Abstract
Objective
Recent studies suggest that the soluble triggering receptor expressed on myeloid cells-like transcript 1 (sTLT-1) facilitate atherothrombosis. Therefore, we evaluated sTLT-1 as a functional measure of atherothrombosis in acute coronary syndrome (ACS).
Methods
Levels of sTLT-1 were determined by enzyme-linked immunosorbent assay on plasma from patients with potential ACS and compared with an age-matched control group with similar risk factors for cardiovascular disease.
Results
Of 53 patients enrolled, 19 patients were undergoing ACS (15 unstable angina, 2 non–ST-segment elevated myocardial infarction, and 2 ST-segment elevated myocardial infarction), 5 patients were found with noncardiac chest pain, and 29 were in the control group. The mean plasma sTLT-1 values in the ACS group were 4.644 ng/mL ± 1.277 standard error of the mean (SEM), in the noncardiac chest pain group were 0.708 ng/mL ± 0.427 SEM, and in the control group were 1.007 ng/mL ± 0.098 SEM.
Conclusion
A statistically significant difference exists between patients experiencing cardiogenic chest pain versus controls (P < .05), suggesting sTLT-1 as a potential tool for understanding atherothrombosis in ACS.
Keywords: acute coronary syndromes, atherosclerosis, cardiology, TREM, sTLT-1, lupus inhibitor
Introduction
Inflammation is the fundamental path in the process of atherosclerosis,1 and this nonlinear process that is negotiated by several pathways and elicited by a myriad of interactions and insults to the vascular system occurs since childhood.2 In this process, the platelets have been regarded as having the starring role in thrombus formation, and only recently they have been recognized as a modulator of inflammatory reactions and immune responses that participate in thrombosis and atherogenesis.3,4 Platelet function is determined by its different receptor moieties and the expression and secretion of the many mediators that participate in platelet activation, allowing platelet interaction with leukocytes and endothelial cells, the vascular endothelium, and other inflammatory cells. One key facilitator in platelet secretion is the α-granule, which derives its function from its content; α-granule content includes both membrane-bound proteins that become expressed on the platelet surface and soluble proteins that are released into the extracellular space.5 Among the many membrane-bound and soluble proteins that are released by the α-granules, the triggering receptor expressed on myeloid cells-like transcript 1 (TLT-1) is a type 1, single Ig domain orphan receptor stored in the platelet and megakaryocyte α-granules6,7 and the focus of our study. Upon activation, α-granules relocate to the platelet granulomere, exposing the TLT-1 receptor to the surface. The TLT-1 is also released as a soluble fragment (sTLT-1).8 This soluble fragment is found in serum but not in the plasma of healthy individuals and can enhance platelet aggregation in vitro.
Patients diagnosed with inflammatory diseases, however, have significantly elevated levels of sTLT-1 in their blood.9 Platelets influence atherogenesis by adhering to endothelial cells and depositing chemotactic mediators on the endothelial surface.10,11 Published studies propose that during inflammation, sTLT-1 may mediate hemostasis by enhancing actin polymerization, resulting in increased platelet aggregation and adherence to the endothelium.9 Therefore, sTLT-1 could in fact be a facilitator in the process of atherothrombosis, which upon its release functions as a chemotactic mediator that not only activates other platelets but also aids in the attachment of organized thrombi to the vascular endothelium.
Platelet aggregation and thrombus formation secondary to plaque disruption are key pathogenic mechanisms underlying acute coronary syndrome (ACS), which typically manifests as chest pain due to myocardial ischemia. The common denominator in the pathophysiology of ACS is the disruption of a labile atherosclerotic plaque. Atherosclerosis develops in a milieu of inflammatory cells and chemokines up until erosion and/or breakage ensues on the thinning fibrotic plaque. Rupture exposes the lipid core to the vascular lumen, and the blood coagulation factors together with tissue factor promote platelet aggregation resulting in decreased coronary perfusion and ischemia. Certainly, this occurs while platelets degranulate and release their contents including sTLT-1.
