Abstract
Objective
Suicide, a major cause of death worldwide, has distinct biological underpinnings. The authors review and synthesize the research literature on biomarkers of suicide, with the aim of using the findings of these studies to develop a coherent model for the biological diathesis for suicide.
Method
The authors examined studies covering a large range of neurobiological systems implicated in suicide. They provide succinct descriptions of each system to provide a context for interpreting the meaning of findings in suicide.
Results
Several lines of evidence implicate dysregulation in stress response systems, especially the hypothalamic-pituitary-adrenal axis, as a diathesis for suicide. Additional findings related to neuroinflammatory indices, glutamatergic function, and neuronal plasticity at the cellular and circuitry level may reflect downstream effects of such dysregulation. Whether serotonergic abnormalities observed in individuals who have died by suicide are independent of stress response abnormalities is an unresolved question.
Conclusions
The most compelling biomarkers for suicide are linked to altered stress responses and their downstream effects, and to abnormalities in the serotonergic system. Studying these systems in parallel and in the same populations may elucidate the role of each and their interplay, possibly leading to identification of new treatment targets and biological predictors.
In 2011, suicide claimed nearly 40,000 lives in the United States (1); worldwide, the number approached 1 million (2). In our model (3, 4), suicide is triggered by a life event or a psychiatric episode in an individual with a predisposing diathesis. The diathesis begins with the individual’s genetic risk and develops further as the accumulation of traumatic events, mental and physical illnesses, and losses leads to neurobiological changes in the organism. We and others have proposed that a key component of the underlying diathesis is an altered stress response. In this review, we assess the evidence for this neurobiological mechanism and other related systems, as well as serotonergic function. These systems typically have been studied using biomarkers, as defined by the National Institutes of Health (5): “a characteristic that is objectively measured and evaluated as an indicator of … pathogenic processes.” We focus here on studies that distinguish biomarkers of suicide from those associated with co-occurring psychiatric disorders.
Suicidal behavior varies in degree of lethality and suicidal intent, and biomarkers will likely depend on these variables to some extent. To reduce this variance, we focus on the most serious manifestation of suicidal behavior, suicide itself. Because understanding the physiology of systems within which putative biomarkers fall is essential, we briefly review pertinent systems.
Stress Response and Its Modulation
Hypothalamic-Pituitary-Adrenal Axis and Locus Ceruleus-Norepinephrine System
Stress elicits a complex array of physiological and behavioral responses, which optimally are rapidly discontinued once the stressor is gone or the organism adapts to it (6). The main components of the stress response system are the corticotropin-releasing hormone/hypothalamic-pituitary-adrenal axis (CRH-HPA) system and the locus ceruleus-based norepinephrine (LC-NE) system (7).
Stress activates the CRH-HPA system via release of corticotropin-releasing hormone (CRH) and vasopressin from the hypothalamus into the portal circulation, which in turn activates CRH receptor 1 (CRHR1) and vasopressin receptors in the pituitary, leading to pituitary release of pro-opiomelanocortin (POMC), the precursor of β-endorphin and adrenocorticotropic hormone (ACTH). Once in the main circulation, ACTH triggers cortisol release from the adrenal cortex, which mobilizes energy, potentiates some catecholamine actions, dampens inflammatory responses, and activates glucocorticoid receptors (GRs). GRs are ubiquitous lower-affinity receptors, binding cortisol when levels are high. GR stimulation increases glucose levels and lipolysis, mobilizing energy resources, and enhances memory storage and consolidation, preparing the organism for similar events in the future (6). Mineralocorticoid receptors (MRs) are higher affinity (~10 times the affinity of GRs) and are occupied under basal conditions, thereby maintaining HPA axis tone. They are present in the hippocampus, the amygdala, the septum, and some cortical areas. When bound to corticosteroids, MRs and GRs regulate gene transcription (see Table 1 for a summary of gene expression findings in suicide), mediating later effects of stressors. They affect gene expression of G-protein coupled receptors, ion channels, and membrane proteins relevant to conductance and hence to cell activation. Downstream, they influence cell structure, metabolism, and synaptic transmission and may be central to apoptosis and neurogenesis in the hippocampus and other limbic and prefrontal areas (6), generating effects at both the neuronal and the neurocircuitry levels (8). Negative feedback commences when corticosteroids bind to MRs and GRs. GRs are also inhibited by the cochaperone, immunophilin FK506 binding protein 5 (FKBP5).
TABLE 1.
Gene Expression Findings in Suicidea
| System | Paper | mRNA | Brain Regions | Findings |
|---|---|---|---|---|
| CRH-HPA | Pandey et al. (15) | GR-α, GR-β, MR | Amygdala, PFC, hippocampus |
In teenage suicide victims compared with deceased healthy controls: 1) GR-α mRNA levels reduced in amygdala and PFC, but not hippocampus, 2) no difference in PFC GR-β mRNA expression, 3) no difference in MR mRNA expression in all three brain regions |
| López et al. (16) | GR, MR | Hippocampus | No difference in GR or MR mRNA levels in depressed suicide victims compared with deceased controls |
|
| Labonte et al. (19) | GR, GR exon splice variants (1B, 1C, 1H) |
Hippocampus, ACC | Hippocampus: decreased GR, GR1B, GR1C, and GR1H mRNA expression in suicide victims with abuse history compared with suicide victims without abuse history and controls; no difference between suicide victims without abuse and controls in GR1B, GR1C, and GR1H levels; ACC: no difference between groups in GR and variant expression |
|
| López et al. (14) | POMC, GR | Pituitary | Increased POMC mRNA density in corticotropic cells of suicide victims compared with controls; no difference in GR mRNA expression |
|
| Sequeira et al. (112) | MT | ACC, nucleus accumbens | Decreased mRNA expression of two MT families, MT-1 and MT-2, in both ACC and nucleus accumbens of depressed suicide victims compared with deceased controls; no difference in MT-3 mRNA expression |
|
| Merali et al. (13) | CRH1, CRH2 | FPC, DMPFC, VLPFC | In FPC, CRH1 mRNA expression lowered in suicide victims compared with controls, no difference in CRH2 mRNA levels; no differences in either CRH1 or CRH2 mRNA expression in DMPFC or VLPFC |
|
| NE-LC | Du et al. (51) | COMT | FPC, OFC | COMT mRNA expression elevated in depressed suicide victims compared with deceased controls in both the FPC and OFC; also, Met carriers who died of suicide showed elevated COMT mRNA levels in FPC compared with control Met carriers |
| Escribá et al. (76) | α2A-adrenoceptor | PFC | Increased mRNA expression levels found in suicide victims compared with controls |
|
| Cytokines | Tonelli et al. (61) | TNF-α, IL-1β, IL-4, IL-5, IL-6, IL-13 |
BA 11 | IL-4 mRNA expression was increased in female suicide victims compared with controls, whereas IL-13 mRNA expression was elevated in male suicide victims; no difference in the remaining factors |
| Endogenous opioids |
Escribá et al. (76) | μ-opioid receptor | PFC | Increased mRNA expression found in suicide victims compared with controls |
| Hurd et al. (77) | Prodynorphin | Caudate nucleus | Increased mRNA expression levels in suicide victims compared with patients with schizophrenia and controls |
|
| Serotonin | Bach-Mizrachi et al. (85) |
TPH2 | DRN, MRN | Higher mRNA expression in DRN (along entire rostrocaudal axis) and MRN of suicide victims compared with deceased nonpsychiatric controls |
| Bach-Mizrachi et al. (88) |
TPH2 | DRN, MRN | Mean TPH2 mRNA expression levels per neuron in DRN and MRN greater in depressed suicide victims than deceased healthy controls; in DRN, expression levels different in caudal but not rostral regions |
|
| De Luca et al. (87) | TPH2 | DLPFC (BA 46) | No difference in mRNA expression between suicide victims and controls |
|
| Perroud et al. (84) | TPH1, TPH2 | VPFC | Increased TPH2 mRNA expression in suicide victims compared with controls; no difference in TPH1 expression |
|
| López et al. (16) | 5-HTR1A | Hippocampus | Decreased mRNA expression in depressed suicide victims compared with deceased controls |
|
| Matthews and Harrison (89) |
