Abstract
In vitro, the overexpression of the bcl-2 protooncogene in cultured neurons has been shown to prevent apoptosis induced by neurotrophic factor deprivation. We have generated transgenic mice overexpressing the Bcl-2 protein in neurons, including motoneurons of the facial nucleus. We have tested whether Bcl-2 could protect these motoneurons from experimentally induced cell death in new born mice. To address this question, we performed unilateral lesion of the facial nerve of wild-type and transgenic 2-day-old mice. In wild-type mice, the lesioned nerve and the corresponding motoneuron cell bodies in the facial nucleus underwent rapid degeneration. In contrast, in transgenic mice, facial motoneurons survived axotomy. Not only their cell bodies but also their axons were protected up to the lesion site. These results demonstrate that in vivo Bcl-2 protects neonatal motoneurons from degeneration after axonal injury. A better understanding of the mechanisms by which Bcl-2 prevents neuronal cell death in vivo could lead to the development of strategies for the treatment of motoneuron degenerative diseases.
Full text
PDF

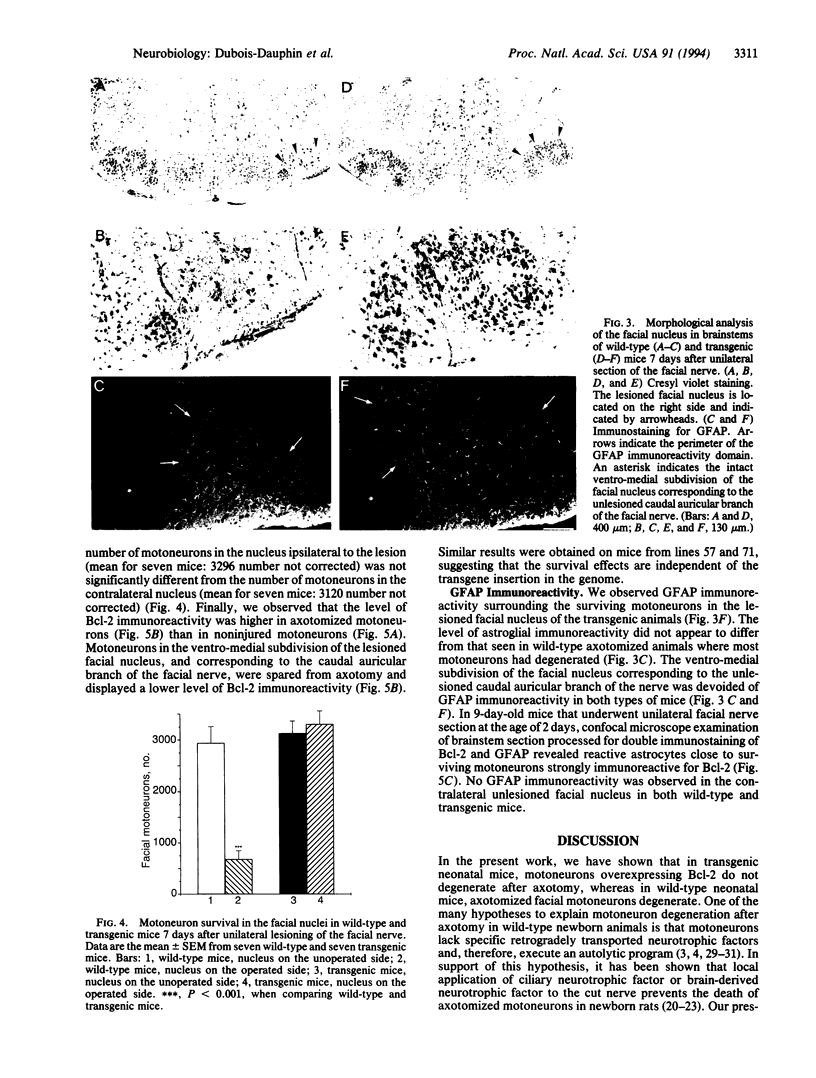


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsopp T. E., Wyatt S., Paterson H. F., Davies A. M. The proto-oncogene bcl-2 can selectively rescue neurotrophic factor-dependent neurons from apoptosis. Cell. 1993 Apr 23;73(2):295–307. doi: 10.1016/0092-8674(93)90230-n. [DOI] [PubMed] [Google Scholar]
- Bakhshi A., Jensen J. P., Goldman P., Wright J. J., McBride O. W., Epstein A. L., Korsmeyer S. J. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985 Jul;41(3):899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- Barde Y. A. Trophic factors and neuronal survival. Neuron. 1989 Jun;2(6):1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Clarke P. G. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl) 1990;181(3):195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7439–7443. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle J. T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993 Oct 29;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Forss-Petter S., Danielson P. E., Catsicas S., Battenberg E., Price J., Nerenberg M., Sutcliffe J. G. Transgenic mice expressing beta-galactosidase in mature neurons under neuron-specific enolase promoter control. Neuron. 1990 Aug;5(2):187–197. doi: 10.1016/0896-6273(90)90308-3. [DOI] [PubMed] [Google Scholar]
- Garcia I., Martinou I., Tsujimoto Y., Martinou J. C. Prevention of programmed cell death of sympathetic neurons by the bcl-2 proto-oncogene. Science. 1992 Oct 9;258(5080):302–304. doi: 10.1126/science.1411528. [DOI] [PubMed] [Google Scholar]
- Griffin W. S., Stanley L. C., Ling C., White L., MacLeod V., Perrot L. J., White C. L., 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Jacobson M. D., Burne J. F., King M. P., Miyashita T., Reed J. C., Raff M. C. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993 Jan 28;361(6410):365–369. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- Kane D. J., Sarafian T. A., Anton R., Hahn H., Gralla E. B., Valentine J. S., Ord T., Bredesen D. E. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993 Nov 19;262(5137):1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliatsos V. E., Clatterbuck R. E., Winslow J. W., Cayouette M. H., Price D. L. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron. 1993 Mar;10(3):359–367. doi: 10.1016/0896-6273(93)90326-m. [DOI] [PubMed] [Google Scholar]
- Komiyama M., Shibata H., Suzuki T. Somatotopic representation of facial muscles within the facial nucleus of the mouse. A study using the retrograde horseradish peroxidase and cell degeneration techniques. Brain Behav Evol. 1984;24(2-3):144–151. doi: 10.1159/000121312. [DOI] [PubMed] [Google Scholar]
- LeBrun D. P., Warnke R. A., Cleary M. L. Expression of bcl-2 in fetal tissues suggests a role in morphogenesis. Am J Pathol. 1993 Mar;142(3):743–753. [PMC free article] [PubMed] [Google Scholar]