Hence, it is our intention to measure sTLT-1 levels in patients undergoing chest pain resulting from ACS to better understand the interactions between the vascular endothelium, platelets, and the formation of the coronary thrombi.
Methods
Participants
After written informed consent was obtained from all participants of the study, we analyzed sTLT-1 in the peripheral blood of 24 patients (14 men and 10 women, 54 to 89 years old with a mean age of 72 years) with potential ACS, by referral of the emergency department (ED) physician and triage nurses. For inclusion into the study, patients had to have chest pain for less than 12 hours. Clinical history and risk factors for coronary artery disease (CAD) were documented for each patient. The patients were stratified into 5 categories: unstable angina (UA) defined as angina at rest for more than 20 minutes of duration, severe new-onset chest pain, or prior angina increasing in severity; non–ST-segment elevation myocardial infarction (NSTEMI) defined as ST-segment depression or prominent T-wave inversion and/or positive biomarkers of myocardial necrosis in the absence of ST-segment elevation in patients with chest pain; ST-segment elevation myocardial infarction (STEMI) defined as ST-segment elevation more than 1 mm (0.1 mV) in 2 or more contiguous precordial leads. Those patients not meeting the above-mentioned criteria were diagnosed as having noncardiac chest pain of undetermined etiology.12,13 An age-matched group with similar risk factors for cardiovascular disease was used as a control group; samples were gathered at a wellness center for the elderly patients in a nonhospital setting.
Blood Sampling
Whole blood was obtained within 12 hours after the onset of symptoms at the moment of standard venipuncture of the peripheral vein with the initial diagnostic workup in all patients and collected in sodium citrate tubes before the performance of coronary angiography. Collected blood was transported to the hospital clinical laboratory in less than 30 minutes for plasma isolation by centrifugation at 1500g for 15 minutes. Supernatant was aliquoted and stored frozen at −20°C. Later these samples were thawed and filtered through a 0.2-μm filter for the sTLT-1 (enzyme-linked immunosorbent assay catalog number DY2394; R&D Systems, Inc Minneapolis, Minnesota). Briefly, a 96-well plate was coated with the capture antibody (goat anti-human TREML1) by overnight incubation, washed with phosphate-buffered saline (PBS), and then blocked with 1% bovine serum albumin. Plates were subsequently washed with PBS and plasma was added and then incubated in the plates for 2 hours, washed with PBS, and detection was realized with a second monoclonal antibody (biotinylated goat anti-human TREML1). A serial dilution of recombinant TLT-1 was used for standard. Then 100 μL of streptavidin-horseradish peroxidase was added to each well and incubated for 20 minutes at room temperature. After washing with PBS, 100 μL of color reagent tetramethylbenzidine was added to each well, and the mixture was left for 20 minutes at room temperature. The enzyme reaction was stopped by the addition of 50 μL of 1N H2SO4, and absorbance at 540 nm was measured in a microplate reader.
Statistical Analysis
The clinical characteristics of the study groups described in Table 1 were compared by chi-square and Fisher Exact tests. Values for sTLT-1 are presented as mean ± standard error of the mean in Table 2. The median value of plasma sTLT-1 levels were compared using Kruskal-Wallis and Mann-Whitney U tests. Differences were considered statistically significant at the .05 level. Statistical analysis was performed using SPSS Statistics 17 and 19.0.
Table 1.
Baseline Characteristics of Study Patients.
| Variables | ACS
|
Control
|
P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | ± SD | N | % | Mean | ± SD | N | % | ||
| Age | 73 | 10 | 19 | 100.0 | 73 | 7 | 29 | 100.0 | |
| Male | 10 | 52.6 | 14 | 48.3 | |||||
| Female | 9 | 47.4 | 15 | 51.7 | |||||
| BMI | 25.5 | 3.9 | 28.1 | 5.3 | |||||
| Family history of CAD | 11 | 57.9 | 6 | 20.7 | <.05 | ||||
| Prior MI | 10 | 52.6 | 4 | 13.8 | .012a | ||||
| Prior CABG | 7 | 36.8 | 4 | 13.8 | |||||
| DM | 10 | 52.6 | 10 | 34.5 | |||||
| Arterial hypertension | 16 | 84.2 | 20 | 69.0 | |||||
| Dyslipidemia | 10 | 52.6 | 14 | 48.3 | |||||
| PAD | 7 | 36.8 | 11 | 37.9 | |||||
| Atrial fibrillation | 5 | 26.3 | 1 | 3.4 | .05a | ||||
| Use ASA or clopidogrel | 10 | 36.8 | 11 | 37.9 | |||||
| Use of ARB or ACE inhibitor | 10 | 52.6 | 14 | 48.3 | |||||
| Use statins | 8 | 50 | 7 | 24.1 | |||||
Abbreviations: ACE, angiotensin-converting enzyme; ACS, acute coronary syndrome; ARB, angiotensin receptor blockers; ASA, acetylsalicylic acid; BMI, body mass index; MI, myocardial infarction; CABG, coronary artery bypass grafting; DM, diabetes mellitus; PAD, peripheral arterial disease; SD, standard deviation; N, total.
Fisher Exact test.
Table 2.
Levels of Plasma sTLT-1 and P-Selectin Among Each Group.
| Groups | sTLT-1 Levels, ng/mL
|
Pa | sP-selectin, ng/mL
|
Pa | N | ||
|---|---|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | ||||
| ACS | 4.644 | 5.57 | <.05 | 10.828 | 3.95 | <.0001 | 19 |
| Control | 1.007 | 0.53 | 15.268 | 1.71 | 29 | ||
| Total | 53 | ||||||
Abbreviations: SD, standard deviation; sTLT-1, soluble triggering receptor expressed on myeloid cells-like transcript 1; sP-selectin, soluble P-selectin.
Mann-Whitney U test (P ≤ .05).
Results
Clinical Characteristics
Of the 24 patients who arrived at the ED, 19 patients were found to have ACS (15 UA, 2 NSTEMI, and 2 STEMI) and 5 patients were found to have noncardiac chest pain. The clinical characteristics of patients (Table 1) with ACS and patients with noncardiac chest pain and the control group were similar for age and major risk factors with the exception of prior myocardial infarction (P = .012) being 52.6% in ACS, 40.0% in noncardiac chest pain, and 13.8% in the control group and atrial fibrillation (P = .050) with 26.3% in ACS and 3.4% in the control group. Of the 19 patients with ACS, a total of 11 had a past history of coronary disease. In all, 7 patients had history of coronary artery bypass surgery, 10 patients had a history of prior coronary angiography with stent placement, and 1 patient had angiography with stent placement at the time of the ED visit. Thus, 8 patients who arrived to the ED with ACS did not have their coronary anatomy defined.