5-HTR1A | DRN | No difference in mRNA expression between suicide victims, three non-suicide groups (schizophrenia, bipolar disorder, depression) and controls |
|
| Escribá et al. (76) | 5-HTR1A, 5-HTR2A | PFC | Increased mRNA expression of both 5-HTR1Aand 5- HTR2A in suicide victims compared with controls |
|
| Sequeira et al. (112) | 5-HTR2A, PER1, ADCY1 | DLPFC | Decreased mRNA expression of 5-HTR2A, ADCY1 (adenylate cyclase gene) and PER1 (period homologue) in suicide victims compared with deceased controls |
|
| Pandey et al. (113) | 5-HTR2C | Multiple brain regions | No difference in mRNA expression levels of 5-HTR2C between suicide victims and controls in any studied brain regions (PFC, hippocampus, choroid plexus, hypothalamus, nucelus accumbens, cerebellum) |
|
| GABA | Merali et al. (13) | GABAA | FPC, DMPFC, VLPFC | In FPC, mRNA levels of GABAA α1, α3, α4, and δ subunits, but not α2, α5, or λ, were lower in suicide victims compared with deceased controls; no difference in GABAA expression between groups in DMPFC or VLPFC |
| Poulter et al. (140) | GABAA | OFC, LC, PVN, hippocampus, amygdala |
Pattern of GABAA subunit expression differed in suicide victims compared with controls; lowered subunit coordination in hippocampus and amygdala, increased coordination in OFC and PVN, unchanged in LC |
|
| Sequeira et al. (139) | Multiple GABA-ergic genes |
17 brain regions | Up-regulation in mulitple GABA-ergic genes in depressed suicide victims, but no differences between nondepressed suicide victims and controls observed |
|
| Klempan et al. (143) | Multiple GABA-ergic genes |
VPFC (BA 44, 45, 46, 47), DPFC |
Multiple GABA-ergic genes (GABAA α5, β1, δ, γ1, and γ2, GABBR2, SLC6A1) differentially expressed in BA 46 of suicide victims compared with controls |
|
| Choudary et al. (150) | Multiple GABA-ergic genes |
ACC | GABAA α1 and GABAA β3 mRNA expression up- regulated in suicide victims with mood disorders compared with controls |
|
| Glutamate | Sequeira et al. (139) | Multiple glutamatergic genes |
Multiple brain regions | GLUL down-regulated in PFC, amygdala of depressed suicide victims; AMPA up-regulated in amygdala, hippocampus, nucleus accumbens, and few cortical regions in depressed suicide victims; GRM3 down-regulated in PFC, parietal cortex of suicide victims (with and without depression) compared with controls |
| Klempan et al. (143) | Multiple glutamatergic genes |
VPFC (BA 44, 45, 46, 47), DPFC |
Multiple glutamatergic genes (GRIA3, GRIN2A, SLC1A2) showed increased expression in BA 46 of suicide victims compared with controls |
|
| Neuronal plasticity |
Pandey et al. (162) | BDNF, TrkB | PFC (BA 9), hippocampus | BDNF and TrkB mRNA levels lower in PFC and hippocampus of teenage suicide victims compared with deceased healthy controls |
| Dwivedi et al. (164) | BDNF, TrkB | PFC (BA 9), hippocampus | BDNF and TrkB mRNA expression lower in PFC and hippocampus of suicide victims compared with deceased healthy controls |
|
| Keller et al. (170) | BDNF promoter/exon IV | Wernicke area | Suicide victims with high BDNF promoter/exon IV methylation patterns showed decreased BDNF mRNA expression compared with intermediate and low methylation pattern groups (including suicide victims and controls) |
|
| Keller et al. (169) | TrkB, TrkB-T1 | Wernicke area | No differences in mRNA expression levels of either TrkB or TrkB-T1 in suicide victims compared with deceased controls |
|
| Dwivedi et al. (166) | TrkA, TrkC, p75(NTR) | PFC, hippocampus | TrkA mRNA expression decreased, whereas p75 (NTR) expression increased, in both PFC and hippocampus of suicide victims compared with deceased nonpsychiatric controls; TrkC expression decreased in hippocampus |
|
| Dwivedi et al. (167) | NGF, NT-3, NT-4/5, NSE | PFC, hippocampus | NGF, NT-3, NT-4/5, and NSE mRNA expression levels lower in hippocampus of suicide victims compared with deceased controls; in PFC, only NT-4/5 mRNA expression lowered in suicide victims |
|
| Methylation | Labonte et al. (19) | GR, GR exon splice variants (1B, 1C, 1H) |
Hippocampus | GR1B: hypermethylation at CpG6 and CpG8, hypomethylation at CpG11 in suicide victims compared with controls; GR1C: hypermethylation at CpG9 and CpG12 (suicide victims), CpG8 (abused suicide victims), hypomethylation at CpG13 (abused suicide victims); GR1H: hypomethylation in abused suicide victims (CpG2, CpG5, CpG10) compared with suicide victims without abuse (CpG3, CpG7, CpG8, CpG12) and controls (CpG1) |
| Poulter et al. (142) | GABAA α1, DNMT | Multiple brain regions | DNMT-3B up-regulation associated with hypermethylation at three sites on GABAA α1 gene in FPC of depressed suicide victims compared with deceased controls |
|
| Keller et al. (169) | TrkB, TrkB-T1, BDNF | Wernicke area | No differences in methylation patterns at any TrkB or TrkB-T1 CpG sites examined in suicide victims compared with deceased controls; hypermethylation of BDNF promoter IV present in suicide |
|
| Keller et al. (170) | BDNF promoter/exon IV | Wernicke area | Significant hypermethylation at two CpG sites of BDNF promoter/exon IV in suicide victims compared with deceased controls |
ACC=anterior cingulate cortex; BA=Brodmann’s area; CRH-HPA=corticotropin-releasing hormone/hypothalamic-pituitary-adrenal axis system; DLPFC=dorsolateral prefrontal cortex; DMPFC=dorsomedial prefrontal cortex; DPFC=dorsal prefrontal cortex; DRN=dorsal raphe nucleus; DVC=dorsal vagal complex; FPC=frontopolar cortex; GR=glucocorticoid receptor; LC=locus ceruleus; LC-NE=locus ceruleus-based norepinephrine system; MR=mineralocorticoid receptor; MRN = median raphe nucleus; MT=metallothionein; OFC=orbitofrontal cortex; PFC=prefrontal cortex; PVN = paraventricular nucleus; VLPFC=ventrolateral prefrontal cortex; VPFC=ventral prefrontal cortex.
In parallel, activation of the LC-NE system leads to norepinephrine release from a dense network of neurons originating in the locus ceruleus and projecting widely to the forebrain, leading to enhanced arousal, vigilance, and anxiety. Several G-protein coupled subtypes of norepinephrine receptors are distributed throughout the neocortex, thalamus, hypothalamus, hippocampus, amygdala, and basal ganglia. Norepinephrine α and β receptors each have several subtypes. Norepinephrine α1 receptors are postsynaptic, found mainly in the thalamus, hippocampus, basal ganglia, and cortical and cerebellar cortices. Norepinephrine α2 receptors are mainly in the locus ceruleus and the cerebral cortex and are either postsynaptic or auto-receptors regulating norepinephrine release. Preclinical models suggest that further subcategories, norepinephrine α2a and α2c, are “stress promoting” and “stress protective,” respectively. Moreover, norepinephrine α1 and α2 have inhibitory and excitatory effects, respectively, on serotonergic function. β receptors found in the basal ganglia, subthalamic nuclei, and deep nuclei of the cerebellum are essential for memory consolidation. Norepinephrine neurotransmission is terminated by reuptake by norepinephrine transporters or catabolism by monoamine oxidase (MAO) or catechol O-methyltransferase (COMT).
Another, slower stress response system is regulated by urocortins II and III, acting mostly through CRH receptor 2 (CRHR2) and with anxiolytic properties, in contrast to the anxiogenic properties of norepinephrine. CRHR2 activation appears to dampen the stress response associated with CRHR1 activation. Of note, brain distributions of CRHR1 and CRHR2 overlap (6).
HPA axis in suicide
Studies comparing depressed individuals who have died by suicide to nonpsychiatric comparison subjects report elevated CRH (9-11) and vasopressin (11) levels in the forebrain, raphe, and locus ceruleus (11); fewer CRHR1s (but not CRHR2s) in the frontal cortex (12), presumably down-regulated in response to elevated CRH (13); increased POMC in the pituitary (14); decreased GR expression (15) in the amygdala and prefrontal cortex, but not the hippocampus; and decreased hippocampal MR mRNA (16), a pattern similar to that observed in rodent models of chronic stress (17, 18). Of interest, one study noted decreased hippocampal GR expression only among suicide victims with childhood abuse (19). Basal cortisol levels are decreased in depressed suicide victims compared with depressed patients (20), implying that although data suggest HPA axis hyperreactivity to stimuli, basal HPA axis tone, generally modulated by MRs, may be lower. Studies showing adrenal cortex hypertrophy in violent suicides are also supportive (21-23) of an overactive HPA axis, although not all studies agree (24). Additionally, in a Japanese population, haplotype analysis of the FKBP5 gene (see Table 2 for a summary of genetic findings in suicide), which encodes a cochaperone that binds to cytosolic GR and inhibits its sensitivity, shows an association with suicide (25). The lack of psychiatric controls in these studies hampers interpretation.
TABLE 2. Genetic Findings in Suicide.