- Lowrie M. B., Vrbová G. Dependence of postnatal motoneurones on their targets: review and hypothesis. Trends Neurosci. 1992 Mar;15(3):80–84. doi: 10.1016/0166-2236(92)90014-y. [DOI] [PubMed] [Google Scholar]
- Mah S. P., Zhong L. T., Liu Y., Roghani A., Edwards R. H., Bredesen D. E. The protooncogene bcl-2 inhibits apoptosis in PC12 cells. J Neurochem. 1993 Mar;60(3):1183–1186. doi: 10.1111/j.1471-4159.1993.tb03275.x. [DOI] [PubMed] [Google Scholar]
- Martin D. P., Schmidt R. E., DiStefano P. S., Lowry O. H., Carter J. G., Johnson E. M., Jr Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol. 1988 Mar;106(3):829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell T. J., Deane N., Platt F. M., Nunez G., Jaeger U., McKearn J. P., Korsmeyer S. J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- Miura M., Zhu H., Rotello R., Hartwieg E. A., Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993 Nov 19;75(4):653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Monaghan P., Robertson D., Amos T. A., Dyer M. J., Mason D. Y., Greaves M. F. Ultrastructural localization of bcl-2 protein. J Histochem Cytochem. 1992 Dec;40(12):1819–1825. doi: 10.1177/40.12.1453000. [DOI] [PubMed] [Google Scholar]
- Negrini M., Silini E., Kozak C., Tsujimoto Y., Croce C. M. Molecular analysis of mbcl-2: structure and expression of the murine gene homologous to the human gene involved in follicular lymphoma. Cell. 1987 May 22;49(4):455–463. doi: 10.1016/0092-8674(87)90448-x. [DOI] [PubMed] [Google Scholar]
- Nuñez G., London L., Hockenbery D., Alexander M., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990 May 1;144(9):3602–3610. [PubMed] [Google Scholar]
- Olanow C. W. A radical hypothesis for neurodegeneration. Trends Neurosci. 1993 Nov;16(11):439–444. doi: 10.1016/0166-2236(93)90070-3. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Prevette D., Tytell M., Homma S. Naturally occurring and induced neuronal death in the chick embryo in vivo requires protein and RNA synthesis: evidence for the role of cell death genes. Dev Biol. 1990 Mar;138(1):104–113. doi: 10.1016/0012-1606(90)90180-q. [DOI] [PubMed] [Google Scholar]
- Pezzella F., Tse A. G., Cordell J. L., Pulford K. A., Gatter K. C., Mason D. Y. Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol. 1990 Aug;137(2):225–232. [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Barres B. A., Burne J. F., Coles H. S., Ishizaki Y., Jacobson M. D. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993 Oct 29;262(5134):695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Sendtner M., Holtmann B., Kolbeck R., Thoenen H., Barde Y. A. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature. 1992 Dec 24;360(6406):757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- Sendtner M., Kreutzberg G. W., Thoenen H. Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature. 1990 May 31;345(6274):440–441. doi: 10.1038/345440a0. [DOI] [PubMed] [Google Scholar]
- Sentman C. L., Shutter J. R., Hockenbery D., Kanagawa O., Korsmeyer S. J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991 Nov 29;67(5):879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Sheard P., McCaig C. D., Harris A. J. Critical periods in rat motoneuron development. Dev Biol. 1984 Mar;102(1):21–31. doi: 10.1016/0012-1606(84)90171-4. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991 Nov 29;67(5):889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Croce C. M. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Finger L. R., Yunis J., Nowell P. C., Croce C. M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984 Nov 30;226(4678):1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Yan Q., Elliott J., Snider W. D. Brain-derived neurotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature. 1992 Dec 24;360(6406):753–755. doi: 10.1038/360753a0. [DOI] [PubMed] [Google Scholar]
- Yuan J., Shaham S., Ledoux S., Ellis H. M., Horvitz H. R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993 Nov 19;75(4):641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]