Levels of Plasma sTLT-1 in ACS, Noncardiac Chest Pain, and Control Group
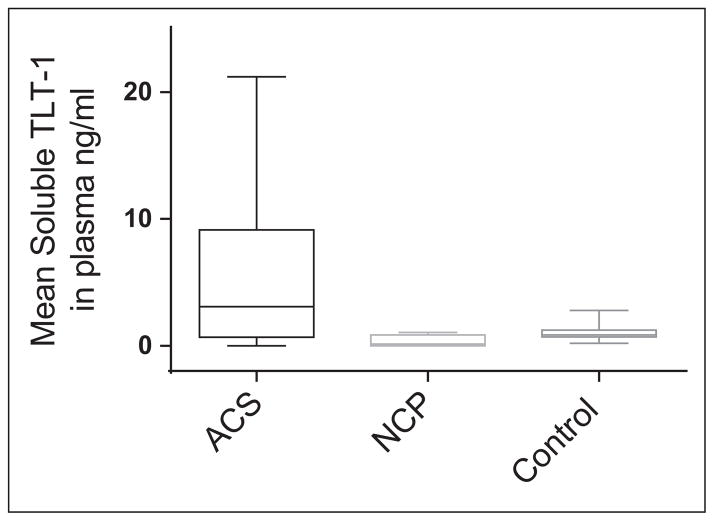
The Kruskal-Wallis test was conducted to evaluate the relationship between median levels of sTLT-1 in the 3 groups (ACS, noncardiac chest pain, and control group) and was deemed significant, χ2 (2, N = 53) = 5.99, P = .05. The proportion of variability in the ranked dependent variable was moderate (η2 = 0.12). A series of Mann-Whitney U tests were conducted to evaluate pairwise differences among the 3 groups. The results of these tests indicated a significant difference between the ACS and the control groups, z = −1.99, P = .046. Significant differences were not found between the other 2 comparisons (ACS vs noncardiac and noncardiac vs control). When comparing mean plasma sTLT-1 levels among the 3 groups (Figure 1), a significant difference between the ACS and the control groups is seen, while in the control and noncardiac chest pain group significant differences are not observed. Troponin levels were measured in all 24 patients who arrived to the ED with chest pain. Elevated levels of troponin were documented in 3 patients in the ACS group (2 with NSTEMI and 1 with STEMI). No elevation in troponin was seen in the noncardiac chest pain group or in the UA group. Of the 3 patients, 2 with high troponin levels also had a high sTLT-1 level. In contrast, 9 of 15 patients with unstable angina had high sTLT-1 level.
Figure 1.
Mean plasma soluble triggering receptor expressed on myeloid cells-like transcript 1 (sTLT-1) levels in the study group. Whiskers represent the highest and lowest levels per group.
Discussion
In the present study, plasma sTLT-1 levels of patients in the ACS group were significantly elevated compared to those in the control group. The levels of sTLT-1 among the control group and noncardiac chest pain group were comparable. In contrast, there was no statistically significant difference between patients undergoing ACS and patients experiencing noncardiac chest pain as evaluated by the Mann-Whitney U test. This finding is most likely due to the limited number of cases in group sample which resulted in low statistical power. Comparison of the mean values of sTLT-1 of the 3 groups shows a difference between the ACS group and both the noncardiac chest pain and the control groups. The significant standard error in the ACS group points to a variability in the levels of sTLT-1, which makes us consider what factors affect the levels of sTLT-1 in each individual and how medications, risk factors for cardiovascular disease, or other environmental issues could affect sTLT-1 levels. Nevertheless, these findings highlight that the pathophysiology of ACS is related to platelet degranulation. As measured, sTLT-1 is a product of platelet α-granules and levels appear to be increased in patients in the ACS group when compared to the control group.
Products of platelet degranulation such as soluble P-selectin (sP-selectin) and its secretome products like the soluble CD40 ligand (sCD40L) have been measured before in the setting of ACS. The sCD40L has been regarded as an inflammatory mediator between neutrophils and platelets,14 displaying outcome-dependent inflammatory patterns after NSTEMI and STEMI,15,16 triggering ACS,17,18 an independent risk factor for major adverse cardiovascular events19 and a marker of coronary artery disease activity.19 sP-selectin also studied in the setting of ACS has been identified as having a role in the pathogenesis of acute coronary events,20 a marker of impending coronary artery insult21 and plaque destabilization.22
These soluble proteins, as in the case of sTLT-1, have been related to deranged inflammatory events such as sepsis.23,24 Ongoing mouse studies in the laboratory demonstrate that TLT-1 plays a role in atherosclerotic lesion development. The laboratory studies were initiated based on the apolipoprotein E (apoeE)-null mouse having exceedingly high levels of sTLT-1. The apoE-null mouse has naturally high levels of cholesterol and develops spontaneous lesions in the aortic sinus. Consistent with these findings, individuals with cardiogenic events have significantly higher levels of sTLT-1 than controls. Knowing that these soluble proteins are a product of platelet activation, it is reasonable to consider the implications of another soluble protein acting as a mediator between platelets, leukocytes, and endothelial cells or its potential to serve as a marker of disease activity in the setting of ACS. Therefore, this study indirectly places sTLT-1 in parallel with other protein products of platelet degranulation and gives it implicit participation in the pathophysiology of atherothrombosis. However, the mechanism by which this soluble protein participates in the pathophysiology of atherothrombosis is still to be elucidated.
Recent studies from our group demonstrated that sTLT-1 mediates actin polymerization through an Rac1/p38 signaling pathway. Although it is not clear how sTLT-1 mediates this pathway, it suggests a means of how sTLT-1 participates in the pathophysiology of plaque generation and/or atherothrombosis.
Study limitations
Study sample was limited by the number of cases and gathered by convenience sampling Therefore, no generalizations were possible from this study group. The group classified as noncardiac chest pain was not evaluated to reach the noncardiac cause of chest pain, leaving room for imprecise assumptions when categorizing patients in this group.
Future Considerations
Being aware of the fact that atherosclerosis is a chronic inflammatory condition challenges us into searching for mediators that participate in such processes. The platelet has many mediators that modulate the inflammatory response in sepsis, in autoimmune diseases, and in atherosclerosis. These mediators of the inflammatory response not only participate in the pathologic process but also serve as markers for disease activity and at times as therapeutic targets to hold or ameliorate a deranged inflammatory response. In identifying the key participants in these processes and understanding their interaction with each other, we may be able to develop new therapeutic options and better management in chronic disease.
Acknowledgments
We would like to thank B. Zimmerman for her helpful comments in reviewing the manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Support for these projects was obtained through grants G12RR-03035 and 8U54M D007587-03 from the National Institute of Minority Health and Health Disparities, a component of the National Institute of Health and (1R01HL090933) from the National Heart Lung and Blood Institute.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Lusis AJ. Atherosclerosis. Nature. 2000;407(6801):233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGill HC, Jr, McMahan CA, Herderick EE, et al. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr. 2000;72(5 suppl):1307S–1315S. doi: 10.1093/ajcn/72.5.1307s. [DOI] [PubMed] [Google Scholar]
- 3.von HP, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res. 2007;100(1):27–40. doi: 10.1161/01.RES.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- 4.van Gils JM, Zwaginga JJ, Hordijk PL. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol. 2009;85(2):195–204. doi: 10.1189/jlb.0708400. [DOI] [PubMed] [Google Scholar]
- 5.Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23(4):177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Washington AV, Gibot S, Acevedo I, et al. TREM-like transcript-1 protects against inflammation-associated hemorrhage by facilitating platelet aggregation in mice and humans. J Clin Invest. 2009;119(6):1489–1501. doi: 10.1172/JCI36175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Washington AV, Schubert RL, Quigley L, et al. A TREM family member, TLT-1, is found exclusively in the alpha-granules of megakaryocytes and platelets. Blood. 2004;104(4):1042–1047. doi: 10.1182/blood-2004-01-0315. [DOI] [PubMed] [Google Scholar]
- 8.Gattis JL, Washington AV, Chisholm MM, et al. The structure of the extracellular domain of triggering receptor expressed on myeloid cells like transcript-1 and evidence for a naturally occurring soluble fragment. J Biol Chem. 2006;281(19):13396–13403. doi: 10.1074/jbc.M600489200. [DOI] [PubMed] [Google Scholar]
- 9.Morales J, Villa K, Gattis J, et al. Soluble TLT-1 modulates platelet-endothelial cell interactions and actin polymerization. Blood Coagul Fibrinolysis. 2010;21(3):229–236. doi: 10.1097/MBC.0b013e3283358116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huo Y, Schober A, Forlow SB, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9(1):61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 11.Schober A, Manka D, von HP, et al. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation. 2002;106(12):1523–1529. doi: 10.1161/01.cir.0000028590.02477.6f. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American college of cardiology/American heart association task force on practice guidelines (writing committee to revise the 2002 guidelines for the management of patients with unstable angina/Non-ST-Elevation myocardial infarction) developed in collaboration with the American college of emergency physicians, the society for cardiovascular angiography and interventions, and the society of thoracic surgeons endorsed by the American association of cardiovascular and pulmonary rehabilitation and the society for academic emergency medicine. J Am Coll Cardiol. 2007;50(7):e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Part 8: Stabilization of the Patient With Acute Coronary Syndromes. Circulation. 2005;112:IV–89. [Google Scholar]
- 14.Setianto BY, Hartopo AB, Gharini PP, Anggrahini DW, Irawan B. Circulating soluble CD40 ligand mediates the interaction between neutrophils and platelets in acute coronary syndrome. Heart Vessels. 2010;25(4):282–287. doi: 10.1007/s00380-009-1199-1. [DOI] [PubMed] [Google Scholar]
- 15.Di Stefano R, Di Bello V, Barsotti MC, et al. Inflammatory markers and cardiac function in acute coronary syndrome: difference in ST-segment elevation myocardial infarction (STEMI) and in non-STEMI models. Biomed Pharmacother. 2009;63(10):773–780. doi: 10.1016/j.biopha.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y, Lee WH, Lee SC, et al. CD40 L activation in circulating platelets in patients with acute coronary syndrome. Cardiology. 1999;92(1):11–16. doi: 10.1159/000006940. [DOI] [PubMed] [Google Scholar]
- 17.Antoniades C, Bakogiannis C, Tousoulis D, Antonopoulos AS, Stefanadis C. The CD40/CD40 ligand system: linking inflammation with atherothrombosis. J Am Coll Cardiol. 2009;54(8):669–677. doi: 10.1016/j.jacc.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 18.Fouad HH, Al-Dera H, Bakhoum SW, et al. Levels of sCD40 ligand in chronic and acute coronary syndromes and its relation to angiographic extent of coronary arterial narrowing. Angiology. 2010;61(6):567–573. doi: 10.1177/0003319709356785. [DOI] [PubMed] [Google Scholar]
- 19.Yan JC, Zhu J, Gao L, et al. The effect of elevated serum soluble CD40 ligand on the prognostic value in patients with acute coronary syndromes. Clin Chim Acta. 2004;343(1–2):155–159. doi: 10.1016/j.cccn.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Xu DY, Zhao SP, Peng WP. Elevated plasma levels of soluble P-selectin in patients with acute myocardial infarction and unstable angina. An inverse link to lipoprotein(a) Int J Cardiol. 1998;64(3):253–258. doi: 10.1016/s0167-5273(98)00075-8. [DOI] [PubMed] [Google Scholar]
- 21.Aref S, Sakrana M, Hafez AA, Hamdy M. Soluble P-selectin levels in diabetes mellitus patients with coronary artery disease. Hematology. 2005;10(3):183–187. doi: 10.1080/10245330500072405. [DOI] [PubMed] [Google Scholar]
- 22.Draz N, Hamdy MS, Gomaa Y, Ramzy AA. Soluble P-selectin is a marker of plaque destabilization in unstable angina. Egypt J Immunol. 2003;10(1):83–87. [PubMed] [Google Scholar]
- 23.Chew M, Rahman M, Ihrman L, et al. Soluble CD40 L (CD154) is increased in patients with shock. Inflamm Res. 2010;59(11):979–982. doi: 10.1007/s00011-010-0213-5. [DOI] [PubMed] [Google Scholar]
- 24.Mosad E, Elsayh KI, Eltayeb AA. Tissue factor pathway inhibitor and P-selectin as markers of sepsis-induced non-overt disseminated intravascular coagulopathy. Clin Appl Thromb Hemost. 2011;17(1):80–87. doi: 10.1177/1076029609344981. [DOI] [PubMed] [Google Scholar]