| Systema | Genes | Paper | Sample | Findings |
|---|---|---|---|---|
| CRH-HPA | FKBP5 | Supriyanto et al. (25) | Japanese | Higher frequency of TC haplotype associated with higher transcription of FKBP5 in suicide victims compared with live nonpsychiatric controls |
| NE-LC | ADRA2 | Fukutake et al. (37) | Japanese | C-allele of C-1291G SNP (unknown function) more prevalent in female suicide victims than in live female nonpsychiatric controls |
| MAO-A | Du et al. (46) | Hungarian | High activity-related allele of EcoRV polymorphism differed in depressed male but not female suicide victims compared with deceased nonpsychiatric controls |
|
| COMT | Ono et al. (48) | Japanese | High activity Val/Val genotype of 158Val/Met polymorphism found less prevalent and Val/Met genotype more prevalent in male but not female suicide victims compared with living nonpsychiatric controls |
|
| COMT | Du et al. (51) | Hungarian | No difference in allele frequency or genotypic distribution in depressed suicide victims compared with deceased nonpsychiatric controls |
|
| COMT | Pivac et al. (49) | Slovenian | Val/Val and Val/Met genotypes of 158Val/Met polymorphism more prevalent in male but not female suicide victims, as compared with deceased controls |
|
| Endogenous opioid |
OPRM1 | Hishimoto et al. (71) | Japanese | The A/A genotype of the A118G SNP, linked to increased binding was more frequent in suicide victims than in live healthy controls |
| Serotonin | TPH2 | Zill et al. (91) | German | SNP rs1386494 (located between exon 5 and exon 7) associated with suicide in a sample of suicide victims compared with live healthy controls |
| TPH2 | Lopez de Lara et al. (92) |
French-Canadian | T allele of SNP rs4448731 and G allele of rs6582071 in upstream region, G allele of rs4641527 in intron 1, and C allele of rs1386497 in intron 8 more prevalent in depressed suicide victims compared with live depressed patients |
|
| TPH2 | Stefulj et al. (93) | Croatian | No difference in genotype or allele frequency of G-703T SNP in sample of suicide victims compared with controls |
|
| TPH2 | Mouri et al. (94) | Japanese | No difference in genotype or allele frequency of 15 tagging SNPs in suicide victims compared with live healthy controls |
|
| TPH1, TPH2, SLC6A4 | Buttenschøn et al. (102) |
Danish | No significant association of either TPH1, TPH2, or 5-HTTLPR with suicide in a sample of suicide victims compared with living controls |
|
| TPH, 5-HTT | Jernej et al. (103) | Croatian | Co-occurrence of 5-HTT intron 2 VNTR 10 allele and CC variant of TPH intron 7 A218C SNP more frequent in suicide victims than in live healthy controls |
|
| 5-HTT | Bondy et al. (171) | German | S allele and homozygous SS genotype of 5-HTTLPR more prevalent in suicide victims than in healthy living controls |
|
| 5-HTT | Lopez de Lara et al. (101) |
French-Canadian | VNTR STin2 allele 10 more frequent in depressed suicide victims compared with depressed controls; no significant genotypic or allelic differences noted at 5-HTTLPR locus |
|
| 5-HTT | Hranilovic et al. (105) | Croatian | No difference in allele and genotype frequency in either 5-HTTLPR or VNTR intron 2 polymorphisms in suicide victims compared with live healthy controls |
|
| 5-HTR1A | Videtic et al. (172) | Slovenian | No difference in C-1019G allele or genotype distributions between suicide victims and deceased controls |
|
| 5-HTR1B | Zouk et al. (114) | French-Canadian | Higher frequency of T allele at A-161T locus in suicide victims compared with live nonpsychiatric controls; no differences atT-261G, C129T, G861C, or A1180G loci |
|
| 5-HTR1B, 5-HTR1Dα, 5-HTR1E, 5-HTR1F, 5-HTR2C, 5-HTR5A, 5-HTR6 |
Turecki et al. (117) | French-Canadian | No difference in allele or genotype frequencies in any loci examined (G861C of 5-HTR1B, T1350C of 5-HTR1Dα, C117T of 5-HTR1E, C-78T and C528T of 5-HTR1F, G-995A of 5-HTR2C, G-19C of 5-HTR5A, and C267T of 5-HTR6) in suicide victims compared with live healthy controls |
|
| 5-HTR2A | Ono et al. (119) | Japanese | No association between 5-HTR2A A-1438G SNP and suicide |
|
| 5-HTR2A | Turecki et al. (111) | French-Canadian | No difference in T102C or A-1438G SNP allele or genotype distribution in suicide victims compared with deceased controls |
|
| 5-HTR2C | Videtic et al. (116) | Slovenian | Increased frequency of G68C G-allele and GG genotype in suicide victims compared with controls; relationship also significant in females but not males; no association between G-995A and suicide |
|
| 5-HTR6 | Azenha et al. (115) | Portuguese | Difference in C267T genotype distribution in male suicide victims compared with deceased nonpsychiatric controls; no association present when females included |
|
| Neuronal plasticity |
BDNF | Pregelj et al. (168) | Slovenian | No difference in Val66Met variant distribution in suicide victims compared with deceased controls; in females, more likely to have Met alleles associated with low activity |
| DISC1 | Ratta-apha et al. (173) |
Japanese | Allele frequency and genotype distribution of Ser704Cys variant differ in suicide victims and live healthy controls |
CRH-HPA=corticotropin-releasing hormone/hypothalamic-pituitary-adrenal axis system; LC-NE=locus ceruleus-based norepinephrine system.
Most data implicating the HPA axis come from studies examining the negative feedback system as tested by the dexamethasone suppression test (DST). In one study (26), depressed DST nonsuppressors were more likely to die by suicide and have higher-lethality attempts than DST suppressors, although a similar study did not confirm this (20). In depressed inpatients, although DST did not distinguish those at risk for suicide, suicide attempters with DST non-suppression were more likely to die by suicide (27). Coryell and Schlesser (28), who followed a depressed cohort for 15 years, found that DST nonsuppression at baseline was associated with a 14-fold higher odds of eventual suicide. This finding was expanded by a meta-analysis of prospective studies of suicide, which generated a prediction model in which DST nonsuppression had a specificity of 55%, rising to 88% when a low CSF level of 5-hydroxyindoleacetic acid (5-HIAA), the main metabolite of serotonin, was included in the model; this, however, came at the expense of sensitivity, which declined to 38% (29).
Together, these data implicate abnormalities in HPA axis function at several levels. Perhaps CRHR1 is down-regulated to compensate for higher CRH levels, but not enough to dampen the CRH-ACTH-cortisol pathway. Higher cortisol levels may also lead to low basal tone and DST nonsuppression, reflecting altered sensitivity of MRs and GRs, respectively, and potentially decreased GR sensitivity.
Noradrenergic-locus ceruleus system in suicide
Postmortem studies suggest alterations at several levels of the noradrenergic system in suicide, although the data are inconsistent. Higher locus ceruleus levels of tyrosine hydroxylase, the rate-limiting enzyme in norepinephrine synthesis, may reflect increased norepinephrine activity and/or effects of chronic stress. However, both higher (30) and lower (31) tyrosine hydroxylase immunocytochemical staining density have been reported in the locus ceruleus of suicide victims compared with matched comparison subjects.
Findings regarding norepinephrine a1 receptor binding abnormalities in suicide are inconsistent as well. Lower norepinephrine α1 binding in the prefrontal cortex, temporal cortex, and head of caudate (32) has been reported in suicide compared with sudden death. However, Arango et al. (33) reported higher norepinephrine a1 binding in the prefrontal cortex in suicide victims and higher norepinephrine levels. Of note, decreased norepinephrine a1 binding in the lateral prefrontal cortex has been reported in alcohol-dependent suicide victims (34). The discrepancies in the aforementioned studies do not appear to be simply due to the use of different ligands.
Presynaptic norepinephrine α2 binding is reportedly higher (35) or similar (36) in hippocampal and frontal regions in suicide victims relative to comparison subjects. Of note, a polymorphism in the α2 norepinephrine receptor gene promoter region of unknown functional significance was more common in Japanese female but not male suicide victims relative to live healthy comparison subjects (37). Thus, whether norepinephrine α2 receptor binding is altered in suicide remains unknown.
Some studies have reported higher norepinephrine β receptors in the frontal (38-40) and temporal (39) cortex of suicide victims, although subjects who died by suicide and comparison subjects were not matched by diagnosis. However, when a suicide group with diverse diagnoses was compared with a non-suicide group matched partly based on diagnosis, decreased norepinephrine β receptor binding was observed in the suicide group (41). Additionally, fewer norepinephrine β receptor binding sites have been reported in the temporal cortex (Brodmann’s area [BA] 38) of depressed suicide victims with no antidepressant treatment in the previous 3 months (42) and in depressed suicide victims with antidepressant treatment (43), compared with nonpsychiatric comparison subjects. Moreover, suicide victims who used violent methods had fewer norepinephrine β receptors compared with those using nonviolent methods (42). Antidepressant-treated depressed suicide victims also had lower norepinephrine β receptor binding in the thalamus compared with nonpsychiatric comparison subjects (43), consistent with animal studies in which antidepressants were found to down-regulate β receptor binding (44). Clearly, reconciling findings regarding norepinephrine β receptors in suicide is challenging.
Molecular abnormalities in the norepinephrine transporter, in related enzymes, and in neuron number also have been reported. Lower norepinephrine transporter binding has been observed in the locus ceruleus but not the hypothalamus in depressed suicide victims relative to nonpsychiatric comparison subjects (45). A monoamine oxidase A (MAO-A) gene polymorphism leading to high activity was found to be more prevalent in male but not female suicide victims with mood disorders compared with nonpsychiatric comparison subjects (46), although MAO-A and MAO-B activity does not seem altered in suicide victims (47). In a study of Japanese suicide victims, the Val/Val genotype of COMT, which renders COMT more efficient at catabolizing norepinephrine, was more prevalent in healthy live male comparison subjects than in suicide victims (48), but this was not observed in females. However, an opposite, protective effect of the Met/Met genotype was found in a sample of Caucasians who died from other causes compared with those who died by suicide, only among males (49). Moreover, in suicide victims compared with nonpsychiatric comparison subjects, decreased density and fewer norepinephrine neurons were observed in the rostral two-thirds of the locus ceruleus (50), as were higher concentrations of COMT in the prefrontal cortex and orbitofrontal cortex (51). Thus, some evidence suggests lower norepinephrine function in suicide, which, if true, could be secondary to down-regulation in response to chronic stress. The lack of psychopathological comparison subjects renders interpretation difficult.
Neuroinflammation
Cytokines
Cytokines are intracellular mediator proteins that act in a paracrine manner on secretion by inflammatory leukocytes and some non-leukocyte cells. Although cortisol inhibits cytokine secretion during acute stress (52), inflammatory cytokines also modulate HPA axis function in a complex manner, activating the CRH-ACTH-cortisol cascade and down-regulating GRs (53). Thus, inflammation may induce a functional glucocorticoid insufficiency, contributing to the blunted responsiveness of the negative feedback loop to glucocorticoids, as measured by the DST (54).
The observation that cytokine therapies such as β-interferon can induce suicidal ideation and behaviors (55) as well as depression has led to work to uncover the mechanism. One pathway may be via influences on the serotonin system. In cell culture, serotonin transporter activity is stimulated by the proinflammatory cytokines interleukin [IL]-1β (56, 57) and tumor necrosis factor (56, 58), enhancing reuptake of intrasynaptic serotonin and reducing signaling. Conversely, the anti-inflammatory cytokine IL-4 induces a dose-dependent decrease in serotonin uptake by the transporter (59).
Cytokine abnormalities in suicide
Suicide victims with a variety of diagnoses exhibit altered levels of cytokines, including increased frontopolar cortex levels of IL-1, IL-6, and tumor necrosis factor alpha (TNF-α) in teen suicide victims (60), increased orbitofrontal cortex levels of IL-4 mRNA in female and IL-13 in male suicide victims (61), and microgliosis (62), another marker of neuroinflammation, compared with nonpsychiatric comparison subjects. Because these study samples had relatively few depressed subjects, cytokine elevations in suicide victims are unlikely to be explained by depression.
Polyunsaturated fatty acids
Another potential path to an inflammatory role in suicide is an imbalance of proinflammatory omega-6 (primarily arachidonic acid) and anti-inflammatory omega-3 (primarily docosahexaenoic acid [DHA] and eicosapentaenoic acid [EPA]) polyunsaturated fatty acids. Omega-3 fatty acids reduce proinflammatory eicosanoids, cytokines, reactive oxygen species, and adhesion molecules via several mechanisms, including competitive inhibition of omega-6, influencing inflammatory gene transcription factors, and through anti-inflammatory effects of their own metabolic products, the resolvins (63). Low omega-3 fatty acid levels are associated with suicide attempts (64) or predicted future attempts (65), suggesting a role in suicide risk.
Polyunsaturated fatty acid status in suicide
A single large retrospective case-control study of deceased U.S. military personnel who had died by suicide and by non-suicide causes found that the suicide group had lower serum concentrations of omega-3 DHA as a percentage of total fatty acids (66). Suicide risk increased 14% with each standard deviation of decrease in DHA concentration. Two other postmortem samples (67, 68) showed no differences between suicide deaths and non-suicide deaths in brain fatty acid composition; however, sample sizes were small, and only one included psychiatric comparison subjects (68). A large population study using dietary questionnaires did not find a link between EPA consumption and suicide either (69).
Endogenous Opioid System
The opioid system plays a key role in stress response and interacts with both the HPA axis and the LC-NE system. Stress causes pituitary release of POMC, the precursor of ACTH and β-endorphin and a major endogenous opioid (70). Endogenous opioids reduce the emotional component of pain, but not pain perception per se. As such, these peptides may attenuate stress effects, and their alteration may contribute to suicide risk.
The opioid system in suicide
Eight studies have examined the opioid system’s role in suicide (71-78). Six of them (71-76) focused on the mu receptor, with inconclusive results. Three suggest higher mu receptor binding in suicide victims compared with dead or live comparison subjects (72, 75, 76); however, they focused on different brain areas (the caudate and frontal cortex, the frontal and temporal cortex, and BA 9 and the prefrontal cortex, respectively), or analyzed only young suicide victims (75). In contrast, Zalsman et al. (74) and González-Maeso et al. (73) found no differences in mu receptor distribution despite examining similar brain regions (the prefrontal cortex and BA 9, and the dorsolateral prefrontal cortex, respectively). Studies of endogenous opioids in suicide are few and difficult to interpret, with one study of prodynorphin showing higher levels in the caudate but not the putamen or nucleus accumbens (77), and one of beta endorphin showing lower levels in the caudate and temporal and frontal cortex (78). Unfortunately, most of these studies had small samples, ranging from 10 or fewer cases to 28 cases per cell. One genetic study (71) comparing suicide victims and comparison subjects found a single-nucleotide polymorphism (SNP) in the mu receptor gene associated with suicide. Drawbacks include the lack of matched psychiatric comparison subjects, variable postmortem intervals, and a variable toxicology status among subjects.
Neurotransmitter Systems Implicated in Suicide
See Table 3 for a summary.
TABLE 3.
Neurochemical, Autoradiographic, Morphological, and Other Findings in Suicidea
| Systema | Focus of Findings | Findings in Suicide | Locationb | Samplesc |
|---|---|---|---|---|
| Neurochemical findings | ||||
|
| ||||
| System | Compound | Findings in suicide | Location | Samples |
|
| ||||
| CRH-HPA | CRH | Elevated concentration | CSF (9), LC (10, 11), caudal raphe nucleus (10), DLPFC, VMPFC (11), FPPFC (11, 13), DMPFC (13) |
DS v. NC |
| Reduced concentration | DVC (11) | DS v. NC | ||
| Vasopressin | Elevated concentration | LC, DMPFC, paraventricular hypothalamic nucelus (11) |
DS v. NC | |
| Reduced concentration | DVC (11) | DS v. NC | ||
| CRHR1 | Reduction in number of binding sites |
Frontal cortex (12) | DS v. NC | |
| Cortisol | Reduced baseline levels | Plasma (20) | MDS v. PC | |
| NE-LC | Tyrosine hydroxylase |
Reduced stain density | LC (31) | |
| NE | Elevated concentration | Temporal cortex (33) | ||
| MAO-A, MAO-B | No difference | Frontal cortex (47) | ||
| MHPG | Reduced concentration | CSF (20) | MDS v. PC | |
| Cytokines | IL-1β, IL-6, TNFα | Elevated expression | BA 10 (60) | |
| Microglia | Elevated density | DLPFC, ACC, MDT (62) | ||
| Fatty acids | DHA | Reduced concentration | Serum (66) | |
| DHA, MUFA, saturated FA |
No difference | BA 10 (67) | ||
| FAs | No difference | OFC, VPFC (68) | S v. DS v. PC | |
| Endogenous opioids |
β-endorphin | Reduced concentration | Temporal cortex, frontal cortex, caudate nucleus (78) |
|
| Serotonin | TPH | Elevated concentration | DRN (86) | |
| No difference | MRN (86) | |||
| 5-HT | Elevated concentration | Brainstem (90) | ||
| 5-HIAA | Reduced concentration | CSF (121-124) | S v. SA (121); MDS v. PC (122, 124) |
|
| Elevated concentration | Hippocampus (125), amygdala (126) |
DS v. NC (126) | ||
| Dopamine | HVA/5-HIAA | Higher ratio | Frontal cortex (134) | |
| DOPAC | Reduced concentration | Caudate, putamen (AFS), nucelus accumbens (AFS) (135) |
AFS v. ATS v. NC | |
| Glutamate/GABA | Glutamine | Reduced concentration | Hypothalamus (146) | |
| Neuronal plasticity | BDNF | Reduced concentration | PFC, hippocampus (165) | |
| NT-3 | Reduced concentration | Hippocampus (165) | ||
| Autoradiographic findings | ||||
| System | Receptor | Ligand | Findings in suicide | Location | Samples |
|---|---|---|---|---|---|
| NE-LC | α1-adrenoceptor | [3H]Prazosin | Decreased binding | Caudate nucleus, PFC, frontoparietal and temporal cortical regions (32) |
|
| [3H]Prazosin | Increased binding | PFC (33) | |||
| [3H]Prazosin | No difference in number of receptors |
PFC, hippocampus, hypothalamus, thalamus, caudate, putamen (36) |
ATS v. NC; AFS v. NC | ||
| α2-adrenoceptor | p-[125I]Iodoclonidine | Increased binding | LC (30) | ||
| [3H]UK 14304 | Increased binding | Hippocampus (CA1), frontal cortex (35) |
|||
| [3H]Rauwolscine | Decreased binding | Occipital cortex, hippocampus (36) |
ATS v. NC | ||
| [3H]Aminoclonidine | No difference | PFC (33) | |||
| [3H]Rauwolscine | Increased binding | Temporal cortex (BA 21/22) (36) |
AFS v. NC | ||
| β-adrenoceptor | [3H]DHA | Increased binding | Frontal cortex (38), PFC (40) | VS v. NC (38) | |
| [125I]PIN | Increased binding | PFC (39) | |||
| [125I]PIN | Decreased binding | Frontal cortex (41) | |||
| [3H]CGP 12177 | Decreased binding | Temporal cortex (42, 43), thalamus (43) |
DS v. NC (42); ATS v. NC (43) |
||
| NET | [3H]Nisoxetine | Decreased binding | LC (45) | DS v. NC | |
| Endogenous opioids |
μ-opioid receptor |
[3H]DAGO | Higher density | Frontal cortex (72, 75), caudate (72); temporal cortex (75) |
Young S v. NC (75) |
| [3H]DAGO | No difference | PFC, PPCG (74) | |||
| DAMGO | No difference | PFC (BA 9) (73) | MDS v. NC | ||
| Serotonin | 5-HTT | [3H]CN-IMI | Decreased binding | VLPFC (95), VPFC (96) | |
| [3H]CN-IMI | No difference | DRN, MRN (99) | DS v. NC | ||
| [3H]Imipramine | Decreased binding | Frontal cortex (97); hippocampus (98); claustrum, insular cortex, PCCG (100) |
DS v. NC (98) | ||
| [3H]Imipramine | Increased binding | Hippocampus (100) | |||
| 5-HTR1A | [3H]8-OH-DPAT | Increased binding | VLPFC (95), DRN (106) | DS v. NC (106) | |
| [3H]8-OH-DPAT | Decreased binding | DRN (99, 107), MRN (99) | DS v. NC | ||
| 5-HTR2 | [3H]Spiroperidol | Increased density | Frontal cortex (110) | ||
| [3H]Spiroperidol | Increased binding | Frontal cortex (38) | |||
| [3H]Spiperone | Increased binding | Frontal cortex (109) | |||
| Dopamine | D1 receptors | [3H]SCH23390 | No difference | Caudate nucleus, putamen, and nucleus accumbens (133) |
DS v. NC |
| D2 receptors | [3H]Raclopride | No difference | Caudate nucleus (132, 133), putamen, nucleus accumbens (133) |
DS v. NC | |
| Dopamine reuptake sites |
[3H]WIN 35428 | No difference | Caudate nucleus (131) | DS v. NC | |
| GABA | GABAA receptors | [3H]-Flunitrazepam | No difference | LC (141) | DS v. NC |
| GABAB receptors | [3H]GABA | No difference | Frontal cortex, temporal cortex, hippocampus (144) |
DS v. NC | |
| Glutamate | NMDA | [3H]Dizocilpine | No difference | Frontal cortex (148, 151, 152), parietal cortex (148), temporal and occipital cortices, hippocampus, thalamus (152), putamen, caudate nucleus (147, 152), nucleus accumbens (147) |
DS v. NC (152); S v. PC, S v. NC (147) |
| [3H]CGP-39653 | Decreased binding | Frontal cortex (151) | |||
| AMPA | [3H]AMPA | Increased binding | Caudate nucleus (149) | ||
| [3H]CNQX | Increased binding | Caudate nucleus (147) | S v. PC, S v. NC | ||
| Morphological findings | |||||
| System | Structure | Findings in suicide | Samples |
|---|---|---|---|
| CRH-HPA | Adrenal glands | Increased adrenal weight (21–23) |
VS v. C (21) |
| No difference in adrenal weight (24) |
|||
| NE-LC | Locus ceruleus | Decreased number of neurons, decreased neuron density (50) |
|
| Serotonin | DRN | Increase in DRN area, decrease in 5-HT neuron size, increased 5-HT neuron density (89) |
S v. PC |
| Neuronal plasticity | Hippocampus | Decreased number of dentate gyrus granule neurons; increased anterior and mid granule cell layer volume (160) |
AFS v. NC |
| No difference (161) | SS v. NC | ||
| Parahippocampus | Decreased right parahippocampal volume (161) |
SS v. NC | |
| PFC | Reduction in cortical and laminar thickness (159) |
S v. PC | |
| Other findings |
| System | Test | Findings in suicide | Samples |
|---|---|---|---|
| CRH-HPA | Dexamethasone suppression test (DST) |
DST nonsuppressors more likely to die by suicide (26-28) |
MDP DST suppressors v. nonsuppressors (26, 28) |
| MDS DST suppressors v. nonsuppressors (27) |
|||
| Dopamine | Apomorphine challenge | Decreased growth hormone response to apomorphine (129) |
DS v. DP |
CRH-HPA=corticotropin-releasing hormone/hypothalamic-pituitary-adrenal axis system; LC-NE=locus ceruleus-based norepinephrine system.
Numbers in parentheses are reference numbers. ACC=anterior cingulate cortex; BA=Brodmann’s area; CSF=cerebrospinal fluid; DLPFC=dorsolateral prefrontal cortex; DMPFC=dorsomedial prefrontal cortex; DRN=dorsal raphe nucleus; DVC=dorsovagal complex; FPPFC=frontopolar prefrontal cortex; LC=locus ceruleus; MDT=mediodorsal thalamus; MRN = median raphe nucleus; OFC=orbitofrontal cortex; PCCG=postcentral cortical gyrus; PFC=prefrontal cortex; PPCG=pre- and postcentral gyri; VLPFC=ventrolateral prefrontal cortex; VMPFC=ventromedial prefrontal cortex; VPFC=ventral prefrontal cortex.
Numbers in parentheses are reference numbers. The samples cited consist of nonspecific suicide victims compared with comparison subjects, unless otherwise specified. AFS=antidepressant-free suicide victims; ATS=antidepressant-treated suicide victims; DP=depressed patients; DS=depressed suicide victims; MDP=patients with mood disorders; MDS=suicide victims with mood disorders; NC=nonpsychiatric comparison subjects; PC=psychiatric comparison subjects; S=suicide victims; SA=suicide attempters; SS=suicide victims with schizophrenia; VS=violent suicides.
Serotonin
Cell bodies in the dorsal and median raphe nuclei provide extensive serotonergic innervation throughout the brain. Seven serotonin (5-hydroxytryptamine [5-HT]) receptor families exist, many with several subtypes. All but one (5-HT3, a ligand-gated ion channel) are G-protein coupled receptors (79). The presynaptic serotonin transporter (5-HTT) removes serotonin from the synaptic cleft. Tryptophan hydroxylase (TPH) is the rate-limiting enzyme in 5-HT synthesis (80, 81), and two isoforms, TPH1 and TPH2, are present in humans. TPH1, found primarily in the periphery after birth, is implicated in intrauterine neurodevelopment (82, 83). TPH2 is specific to brain (82). Serotonin breakdown involves oxidation of 5-HT by MAO-A and aldehyde dehydrogenase to produce 5-HIAA. Given the serotonergic system’s involvement in regulation of sleep, appetite, anxiety, mood, cognition, aggression, and memory, all of which have a role among the clinical features associated with suicide, it is a key candidate for study.
Tryptophan hydroxylase and serotonin synthesis in suicide
Elevated TPH2 levels have been reported in the ventral prefrontal cortex of suicide victims (84) relative to comparison subjects, even controlling for psychopathology; this elevation is more pronounced in subjects who died in nonviolent suicides (84). Additionally, increased TPH2 expression and protein have been noted in suicide victims in the dorsal (85, 86) and median raphe nucleus (85) and along the entire rostrocaudal axis of the dorsal raphe nucleus (85), but not in the dorsolateral prefrontal cortex (87). Whether these elevations reflect a compensation for decreased serotonin availability (85, 86) is unknown. In addition to increased expression of TPH2, the presence of more serotonin neurons in the raphe nuclei of suicide victims (88, 89) indicates that serotonin synthesis capacity is enhanced, perhaps in response to decreased serotonergic tone. Indeed, Bach et al. (90) recently reported more serotonin in the raphe nuclei of suicide victims. In addition, links have been reported between suicide and SNPs located between TPH2 exons 5 and 7 (91), in intron 1, intron 8, and the 5′ upstream region (92). However, no link was found between suicide and the G703T SNP (93) or multiple other TPH2 polymorphisms (94).
Serotonin transporter in suicide
Most studies report a decrease (95-98) or no change (99) in 5-HTT binding affinity in the hippocampus and prefrontal cortex, although an increase has also been reported (100). Fewer neurons express the serotonin transporter in the raphe nuclei of suicide victims. The deficit in transporter binding has been shown to differ from that seen in major depression, where it extends over most of the prefrontal cortex, whereas in suicide it is restricted to the ventromedial prefrontal cortex and anterior cingulate regions (95), which are implicated in decision making and willed action. Of note, a serotonin transporter intron 2-variable nucleotide tandem repeat (STin2-VNTR) 10-allele was more common in depressed Canadians who died by suicide compared with depressed live comparison subjects (101), but no other 5-HTT polymorphisms have been shown to confer risk, including promoter (5-HTTLPR) alleles in the Canadian study (101) and a more recent one (102) examining the SCL6A4 site. One study (103) examining the co-occurrence of the 10-allele of the STin2-VNTR polymorphism and the CC variant of the TPH A218C polymorphism in suicide found that concurrent presence of these genotypes increased suicide risk, but a study of alcohol-dependent suicide victims (104) did not find effects for them, either separately or together. Not all studies concur (105), but most data suggest fewer serotonin transporters in suicide victims, possibly as a homeostatic adaptation to low serotonergic tone, particularly in the ventral prefrontal cortex and anterior cingulate.
Serotonin receptors in suicide
Higher rostral dorsal raphe nucleus 5-HT1A binding has been identified in depressed suicide victims (106-108), although a study of 5-HT1A gene expression (89) did not find an abundance of mRNA. Suicide victims also appear to have more prefrontal cortex 5-HT2A receptors and gene expression relative to nonpsychiatric comparison subjects (38, 109-112), even after accounting for the sex effect on 5-HT2A binding sites (males > females) (109), although one study (108) detected no difference. Similarly, 5-HT2C is reported to be more abundant in the prefrontal cortex of suicide victims relative to comparison subjects (113). No relationship to the violence of the suicide method has been noted for 5-HT2 binding (109).
Genetic association studies of 5-HT receptors have yielded disappointing results. One study examining polymorphisms at five 5-HTR1B loci found an association between the A161T promoter variant and suicide (114). Individuals who died by suicide were more likely to have homozygous TT genotype and score higher on aggression and impulsivity measures (114). Another study investigating the 267C/T SNP of 5-HTR6 found a significant association between this SNP and suicide in males only (115). However, other studies found no association between suicide and genetic variants of the 5-HTR1B, 5-HTR1D, 5-HTR1E, 5-HTR1F, 5-HT2A, 5-HTR5A, 5-HTR6, or 5-HTR2C genes (112, 116-119). Genome-wide association studies have used larger samples, although these have still been insufficient to detect small effects and have not detected any serotonin receptor associations (120).
Serotonin turnover in suicide
Several studies have shown that low CSF levels of 5-HIAA, an index of 5-HT turnover (121), predict suicide (122-124), although one study found this to be true only for males (124). Similarly, most studies of brainstem 5-HT or 5-HIAA in suicide victims report low 5-HIAA levels. Interestingly, Bach et al. (90) reported higher 5-HIAA levels in the brainstem in a pilot study that sampled the raphe nuclei systematically, from rostral to caudal, identifying differences in the ratio of 5-HT to 5-HIAA based on the anatomical level of the raphe. Yet, no such differences were detected in the prefrontal cortex. Because the study excluded subjects exposed to long-term use of antidepressants, which tends to increase 5-HIAA, and found increases in 5-HT or 5-HIAA along the length of the raphe nuclei but not in the prefrontal cortex, it suggests that the impairment is in serotonergic neurotransmission rather than synthesis. In fact, other studies of depressed suicide victims and comparison subjects have found that suicide victims have increased 5-HIAA levels in the hippocampus (125) and amygdala (126) and no differences in 5-HT levels in the cortex or amygdala (126). One study reported that drug-free nonviolent suicide victims had significantly lower 5-HIAA and 5-HT concentrations in the hippocampus than did violent suicide victims (121, 126).
Thus, the most extensive corpus of work in suicide is focused on the serotonergic system, and many studies link altered serotonin transmission to suicide risk, with the most compelling case being made by follow-up studies of individuals with low 5-HIAA.
Norepinephrine
See the section “Noradrenergic-locus ceruleus system in suicide,” above.
Dopamine
Dopamine is synthesized from l-dopa in the ventral tegmental area and substantia nigra (127). Dopamine binds to five receptor subtypes, D1, D2, D3, D4, and D5, each belonging to one of two metabotropic G-protein coupled receptor families, the D1-like and the D2-like families (128), activated by apomorphine, a dopamine agonist (129). Dopamine, when metabolized via MAO, yields 3,4-dihydroxyphenylacetic acid (DOPAC), which COMT then breaks down to homovanillic acid (HVA) (130). In noradrenergic and adrenergic neurons, dopamine β-hydroxylase metabolizes dopamine to norepinephrine (130). Dopamine is implicated in mood, motivation, aggression, reward, working memory, and attention, making it a prime candidate for study.
Dopamine in suicide
Suicide studies have examined dopamine receptors (131-133) and metabolites (134, 135) and growth hormone (GH) response to apomorphine (129), but only some found any association (129, 134, 135). A retrospective study of GH response to apomorphine in depressed inpatients found that suicide victims had lower mean GH peak responses compared with depressed patients who did not die by suicide, suggesting a potential role of the D2 receptor (129). However, studies using [3H]raclopride found no differences in the number or binding affinity of D1 or D2 receptors in the nucleus accumbens, putamen, and caudate nucleus of depressed suicide victims relative to comparison subjects (132, 133). Dopamine transporter availability in the caudate nucleus does not differ either (131). Data regarding dopamine turnover is scant. Compared with persons who died of physical diseases, those who died by suicide have higher cortical HVA concentrations, unrelated to violence of method, an observation also reported for homicide victims (134). Nonviolent depressed suicide victims appear to have lower DOPAC concentrations in the nucleus accumbens, caudate, and putamen, but not in the amygdala or the hippocampus (135). Unfortunately, comparison subjects in these studies were often psychopathology free, so interpretation is challenging. The paucity and inconsistency of results preclude identifying dopaminergic function as a marker of suicide risk.
GABA
GABA inhibition is mediated by two receptor types: GABAA, the ligand-gated ion channel, and GABAB, the metabotropic G-protein coupled receptor (136). GABAA has multiple isoforms based on subunit combinations (137). GABAB has only two subtypes: GABAB1 and GABAB2 (138). It is implicated in anxiety, addictive, and convulsive disorders.
GABA in suicide
Few suicide studies have focused on GABAA receptors (13, 139-141). One study comparing GABAA α1, α2, α3, α4, α5, δ, and γ2 subunit mRNA expression in the frontopolar cortex of suicide victims and comparison subjects found decreased expression of α1, α3, α4, and δ mRNA in suicide victims (13), possibly linked to methylation patterns (142). Furthermore, among suicide victims, the coordination of GABAA subunit expression seen in comparison subjects was not present, presumably hindering configuration of subunits into a functional receptor (140). Whether these findings relate to depression or suicide cannot be determined. Of interest, a gene expression study compared nonpsychiatric comparison subjects and both depressed and nondepressed suicide victims and found no differences between nondepressed suicide victims and comparison subjects in GABA receptors, subunits, or receptor-linked protein genes across several brain regions (139, 143). However, greater gene expression or binding in the hippocampus and prefrontal cortex of the depressed group (139, 141) suggests a potential role of GABA in depression. Two neuroreceptor binding studies did not confirm abnormalities in GABAA or GABAB in either suicide or depression compared with nonpsychiatric comparison subjects (141, 144). Together, these findings do not suggest GABA-ergic dysfunction in suicide.
Glutamate
The role of glutamate in the CNS is generally excitatory, with receptors falling into three major categories: metabotropic, ionotropic, and transporters. The metabotropic receptor family comprises at least eight different receptor subtypes (mGluR1–mGluR8, encoded by genes GRM1–GRM8). The ionotropic receptor family is more complex, with three subgroups, each with several subtypes: α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA) sensitive receptors (GluR1–GluR4, encoded by genes GRIA1–GRIA4); kainate-sensitive receptors (GluR5–GluR7, KA1, and KA2, encoded by GRIK1–GRIK5); and N-methyl-d-aspartate (NMDA) sensitive (NR1, NR2A–NR2D, NR3A, and NR3B, encoded by genes GRIN1–GRIN3B). Two major glial high-affinity transporters, SLC1A2 and SLC1A3, have been identified. They regulate glutamate reuptake, affecting synaptic activation (145).
Glutamate in suicide
Few, mostly small (<15 suicide victims) studies have been conducted (146–150). We could find none with samples larger than 26 (139). In all but two studies (139, 149), suicide victims were not matched to comparison subjects on diagnosis. All studies included individuals exposed to various drugs at the time of death. One reported fewer metabotropic (GRM3) receptors in the prefrontal cortex (139) in suicide victims relative to nonpsychiatric comparison subjects. Higher AMPA binding in the caudate has been observed (147, 149), but lower GRIA3 binding in the prefrontal cortex (139) has also been found. Studies of NMDA binding in the prefrontal cortex in suicide report decreases (151) or no difference (148, 152). Glutamate levels measured by high-performance liquid chromatography in eight brain regions (146) and in homogenized frontal and parietal cortex (148) appear no different between suicide victims and comparison subjects. Thus, the role of glutamatergic alterations in suicide is unclear, but it may, given ketamine’s putative antisuicidal properties, be a potential subject for further study.
Molecular Markers of Neuronal Plasticity in Suicide
Structural and functional adaptation to environmental demands occurs through synaptic plasticity and neurogenesis, processes regulated by neurotrophins (153, 154). Of four identified neurotrophin classes, brain-derived neurotrophic factor (BDNF) and its receptor, tropomyosin-receptor kinase B (TrkB), are critical mediators of plasticity (155, 156). Besides BDNF, compounds such as adenosine, pituitary adenylate cyclase-activating polypeptide (PACAP), anandamide (an endocannabinoid), kainate, glucocorticoids, and dopamine also activate TrkB receptors (157). Putative rapid antidepressant and antisuicidal actions of compounds like ketamine, proposed to act through NMDA receptor antagonism and AMPA/kainate receptor activation, may ultimately be attributable to AMPA/kainate receptor effects increasing TrkB gene expression (158).
Neuronal Plasticity in Suicide
Findings in suicide include cortical thinning in dorsolateral prefrontal cortex neurons (159), fewer dentate gyrus granule neurons in depressed suicide victims (160), and smaller right parahippocampal volume (161), although in the latter study, the finding was attributed to depression rather than suicide. Nonetheless, volume loss suggests reduced neurogenesis, accelerated neuron loss due to apoptosis, or loss of neuropil. Teenage suicide victims with diverse diagnoses have been reported to have lower BDNF protein expression in the prefrontal cortex, fewer TrkB full-length receptors in the prefrontal cortex and hippocampus, and less BDNF and TrkB mRNA (162), possibly linked to microRNA interference with gene transcription (163). Similar findings have been reported in adult suicide victims with diverse diagnoses (164). Another study demonstrated less BDNF and neurotrophin-3 in the hippocampus and ventral prefrontal cortex, but not the entorrhinal cortex, in both depressed and nondepressed suicide victims (165) compared with nonpsychiatric comparison subjects, suggesting that alterations are not due to depression. Of note, BDNF levels were similar between antidepressant-treated suicide victims and nonpsychiatric comparison subjects, suggesting normalization of neurotrophin levels with antidepressants (165). Expression of neurotrophins and proteins in the neurotrophin cascades in the prefrontal cortex and hippocampus of suicide victims is altered (166, 167), indicating impairments in neurogenesis. Furthermore, female suicide victims have been reported to be more likely to carry the BDNF Val66Met (Val/Met or Met/ Met) polymorphism, a gene variant associated with lower activity-dependent secretion of BDNF (168). Epigenetic changes, possibly reflective of early-life adversity, may also be relevant to BDNF-TrkB system dysfunction, as suicide victims have been reported to have higher BDNF-gene DNA methylation (169) and, correspondingly, lower BDNF mRNA relative to non-suicide comparison subjects (170). Thus, this small literature supports a potential role of neuroplasticity in suicide, independent of its role in depression.
Conclusions
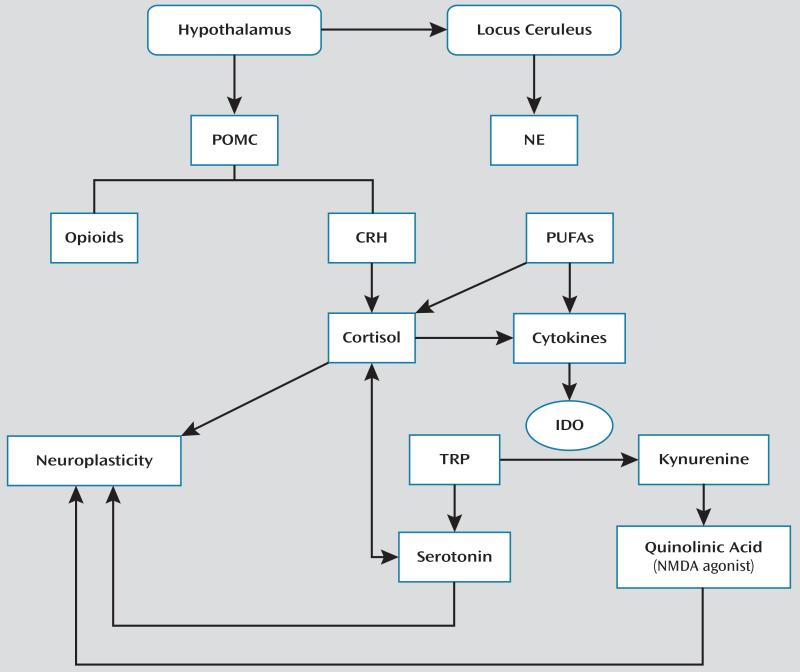
Biological systems implicated in suicide are interrelated (Figure 1), suggesting that a coherent model can be built. However, its components require further development. Currently, data demonstrating multilevel HPA axis dysfunction make this a top candidate biomarker. Some abnormalities, such as high CRH levels, appear to be primary and may result in downstream compensatory changes, such as DST nonsuppression. Abnormalities in the LC-NE stress response system require further elucidation, although some findings suggest lower norepinephrine function, which, if confirmed, could be secondary to norepinephrine depletion due to excessive response to stress. Linked to stress response through POMC release is the opioid system. Although postmortem evidence for opioid involvement is currently weak, its role in pain alleviation in the context of stress may be relevant. The serotonergic system, so extensively studied in suicide, exhibits changes at several levels, with the most compelling data being the prediction of suicide by low CSF levels of 5-HIAA. The serotonergic system has a close, bidirectional relationship to the HPA axis and is linked to inflammatory processes. Indeed, the small but convincing cytokine and suicide literature helps collate data implicating the HPA axis and polyunsaturated fatty acids and provides a basis for connections between these systems and their effects on suicide. In contrast, scant work examining GABA, dopamine, and glutamate appears negative. Yet, despite less-than-compelling data about glutamate’s role, emerging data regarding “antisuicidal effects” of ketamine suggest that more studies are needed. Furthermore, the glutamatergic and serotonergic system and cytokines have a role in neuroplasticity and neurotoxicity, which is key to suicide independent of their role in depression, thus also supporting a role for these systems in suicide.
FIGURE 1. A Model for Relationships Between Putative Suicide Biomarkersa.
a CRH=corticotropin releasing hormone; IDO=2,3-iododeoxygenase; NE=norepinephrine; NMDA=N-methyl d-aspartate receptor; POMC=proopiomelonocortin; PUFAs=polyunsaturated fatty acids; TRP=tryptophan.
Future work on the neurobiology of suicide must overcome challenges such as use of small samples; examination of different brain regions; use of agonists, antagonists, and partial antagonists in neuroreceptor assays; confounding due to damage to the brain; presence of psychotropics that potentially affect biomarkers; variable postmortem intervals; lack of well-matched cases including psychiatric controls; and predominantly male samples. Another key issue is that there are likely differing biological pathways to suicide, and the lack of studies delineating suicide subtypes remains an issue for data interpretation. Thus, defining a precise mechanism underlying suicide is beyond available methodology, and we lack biomarkers with diagnostic utility, let alone treatment targets. The few biomarkers measurable in vivo, such as DST or CSF 5-HIAA level, lack the positive predictive value essential for clinical use.
In summary, a dysregulated CRH-HPA axis is linked to other systems implicated in suicide: the serotonin, opioid, and glutamate systems, inflammatory pathways, lipid status, and neuroplasticity or neurogenesis. Therefore, a battery of biomarkers, rather than a single one, will likely be needed to identify individuals at risk. The silo approach to biomarkers should be phased out in favor of the study of multiple systems in parallel and in the same populations. Only in that way will the role of each and their interplay be clarified, with the goal of identifying new treatment targets and improving suicide risk prediction.
Acknowledgments
Supported by NIMH grants P50 MH090964 and R01 MH48514.
Dr. Oquendo receives royalties for the use of the Columbia Suicide Severity Rating Scale (C-SSRS) and has received financial compensation from Pfizer for the safety evaluation of a clinical facility; she was the recipient of a grant from Eli Lilly to support a year’s salary for the Lilly Suicide Scholar, Enrique Baca-Garcia; she has received unrestricted educational grants and/or lecture fees from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Otsuko, Pfizer, Sanofi-Aventis, and Shire; and her family owns stock in Bristol-Myers Squibb. Dr. Sullivan is employed by Tonix Pharmaceuticals, and previously served as an adviser and as a consultant for Tonix Pharmaceuticals. Dr. Sublette received a grant of nutritional supplements from Unicity International. Dr. Mann receives royalties from the Research Foundation for Mental Hygiene for commercial use of the C-SSRS; he owns stock options in Qualitas, a producer of EPA supplements. Dr. Stanley receives royalties from the Research Foundation for Mental Hygiene for commercial use of the C-SSRS.
Footnotes
The other authors report no financial relationships with commercial interests.
References
- 1.Centers for Disease Control and Prevention . Suicide and Self-Inflicted Injury. http://www.cdc.gov/nchs/fastats/suicide.htm. [Google Scholar]
- 2.World Health Organization Suicide Prevention (SUPRE) http://www.who.int/mental_health/prevention/suicide/suicideprevent/en/
- 3.Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry. 1999;156:181–189. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- 4.Mann JJ, Arango V. Integration of neurobiology and psychopathology in a unified model of suicidal behavior. J Clin Psychopharmacol. 1992;12(suppl):2S–7S. [PubMed] [Google Scholar]
- 5.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual frame-work. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 6.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 7.Chrousos GP, Gold PW. The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 8.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 9.Arató M, Bánki CM, Bissette G, Nemeroff CB. Elevated CSF CRF in suicide victims. Biol Psychiatry. 1989;25:355–359. doi: 10.1016/0006-3223(89)90183-2. [DOI] [PubMed] [Google Scholar]
- 10.Austin MC, Janosky JE, Murphy HA. Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol Psychiatry. 2003;8:324–332. doi: 10.1038/sj.mp.4001250. [DOI] [PubMed] [Google Scholar]
- 11.Merali Z, Kent P, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Bédard T, Anisman H. Corticotropin-releasing hormone, arginine vasopressin, gastrin-releasing peptide, and neuromedin B alterations in stress-relevant brain regions of suicides and control subjects. Biol Psychiatry. 2006;59:594–602. doi: 10.1016/j.biopsych.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry. 1988;45:577–579. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- 13.Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Anisman H. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA (A) receptor subunits in frontal cortical brain region. J Neurosci. 2004;24:1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López JF, Palkovits M, Arató M, Mansour A, Akil H, Watson SJ. Localization and quantification of pro-opiomelanocortin mRNA and glucocorticoid receptor mRNA in pituitaries of suicide victims. Neuroendocrinology. 1992;56:491–501. doi: 10.1159/000126266. [DOI] [PubMed] [Google Scholar]
- 15.Pandey GN, Rizavi HS, Ren X, Dwivedi Y, Palkovits M. Region-specific alterations in glucocorticoid receptor expression in the postmortem brain of teenage suicide victims. Psychoneuroendocrinology. 2013;38:2628–2639. doi: 10.1016/j.psyneuen.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.López JF, Chalmers DT, Little KY, Watson SJ. AE Bennett Research Award: Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- 17.Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- 18.Herman JP, Watson SJ. Glucocorticoid regulation of stress-induced mineralocorticoid receptor gene transcription in vivo. Ann N Y Acad Sci. 1994;746:485–488. doi: 10.1111/j.1749-6632.1994.tb39292.x. [DOI] [PubMed] [Google Scholar]
- 19.Labonte B, Yerko V, Gross J, Mechawar N, Meaney MJ, Szyf M, Turecki G. Differential glucocorticoid receptor exon 1(B), 1(C), and 1(H) expression and methylation in suicide completers with a history of childhood abuse. Biol Psychiatry. 2012;72:41–48. doi: 10.1016/j.biopsych.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 20.Jokinen J, Ouda J, Nordström P. Noradrenergic function and HPA axis dysregulation in suicidal behaviour. Psychoneuroendocrinology. 2010;35:1536–1542. doi: 10.1016/j.psyneuen.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Dorovini-Zis K, Zis AP. Increased adrenal weight in victims of violent suicide. Am J Psychiatry. 1987;144:1214–1215. doi: 10.1176/ajp.144.9.1214. [DOI] [PubMed] [Google Scholar]
- 22.Dumser T, Barocka A, Schubert E. Weight of adrenal glands may be increased in persons who commit suicide. Am J Forensic Med Pathol. 1998;19:72–76. doi: 10.1097/00000433-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Szigethy E, Conwell Y, Forbes NT, Cox C, Caine ED. Adrenal weight and morphology in victims of completed suicide. Biol Psychiatry. 1994;36:374–380. doi: 10.1016/0006-3223(94)91212-2. [DOI] [PubMed] [Google Scholar]
- 24.Stein E, McCrank E, Schaefer B, Goyer R. Adrenal gland weight and suicide. Can J Psychiatry. 1993;38:563–566. doi: 10.1177/070674379303800807. [DOI] [PubMed] [Google Scholar]
- 25.Supriyanto I, Sasada T, Fukutake M, Asano M, Ueno Y, Nagasaki Y, Shirakawa O, Hishimoto A. Association of FKBP5 gene haplotypes with completed suicide in the Japanese population. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:252–256. doi: 10.1016/j.pnpbp.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Yerevanian BI, Feusner JD, Koek RJ, Mintz J. The dexamethasone suppression test as a predictor of suicidal behavior in unipolar depression. J Affect Disord. 2004;83:103–108. doi: 10.1016/j.jad.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Jokinen J, Carlborg A, Mårtensson B, Forslund K, Nordström AL, Nordström P. DST non-suppression predicts suicide after attempted suicide. Psychiatry Res. 2007;150:297–303. doi: 10.1016/j.psychres.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Coryell W, Schlesser M. The dexamethasone suppression test and suicide prediction. Am J Psychiatry. 2001;158:748–753. doi: 10.1176/appi.ajp.158.5.748. [DOI] [PubMed] [Google Scholar]
- 29.Mann JJ, Currier D, Stanley B, Oquendo MA, Amsel LV, Ellis SP. Can biological tests assist prediction of suicide in mood disorders? Int J Neuropsychopharmacol. 2006;9:465–474. doi: 10.1017/S1461145705005687. [DOI] [PubMed] [Google Scholar]
- 30.Ordway GA, Widdowson PS, Smith KS, Halaris A. Agonist binding to alpha 2-adrenoceptors is elevated in the locus coeruleus from victims of suicide. J Neurochem. 1994;63:617–624. doi: 10.1046/j.1471-4159.1994.63020617.x. [DOI] [PubMed] [Google Scholar]
- 31.Biegon A, Fieldust S. Reduced tyrosine hydroxylase immunoreactivity in locus coeruleus of suicide victims. Synapse. 1992;10:79–82. doi: 10.1002/syn.890100111. [DOI] [PubMed] [Google Scholar]
- 32.Gross-Isseroff R, Dillon KA, Fieldust SJ, Biegon A. Autoradiographic analysis of alpha 1-noradrenergic receptors in the human brain postmortem: effect of suicide. Arch Gen Psychiatry. 1990;47:1049–1053. doi: 10.1001/archpsyc.1990.01810230065010. [DOI] [PubMed] [Google Scholar]
- 33.Arango V, Ernsberger P, Sved AF, Mann JJ. Quantitative autoradiography of alpha 1- and alpha 2-adrenergic receptors in the cerebral cortex of controls and suicide victims. Brain Res. 1993;630:271–282. doi: 10.1016/0006-8993(93)90666-b. [DOI] [PubMed] [Google Scholar]
- 34.Underwood MD, Mann JJ, Arango V. Serotonergic and noradrenergic neurobiology of alcoholic suicide. Alcohol Clin Exp Res. 2004;28(suppl):57S–69S. doi: 10.1097/01.alc.0000127415.15000.ca. [DOI] [PubMed] [Google Scholar]
- 35.González AM, Pascual J, Meana JJ, Barturen F, del Arco C, Pazos A, García-Sevilla JA. Autoradiographic demonstration of increased alpha 2-adrenoceptor agonist binding sites in the hippocampus and frontal cortex of depressed suicide victims. J Neurochem. 1994;63:256–265. doi: 10.1046/j.1471-4159.1994.63010256.x. [DOI] [PubMed] [Google Scholar]
- 36.De Paermentier F, Mauger JM, Lowther S, Crompton MR, Katona CL, Horton RW. Brain alpha-adrenoceptors in depressed suicides. Brain Res. 1997;757:60–68. doi: 10.1016/s0006-8993(97)00138-8. [DOI] [PubMed] [Google Scholar]
- 37.Fukutake M, Hishimoto A, Nishiguchi N, Nushida H, Ueno Y, Shirakawa O, Maeda K. Association of alpha2A-adrenergic receptor gene polymorphism with susceptibility to suicide in Japanese females. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1428–1433. doi: 10.1016/j.pnpbp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Mann JJ, Stanley M, McBride PA, McEwen BS. Increased serotonin2 and beta-adrenergic receptor binding in the frontal cortices of suicide victims. Arch Gen Psychiatry. 1986;43:954–959. doi: 10.1001/archpsyc.1986.01800100048007. [DOI] [PubMed] [Google Scholar]
- 39.Arango V, Ernsberger P, Marzuk PM, Chen JS, Tierney H, Stanley M, Reis DJ, Mann JJ. Autoradiographic demonstration of increased serotonin 5-HT2 and beta-adrenergic receptor binding sites in the brain of suicide victims. Arch Gen Psychiatry. 1990;47:1038–1047. doi: 10.1001/archpsyc.1990.01810230054009. [DOI] [PubMed] [Google Scholar]
- 40.Biegon A, Israeli M. Regionally selective increases in beta-adrenergic receptor density in the brains of suicide victims. Brain Res. 1988;442:199–203. doi: 10.1016/0006-8993(88)91453-9. [DOI] [PubMed] [Google Scholar]
- 41.Little KY, Clark TB, Ranc J, Duncan GE. Beta-adrenergic receptor binding in frontal cortex from suicide victims. Biol Psychiatry. 1993;34:596–605. doi: 10.1016/0006-3223(93)90151-3. [DOI] [PubMed] [Google Scholar]
- 42.De Paermentier F, Cheetham SC, Crompton MR, Katona CL, Horton RW. Brain beta-adrenoceptor binding sites in antidepressant-free depressed suicide victims. Brain Res. 1990;525:71–77. doi: 10.1016/0006-8993(90)91321-7. [DOI] [PubMed] [Google Scholar]
- 43.De Paermentier F, Cheetham SC, Crompton MR, Katona CL, Horton RW. Brain beta-adrenoceptor binding sites in depressed suicide victims: effects of antidepressant treatment. Psychopharmacology (Berl) 1991;105:283–288. doi: 10.1007/BF02244323. [DOI] [PubMed] [Google Scholar]
- 44.Baron BM, Ogden AM, Siegel BW, Stegeman J, Ursillo RC, Dudley MW. Rapid down regulation of beta-adrenoceptors by co-administration of desipramine and fluoxetine. Eur J Pharmacol. 1988;154:125–134. doi: 10.1016/0014-2999(88)90089-1. [DOI] [PubMed] [Google Scholar]
- 45.Klimek V, Stockmeier C, Overholser J, Meltzer HY, Kalka S, Dilley G, Ordway GA. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci. 1997;17:8451–8458. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du L, Faludi G, Palkovits M, Sotonyi P, Bakish D, Hrdina PD. High activity-related allele of MAO-A gene associated with depressed suicide in males. Neuroreport. 2002;13:1195–1198. doi: 10.1097/00001756-200207020-00025. [DOI] [PubMed] [Google Scholar]
- 47.Mann JJ, Stanley M. Postmortem monoamine oxidase enzyme kinetics in the frontal cortex of suicide victims and controls. Acta Psychiatr Scand. 1984;69:135–139. doi: 10.1111/j.1600-0447.1984.tb02477.x. [DOI] [PubMed] [Google Scholar]
- 48.Ono H, Shirakawa O, Nushida H, Ueno Y, Maeda K. Association between catechol-O-methyltransferase functional polymorphism and male suicide completers. Neuropsychopharmacology. 2004;29:1374–1377. doi: 10.1038/sj.npp.1300470. [DOI] [PubMed] [Google Scholar]
- 49.Pivac N, Pregelj P, Nikolac M, Zupanc T, Nedic G, Muck Seler D, Videtic Paska A. The association between catechol-O-methyltransferase Val108/158Met polymorphism and suicide. Genes Brain Behav. 2011;10:565–569. doi: 10.1111/j.1601-183X.2011.00695.x. [DOI] [PubMed] [Google Scholar]
- 50.Arango V, Underwood MD, Mann JJ. Fewer pigmented locus coeruleus neurons in suicide victims: preliminary results. Biol Psychiatry. 1996;39:112–120. doi: 10.1016/0006-3223(95)00107-7. [DOI] [PubMed] [Google Scholar]
- 51.Du L, Merali Z, Poulter MO, Palkovits M, Faludi G, Anisman H. Catechol-O-methyltransferase Val158Met polymorphism and altered COMT gene expression in the prefrontal cortex of suicide brains. Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:178–183. doi: 10.1016/j.pnpbp.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 52.Ruud TE, Gundersen Y, Krohn CD, Sveen O, Aasen AO. Effects of infliximab and hydrocortisone on in vitro cytokine responses after stimulation with lipopolysaccharide. Surg Infect (Larchmt) 2013;14:30–34. doi: 10.1089/sur.2011.093. [DOI] [PubMed] [Google Scholar]
- 53.Pace TW, Gaylord RI, Jarvis E, Girotti M, Spencer RL. Differential glucocorticoid effects on stress-induced gene expression in the paraventricular nucleus of the hypothalamus and ACTH secretion in the rat. Stress. 2009;12:400–411. doi: 10.1080/10253890802530730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raison CL, Miller AH. Depression in cancer: new developments regarding diagnosis and treatment. Biol Psychiatry. 2003;54:283–294. doi: 10.1016/s0006-3223(03)00413-x. [DOI] [PubMed] [Google Scholar]
- 55.Fragoso YD, Frota ER, Lopes JS, Noal JS, Giacomo MC, Gomes S, Gonçalves MV, da Gama PD, Finkelsztejn A. Severe depression, suicide attempts, and ideation during the use of interferon beta by patients with multiple sclerosis. Clin Neuropharmacol. 2010;33:312–316. doi: 10.1097/WNF.0b013e3181f8d513. [DOI] [PubMed] [Google Scholar]
- 56.Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- 57.Ramamoorthy S, Ramamoorthy JD, Prasad PD, Bhat GK, Mahesh VB, Leibach FH, Ganapathy V. Regulation of the human serotonin transporter by interleukin-1 beta. Biochem Biophys Res Commun. 1995;216:560–567. doi: 10.1006/bbrc.1995.2659. [DOI] [PubMed] [Google Scholar]
- 58.Mössner R, Heils A, Stöber G, Okladnova O, Daniel S, Lesch KP. Enhancement of serotonin transporter function by tumor necrosis factor alpha but not by interleukin-6. Neurochem Int. 1998;33:251–254. doi: 10.1016/s0197-0186(98)00026-6. [DOI] [PubMed] [Google Scholar]
- 59.Mössner R, Daniel S, Schmitt A, Albert D, Lesch KP. Modulation of serotonin transporter function by interleukin-4. Life Sci. 2001;68:873–880. doi: 10.1016/s0024-3205(00)00992-9. [DOI] [PubMed] [Google Scholar]
- 60.Pandey GN, Rizavi HS, Ren X, Fareed J, Hoppensteadt DA, Roberts RC, Conley RR, Dwivedi Y. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J Psychiatr Res. 2012;46:57–63. doi: 10.1016/j.jpsychires.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K, Schnabel A, Möller HJ, Chen HH, Postolache TT. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr Scand. 2008;117:198–206. doi: 10.1111/j.1600-0447.2007.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 63.Calder PC. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(suppl):1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 64.Huan M, Hamazaki K, Sun Y, Itomura M, Liu H, Kang W, Watanabe S, Terasawa K, Hamazaki T. Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biol Psychiatry. 2004;56:490–496. doi: 10.1016/j.biopsych.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 65.Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163:1100–1102. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- 66.Lewis MD, