 2, Fig. 1). In addition, we co-transformed the suicide plasmid pInt containing the int gene (Fig. 1, Table 2) with plasmid pOHP containing the attP site, the luxCDABE (under the regulation of the strong Hsp60 promoter, Hsp60-luxCDABE) [15], and the dif-ΩHYG-dif cassette (Fig. 1, Table 2).
Table 2. Plasmids in this study.
| The plasmids | Relevant characteristic(s) | Source or reference |
|---|---|---|
| pInt | int, AMPr, ori E, can not multiply in mycobacteria | Fig. 1. |
| pblueInt | The pbluescript SK(+) inserted with attP:int from pMH94 | S3 Fig. [7] |
| pTYP | attP, AMPr, ori E, can not multiply in mycobacteria | S3 Fig. |
| pluxOK | Hsp60-luxCDABE, ori E, KANr, AMPr | [7] |
| pTYOP | Hsp60-luxCDABE, attP, AMPr, ori E, can not multiply in mycobacteria | S2 and S3 Figs |
| pUC19 | AMPr, ori E, general-purpose cloning vector | S1 Fig. |
| pTYdHm | pUC19 containing dif-ΩHYG-dif at KpnI-HindIII sites | [12] |
| pdH3 | pUC19 containing dif-ΩHYG-dif at HindIII site | S1 Fig. |
| pTYOHd | pdH3 inserted with Hsp60-luxCDABE | S1 Fig. |
| pOHIhd | pTYOHd inserted with attP:int | Fig. 1, S1 Fig. |
| pTYd | pUC19 containing dif-dif at KpnI-HindIII sites | S2 Fig. [12] |
| pTYdI | pTYd inserted with int in between dif-dif | S2 Fig. |
| pTYdIH | pUC19 containing dif-Hyg-int-dif | S2 Fig. |
| pOPHI | pTYOP inserted with dif-Hyg-int-dif | Fig. 1, S2 Fig. |
| pOHP | pTYOP inserted with dif-ΩHYG-dif | Fig. 1 |
| pBluescript II SK(+) | AMPr, ori E, general-purpose cloning vector | [7] |
| pBlueI | Derived from pInt for giving int | S2 Fig. |
AMP: ampicillin; KAN: kanamycin; HYG: hygromycin.
Fig 1. The plasmids constructed in this study for transforming into mycobacteria to create unmarked autoluminescent mycobacteria.
oriE, origin region of E. coli; Hsp60, the strong mycobacterial promoter; luxCDABE, the operon for producing autoluminescence; bla, ampicillin resistance gene; Kan, KAN resistance gene; res, the transposonγδ resolvase action site; attP, mycobacteriophage L5 attachment site; int, integrase gene; int’, the remaining part of integrase gene; attB, attachment site from the mycobacterial genome corresponding to attP; oriM, origin region of mycobacteria; Hyg, HYG resistance gene; dif, the recombinases XerCD action site.
To construct plasmid pOHIhd, the dif-ΩHYG-dif cassette was excised with HindIII from plasmid pTYdHm (Table 2) [12] and inserted into plasmid pUC19 (Table 2) digested with the same enzyme to form pdH3 (Table 2, S1 Fig.). The KpnI-Hsp60-luxCDABE-PstI was then excised from pluxOK (Table 2) [7] and inserted into pdH3 (Table 2) digested with the same enzymes to form pTYOHd (S1 Fig., Table 2). The attP:Int excised with KpnI and SmaI from pblueInt (S1 Fig., Table 2) [7] was then inserted into pTYOHd digested with KpnI and ScaI to form pOHIhd (S1 Fig. and Fig. 1, Table 2). To construct the plasmid pOPHI (Table 2), the int gene, amplified with primers Intf and Intr (Table 3) from the plasmid pblueInt (Table 2), was excised with XbaI and ClaI, inserted into the common plasmid pBluescript II SK(+) to form pBlueI (Table 2), and then sequenced. The int gene in pBlueI was then excised with XbaI and ClaI and inserted into pTYd [12] to form pTYdI (S2 Fig., Table 2). The optimized Hyg gene [12] in pTYdHm (Table 2) was cut with XbaI and then inserted into the XbaI site of pTYdI to form pTYdIH (S2 Fig., Table 2). The direction of the Hyg gene was verified by restriction mapping analysis. The dif-ΩHYG-int-dif cassette was then excised from pTYdIH and inserted into pTYOP to form pOPHI (S2 Fig. and Fig. 1, Table 2). To construct plasmid pTYOP, the plasmid pblueInt was digested with PstI and self-ligated to form pTYP (S3 Fig., Table 2) with int gene removed. The Hsp60-luxCDABE excised from pluxOK (Table 2) [7] with KpnI-XhoI was inserted into pTYP to give pTYOP (S3 Fig.).The dif-ΩHYG-dif cassette was excised from the plasmid pTYd constructed in our previous work (Table 2) [12] with XhoI and inserted into the same site of pTYOP to give pOHP (Fig. 1, Table 2).
Table 3. DNA primers used in this study.
| Primer pairs | The function of the primers | Nucleotide sequence (5'-3') with enzyme sites underlined (forward primer/reverse primer) |
|---|---|---|
| Intf/ Intr | Flanking the int gene for cloning it without the attP site. | GCTCTAGACTAGTTTGGAAGAATGGGTGTCT/CCATCGATCTCAGTGTCCTTGGGAGGG |
| Hyg0702-f/Hyg0702-r | Corresponding to an inner part of Hyg for detecting existence of this gene. | AGAGCACCAACCCCGTACTG/GTGAAGTCGACGATCCCGGT |
| Int0702-f/Int0702-r | Corresponding to an inner part of Int for detecting existence of this gene. | TTCATGTGCGCTCGGATCAT/TCACGCTGGAGGAGTACACC |
| noHI-f/noHI-r | Flanking Int-Hyg in the plasmid pOPHI for detecting existence of these 2 genes. | TGGATGCGTCAGCAACCAGT/ CAGAGATGGTGCCCTTGGTG |
| attB1210-f/ attB1210-r | MTB for verifying if the plasmid integrated was dissociated from the genome. | CCTGTTTGGCCAGCTCTTTG/TGCCTTGGTACCGGACAGCA |
| luxAB-f/luxAB-r | Corresponding to an inner part of luxAB for detecting existence of these genes or the luxCDABE operon. | GGTTTATGTGGTGGCTGAAT/GCCGACAACACCATTATCTG |
Construction and verification of the target autoluminescent strains
Mycobacteria transformed with pOHIhd or pOPHI or co-transformed with pInt and pOHP (Table 2) were selected on HYG-containing plates. The autoluminescent mycobacterial colonies with deleted HYG-resistant gene were selected by streaking them in the presence and absence of HYG after several passages in plain 7H9 broth and further tested by PCR using appropriate primers (Table 3).The primer pair Hyg0702-f and Hyg0702-r was for testing the loss of the Hyg gene; Int0702-f and Int0702-r for testing the loss of the int gene; while noHI-f (corresponding to 170 bp from the end of luxE gene) and noHI-r (corresponding to the end of attP near luxCDABE in plasmid pTYOP) was for testing the loss of both Hyg and int genes. A 579-bp fragment was expected from amplification of the Hyg gene open reading frame with Hyg0702-f and Hyg0702-r; a 586-bp fragment from int with Int0702-f and Int0702-r; and a 367-bp fragment from the genome of MTB::pOPHI with deleted dif-ΩHYG-int-dif using primers noHI-f and noHI-r. Three randomly selected MTB H37Rv::pOHIhd colonies with lost HYG resistance gene were amplified with primers attB1210f and attB1210r (Table 3) and sequenced to verify if the whole pOHIhd (Table 2) plasmid had been lost in these strains. Three MSM colonies co-transformed with pInt and pOHP (Table 2) were verified further by amplification with primers luxAB-f and luxAB-r (Table 3), and the expected PCR product was 750bp. The bioluminescence of the autoluminescent MSM/BCG/MTB H37Ra transformants was detected by GloMax 20/20 Luminometer (Promega) while for the autoluminescent MTB H37Rv transformants was detected by Orion II Microplate Luminometer (Titertek-Berthold).
Testing the stability of the selectable marker-free autoluminescent MSM, BCG and MTB
Three single colonies of selectable marker-free autoluminescent MSM, BCG and MTB H37Rv were separately inoculated into 30 mL 7H9 medium and incubated at 37°C with shaking until the OD600 reached over 0.7. An aliquot of 0.3 ml of the culture was then sub-cultured into 30 mL 7H9 medium under the same conditions. An appropriate dilution of the broth culture was obtained after several passages and then plated on plain 7H11 plates. The RLUs of approximately 200 individual colonies picked up at each time point was detected using the above mentioned luminometers. The proportion of autoluminescent colonies was then calculated as: the No. of positive colonies/the total number of colonies detected×100%. If >99% colonies were still autoluminescent after 3 passages (~20 generations), this indicated that the strain was very stable.
Results
Construction of marker-free autoluminescent mycobacteria
We endeavored to create selectable marker-free mycobacteria by removing the resistance marker using the exogenous resolvase or the endogenous mycobacterial recombinases XerCD. In the first strategy, target strains were to be created by integrating the Hsp60-luxCDABE and the res-ΩKAN-res cassette containing plasmids into the genomic attB site with int gene in a separate plasmid. This was to be followed by the removal of the KAN resistance maker gene by the tnpR from resolvase of transposon γδ system, and subsequent removal of the plasmid expressing the resolvase [22]. Even though this tnp/res system had been proved successful in MSM [22], it was unsuccessful in this study using autoluminescent MSM and therefore we did not proceed with it using MTB.
On the other hand, we succeeded using the second strategy in which the target selectable marker-free autoluminescent strains were constructed by integrating the Hsp60-luxCDABE into the genome, followed by the removal of the resistance gene together with the int gene by the endogenous recombinases XerC and XerD [14].
Both MSM and MTB H37Rv were transformed with pOHIhd or pOPHI successfully (Fig. 1, Table 2). Thereafter, BCG and MTB H37Ra were also transformed with pOPHI successfully. All transformants colonies were verified further by detecting bioluminescence. We co-transformed pInt and pOHP (Fig. 1, Table 2) into MSM successfully and obtained MSM-OHP (Table 1). However, none of them was bioluminescent. We therefore verified by PCR if the luxCDABE and Hyg fragments had been integrated into the MSM genome using primer pairs luxAB-f and luxAB-r (750-bp band), and Hyg0702-f and Hyg0702-r (579-bp band), respectively (Table 3). All the 3 randomly selected MSM-OHP colonies gave right sized bands, which meant that the plasmid pOHP (Table 2) had been integrated into the MSM genome.
Counter-selection of the selectable marker-free autoluminescent mycobacteria
The selectable marker-free autoluminescent mycobacterial colonies with HYG-resistant gene rescued were screened by passing the corresponding parent strains several times in antibiotic-free broth culture, testing HYG susceptibility and the autoluminescence of each individual colony. For MSM transformants containing pOHIhd (Fig. 1, Table 2) and designated as AlMSMT1 (Table 1), 90% colonies did not grow on HYG-containing plates anymore after just one passage, and had also lost their autoluminescence. No selectable marker-free autoluminescent MSM was obtained through this technique route after multiple attempts. A similar phenomenon was observed in MTB H37Rv strain transformed with the same plasmid and designated as AlRvT1 (Table 1).
We suspected that the plasmid pOHIhd could have been dissociated from the genome of AlMSMT1 or AlRvT1 much faster than the dissociation of dif-ΩHYG-dif cassette by the endogenous XerCD. Therefore primers attB1210-f and attB1210-r (Table 3) were designed for amplification of the 700-bp fragment containing attB in the middle. Genomic DNA from three randomly selected AlRvT1 colonies without bioluminescence was used as templates with that of wild-type H37Rv as the control. All gave a ~700-bp fragment (S4 Fig.), and the sequence of the randomly selected PCR product from lane 3 was the same as that of wild-type H37Rv.
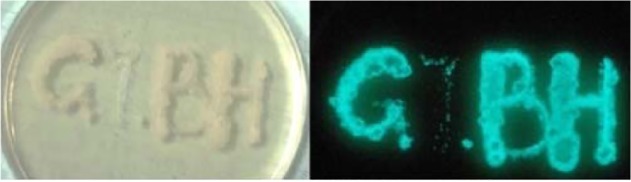
MSM transformed with pOPHI and designated as AlMSMT2 (Table 1) was passed twice in drug-free 7H9 broth and plated on plain agar. 56% colonies lost their HYG resistance and were still autoluminescent and one representative strain was designated as UAlMSM (Table 1). MTB H37Rv transformed with pOPHI was designated as AlRvT2 (Table 2), and all 200 AlRvT2 colonies had lost the HYG resistance and were also still autoluminescent after just one passage. One representative selectable marker-free autoluminescent MTB H37Rv strain was designated as UAlRv (Table 1). Similarly, we obtained AlRaT2 and AlBCGT2 by transforming MTB H37Ra and BCG respectively with pOPHI (Table 2) and the corresponding selectable marker-free autoluminescent UAlRa and UABCG (Fig. 1, Table 1). Two randomly selected UAlMSM, UABCG (Fig. 2), UAlRa and UAlRv colonies (Table 1) were verified further for the loss of the Hyg and int genes by PCR using 3 primer pairs (Table 3): Hyg0702-f and Hyg0702-r for detecting loss of Hyg, Int0702-f and Int0702-r for detecting loss of int, and noHI-f and noHI-r for detecting the loss of both Hyg and int genes. As expected, no right sized PCR products were obtained using the first 2 primer pairs and a 367-bp fragment was obtained using the primers noHI-f and noHI-r (S5 Fig.). Sequence analysis showed that the randomly selected target band from UAlRv1 (lane 2) was the same as deduced from AlRvT2 genome with the dif-ΩHYG-int-dif cassette lost (Table 1). At last, we obtained the selectable marker-free autoluminescent MSM, BCG and MTB H37Rv (Table 1) with int gene lost using pOPHI. The selectable marker-free autoluminescent mycobacterial colonies were visible with naked eyes in a dark room and could be imaged using a normal camera.
Fig 2. Photograph of the more stable, selectable marker-free, autoluminescent BCG (UABCG).
Stability of the selectable marker-free autoluminescent mycobacteria
The stability of UAlMSM, UAlRa, UAlRv and UABCG was tested. For UAlMSM, >99% colonies were still autoluminescent after 6 passage (~40 generations) in about 4 weeks, which implied that the strain was very stable. About 100% (at least more than 99%) of the UAlRv and UAIRa colonies were still strongly autoluminescent after 2 (about 1 month, (~15 generations) and 5 (about 94 days, >35 generations) passages respectively. The UABCG was also very stable as it retained autoluminescence after several passages within 3 months.
Discussion
Studies in MTB and other mycobacteria, and especially the discovery of new anti-mycobacterial drugs and the mechanism of drug action are heavily hampered by their slow growth and the need of expensive biosafety laboratory at higher levels. Rapid, convenient, inexpensive and sensitive reporter strains would facilitate such studies in mycobacteria. We previously demonstrated that a very sensitive autoluminescent MTB grew as fast and was as virulent as its parent strain, in which the RLUs produced by this strain accurately correlated with the CFU counts. The strain could not only be used in vitro for rapid evaluation but also in vivo for rapid drug and even vaccine testing noninvasively using the same batch of live mice in a larger scale [7]. However, there were 2 deficiencies in the autoluminescent strain. Firstly, the strain was not stable, which would limit its applications, and secondly, it contained a KAN resistance marker gene, which further limited its utility in many fields, especially for MTB which has only Hyg and KAN resistance markers [12].
In this study, we demonstrate for the first time the successful construction of selectable marker-free autoluminescent mycobacteria including MTB, BCG and MSM (Table 1). More importantly, all the target strains were extremely stable. For example, 100% (at least >99%) of randomly selected individual colonies of the UAlRv were still autoluminescent after 5 passages with >35 generations in 94 days comprising both log phase and stationary phase culture. In contrast, only 95.7% colonies of the KAN-resistant autoluminescent AlRv (Table 1) were autoluminescent after 37 days of in vitro growth in broth without passage [7]. These results were accordant with a previous study describing the construction of recombinant MTB expressing firefly luciferase gene in which the strains whose int gene was removed were more stable than those whose int gene was not removed [11]. One limitation of this study is that we did not test stability under diverse growth conditions, such as, low pH, macrophage infection model, non-replicating persistence as well as infection animal models. The macrophage infection model can not last for a very long time (usually within 14 days), and no loss of bioluminescence because of instability was observed in our study thus indicating sufficient stability of our strain in the model. The integration of the transforming plasmid into the genomes of mycobacteria and subsequent removal of the integrase gene which excises the plasmid at a very low rate contributed to the stability of the target strains in this study. However, further stability testing of such mycobacterial strains under the above diverse conditions would be needful.
The dif-ΩHYG-int-dif cassette could be widely used in constructing selectable marker-free and more stable recombinant MTB or BCG strains in just one transformation step, such as BCG-based vaccines and recombinant MTB reporter strains.
Previously, no autoluminescent mycobacteria were successfully constructed using extrachromosomal plasmids as delivery vectors [7]. Whether this arose from the reaction of bioluminescence triggering some unknown mechanism to eliminate the plasmids is unknown. Additionally, whether the extra-chromosomal plasmids are affected by the luminescence produced in the autoluminescent mycobacteria challenging their stable existence is also unknown. Another possible cause of instability of the extrachromosomal plasmids expressing luxCDABE in recombinant mycobacteria is the lack of enough energy and toxicity arising from the strong autoluminescence reaction [15]. An earlier study reported that the GFP is expressed at a much higher level when its gene is carried in an extrachromosomal plasmid than when carried in an integrative plasmid [23]. We also reported in our previous study that if a strong promoter is in front of luxAB, such a plasmid could not be obtained even in E. coli because of high toxicity [15]. In this study, we also transformed several types of extrachromosomal plasmids into the selectable marker free autoluminescent mycobacteria and found they could stably exist in them (data not shown). The findings of this study and the other two studies mentioned above support the latter hypothesis about the instability of extrachromosomal plasmids expressing luxCDABE. However, the real reason for this phenomenon still needs further verification as such extrachromosomal plasmids could be used in autoluminescent mycobacteria for studying mechanisms of drug action, such as over-expression and gene complementary experiments.
In a previous study, the authors reported the inability to recover the wild-type attB sequence of MSM in E. coli acceptor cells due to consistent rearrangements [24]. They hypothesized that the wild-type attB sequence of MSM is toxic to E. coli due to the presence of the mycobacterial tRNAgly within the attB site. However, using our failed strategy, we obtained the wild-type attB site-containing plasmid from E. coli without any problem in this study. One difference between these 2 studies is the type of E. coli strains used, as they had used E. coli strain SH288 while we used E. coli DH5α. The other difference is that the fragment containing attB site in our study contained an intact tRNAgly, while in the reported study it only contained a partial tRNAgly [24], which could have been toxic to E. coli.
One interesting observation is that when the mycobacteria were transformed with pOHIhd (Fig. 1, Table 2) in which the Hyg was supposed to be removed by the XerCD, the transformants were autoluminescent. However, all the selectable marker-free derivatives could not give out light. When checked, the selectable marker-free colonies had lost the pOHIhd plasmid at the attB site and were recovered as wild-type. Therefore, selectable marker-free autoluminescent mycobacteria strains could not be obtained by this method. This phenomenon was however not observed with pOPHI (Fig. 1, Table 2) in which the int and Hyg genes were lost together. The autoluminescence together with dif sequence (XerCD) could have affected the activity of the integrase in pOHIhd. Besides, we did not encounter a similar phenomenon in our previous study using eGFP contained in the integrative plasmid pTYGi9 instead of the luxCDABE [12]. The mycobacteria strains transformed with pTYGi9 can just lose the dif-ΩHYG-dif cassette alone successfully instead of the whole plasmid. However, the exact reason for the above strange phenomenon is not fully established and still needs to be further investigated.
The selectable marker-free and more stable mycobacteria present several obvious advantages: Firstly, there is no need of regrowing the original autoluminescent MTB very often to avoid loss of bioluminescence during drug screening and evaluation. Secondly, the potential cross-resistance during drug screening arising from the Kan gene is eliminated. Additionally, the strains can be used to test regimens containing KAN or any drug combinations with KAN. Thirdly the selectable marker-free autoluminescent mycobacterial strains can be used for mycobacterial recombineering [25] or for creating unmarked deletions in autoluminescent strains [26]. Mycobacterial recombineering is a very useful tool that was recently developed for knocking out mycobacterial gene(s) [25], and requires mycobacteria containing a plasmid expressing the gp60/61 genes for increasing the recombination rate. The substrate for homologous exchange usually contains another resistance marker, and as mentioned above, only Kan and Hyg marker genes are utilized in MTB which means that the parent strain should be selectable marker-free. Fourthly, the strains created here can be used for high efficient transposition experiments directly. The mycobacteriophage carrying the highly efficient transposon harbor a Kan marker gene, and when used to transpose MTB [27], the subsequent complementary experiments require the use of another drug resistant marker. Fifthly, some clinical isolates could already be resistant to KAN, and thus after transformation of such a KAN-resistant strain with a Hyg gene to make it autoluminescent; it would be very hard to do any further transformation. Sixthly, the strains have the potential to study the mechanism of drug action related genes more efficiently and quickly. For example, in the knocking out of a gene and complementing it with the corresponding mutant; or overexpressing a gene in the selectable marker-free stable autoluminescent mycobacteria; and then testing their susceptibilities to the corresponding drugs. According to our previous published data on the resistance gene marked autoluminescent strains, it is very reasonable to infer that the new version of strains are more suitable for anti-mycobacterial drug research and for studing the functions or virulence of genes rapidly and more intuitively.
In summary, there existed some unresolved problems associated with autoluminescence, stability and the integration system of L5 mycobacteriophage in mycobacteria. In this study, however, we have successfully created selectable marker-free autoluminescent mycobacteria by just one transformation which presents many advantages than the previous versions.
Supporting Information
oriE, origin region of E. coli; bla, ampicillin resistance gene; Hyg, HYG resistance gene; dif, the recombinases XerCD action site; Hsp60, the strong mycobacterial promoter; luxCDABE, the operon for producing autoluminescence; attP, mycobacteriophage L5 attachment site; int, integrase gene. Commonly used restriction enzyme sites are indicated.
(TIF)
oriE, origin region of E. coli; bla, ampicillin resistance gene; lacZ, the beta-galactosidase gene; lacZ’ and lacZ”, the remaining parts of beta-galactosidase gene; dif, the recombinases XerCD action site; int, integrase gene; int’, the remaining part of integrase gene; Hyg, HYG resistance gene; Hsp60, the strong mycobacterial promoter; luxCDABE, the operon for producing autoluminescence.
(TIF)
oriE, origin region of E. coli; bla, ampicillin resistance gene; attP, mycobacteriophage L5 attachment site; int, integrase gene; int’, the remaining part of integrase gene; Hsp60, the strong mycobacterial promoter; luxCDABE, the operon for producing autoluminescence was from plasmid pluxOK. Commonly used restriction enzyme sites are indicated.
(TIF)
MTB H37Rv transformed the pOHIhd colonies that lost the autoluminescnece by PCR with primers attB1210-f and attB1210-r. M, DNA marker; 1, wild type MTB H37Rv as a control; 2–4, three randomly selected AlRvT1 colonies from that lost the autoluminescnece.
(TIF)
Lane M, DNA marker (bp); Lane 1, PCR product from water as a control (no template); Lane 2,3, PCR products from UAlRv colony 1 and colony2; Lane 4,5, PCR products from UABCG colony 1 and colony2; Lane 6,7, PCR products from UAlMSM colony 1 and colony2; Lane 8, product from wild-type BCG as a control. The right band from lane 2 was sequenced.
(TIF)
Acknowledgments
We are grateful to Professor Christophe Guilhot and Professor Brigitte Gicquel from Institut Pasteur, for providing us with thermosensitive plasmid pCG122 and pWM19 for use in the strategy using transposon γδ system. We thank Professor Eric Nuermberger, William Bishai and Jacques Grosset from the Johns Hopkins University for providing plasmids such as pTYOK, pluxOK and pblueInt and the BCG Tice strain. We thank Professor Jiaoyu Deng at Wuhan Institute of Virology, Chinese Academy of Sciences for providing us with the M. smegmatis mc2 155.
Data Availability
All relevant data are within the paper and its Supporting Information files
Funding Statement
This work was supported by the Chinese Academy of Sciences 'One Hundred Talents Program' (Category A, to TZ), the National Great Research Program of China (2013ZX10003006) and the Key Program of the Chinese Academy of Sciences (KJZD-EW-L02). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. WHO.Global tuberculosis report 2014 World Health Organization, Geneva, Switzerland: 2014. Available: http://www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 2. Hoffner S. Unexpected high levels of multidrug-resistant tuberculosis present new challenges for tuberculosis control. Lancet. 2012; 380:1367–1369. 10.1016/S0140-6736(12)61069-1 [DOI] [PubMed] [Google Scholar]
- 3. WHO. Global tuberculosis report 2012 World Health Organization, Geneva, Switzerland: 2012. [Google Scholar]
- 4. Loewenberg S. India reports cases of totally drug-resistant tuberculosis. Lancet. 2012; 379: 205 [DOI] [PubMed] [Google Scholar]
- 5. Victor TC, van Helden PD, Warren R. Prediction of drug resistance in M.tuberculosis: molecular mechanisms, tools, and applications. IUBMB Life. 2002; 53: 231–237. [DOI] [PubMed] [Google Scholar]
- 6. van Rie, Warren AR, Mshanga I, Jordaan AM, van der Spuy GD, Richardson M, et al. Analysis for a limited number of gene codons can predict drug resistance of Mycobacterium tuberculosis in a high-incidence community. J Clin Microbiol. 2001; 39: 636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang T, Li SY, Nuermberger EL. Autoluminescent Mycobacterium tuberculosis for rapid, real-time, non-invasive assessment of drug and vaccine efficacy. PLoS One. 2012; 7:e29774 10.1371/journal.pone.0029774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang T, Li SY, Converse PJ, Grosset JH, Nuermberger EL. Rapid, Serial, Non-invasive Assessment of Drug Efficacy in Mice with Autoluminescent Mycobacterium ulcerans Infection. PLoS Negl Trop Dis. 2013;7(12): e2598 10.1371/journal.pntd.0002598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Winson MK, Swift S, Hill PJ, Sims CM, Griesmayr G, Bycroft BW, et al. Engineering the luxCDABE genes from Photorhabdus luminescens to provide a bioluminescent reporter for constitutive and promoter probe plasmids and mini-Tn5 constructs. FEMS Microbiol Lett. 1998;163: 193–202. [DOI] [PubMed] [Google Scholar]
- 10. Springer B, Sander P, Sedlacek L, Ellrott K, Bottger EC. Instability and site-specific excision of integration-proficient mycobacteriophage L5 plasmids: Development of stably maintained integrative vectors. Int J Med Microbiol. 2001; 290: 669–675. [DOI] [PubMed] [Google Scholar]
- 11. Andreu N, Zelmer A, Sampson SL, Ikeh M, Bancroft GJ, Schaible UE, et al. Rapid in vivo assessment of drug efficacy against Mycobacterium tuberculosis using an improved firefly luciferase. J Antimicrob Chemother. 2013; 68: 2118–2127. 10.1093/jac/dkt155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang F, Tan Y, Liu J, Liu T, Wang B, Cao Y, et al. Efficient construction of unmarked recombinant mycobacteria using an improved system. J Microbiol Methods. 2014; 103:29–36. 10.1016/j.mimet.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 13. Stover CK, de la Cruz VF, Fuerst TR, Burlein JE, Benson LA, Bennett LT, et al. New use of BCG for recombinant vaccines. Nature. 1991; 351: 456–460. [DOI] [PubMed] [Google Scholar]
- 14. Cascioferro A, Boldrin F, Serafini A, Provvedi R, Palu G, Manganelli R. Xer site-specific recombination, an efficient tool to introduce unmarked deletions into mycobacteria. Appl Environ Microb. 2010; 76: 5312–5316. 10.1128/AEM.00382-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang T, Bishai WR, Grosset JH, Nuermberger EL. Rapid assessment of antibacterial activity against Mycobacterium ulcerans by using recombinant luminescent strains. Antimicrob Agents Chemother. 2010; 54: 2806–2813. 10.1128/AAC.00400-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tan Y, Hu Z, Zhang T, Cai X, Kuang H, Liu Y, et al. Role of pncA and rpsA Gene Sequencing in Detection of Pyrazinamide Resistance in Mycobacterium tuberculosis Isolates from Southern China. J Clin Microbiol. 2014;52: 291–297. 10.1128/JCM.01903-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng H, Lu L, Wang B, Pu S, Zhang X, Zhu G, et al. Genetic basis of virulence attenuation revealed by comparative genomic analysis of Mycobacterium tuberculosis strain H37Ra versus H37Rv. PLoS One. 2008; 3: e2375 10.1371/journal.pone.0002375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horwitz MA, Harth G. A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect Immun. 2003; 71: 1672–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang T, Zhang M, Rosenthal IM, Grosset JH, Nuermberger EL. Short-course therapy with daily rifapentine in a murine model of latent tuberculosis infection. Am J Respir Crit Care Med. 2009; 180: 1151–1157. 10.1164/rccm.200905-0795OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Snapper SB, Melton RE, Mustafa S, Kieser T, Jacobs WR Jr. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis . Mol Microbiol. 1990; 4: 1911–1919. [DOI] [PubMed] [Google Scholar]
- 21. Wards BJ, Collins DM. Electroporation at elevated temperatures substantially improves transformation efficiency of slow-growing mycobacteria. FEMS Microbiol. Lett. 1996; 145:101–105. [DOI] [PubMed] [Google Scholar]
- 22. Malaga W, Perez E, Guilhot C. Production of unmarked mutations in mycobacteria using site-specific recombination. FEMS Microbiol Lett. 2003; 219: 261–268. [DOI] [PubMed] [Google Scholar]
- 23. Huff J, Czyz A, Landick R, Niederweis M. Taking phage integration to the next level as a genetic tool for mycobacteria. Gene. 2010; 468:8–19. 10.1016/j.gene.2010.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saviola B, Bishai WR. Method to integrate multiple plasmids into the mycobacterial chromosome. Nucleic Acids Res. 2004; 32: e11 10.1093/nar/gnh005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Kessel JC, Hatfull GF. Recombineering in Mycobacterium tuberculosis . Nat Methods. 2007; 4: 147–152. [DOI] [PubMed] [Google Scholar]
- 26. Jain P, Hsu T, Arai M, Biermann K, Thaler DS, Nguyen A, et al. Specialized transduction designed for precise high-throughput unmarked deletions in Mycobacterium tuberculosis . MBio. 2014; 5:e01245–14. 10.1128/mBio.01245-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rubin EJ, Akerley BJ, Novik VN, Lampe DJ, Husson RN, Mekalanos JJ. In vivo transposition of mariner-based elements in enteric bacteria and mycobacteria. Proc Natl Acad Sci U S A. 1999; 96: 1645–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
oriE, origin region of E. coli; bla, ampicillin resistance gene; Hyg, HYG resistance gene; dif, the recombinases XerCD action site; Hsp60, the strong mycobacterial promoter; luxCDABE, the operon for producing autoluminescence; attP, mycobacteriophage L5 attachment site; int, integrase gene. Commonly used restriction enzyme sites are indicated.
(TIF)
oriE, origin region of E. coli; bla, ampicillin resistance gene; lacZ, the beta-galactosidase gene; lacZ’ and lacZ”, the remaining parts of beta-galactosidase gene; dif, the recombinases XerCD action site; int, integrase gene; int’, the remaining part of integrase gene; Hyg, HYG resistance gene; Hsp60, the strong mycobacterial promoter; luxCDABE, the operon for producing autoluminescence.
(TIF)
oriE, origin region of E. coli; bla, ampicillin resistance gene; attP, mycobacteriophage L5 attachment site; int, integrase gene; int’, the remaining part of integrase gene; Hsp60, the strong mycobacterial promoter; luxCDABE, the operon for producing autoluminescence was from plasmid pluxOK. Commonly used restriction enzyme sites are indicated.
(TIF)
MTB H37Rv transformed the pOHIhd colonies that lost the autoluminescnece by PCR with primers attB1210-f and attB1210-r. M, DNA marker; 1, wild type MTB H37Rv as a control; 2–4, three randomly selected AlRvT1 colonies from that lost the autoluminescnece.
(TIF)
Lane M, DNA marker (bp); Lane 1, PCR product from water as a control (no template); Lane 2,3, PCR products from UAlRv colony 1 and colony2; Lane 4,5, PCR products from UABCG colony 1 and colony2; Lane 6,7, PCR products from UAlMSM colony 1 and colony2; Lane 8, product from wild-type BCG as a control. The right band from lane 2 was sequenced.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files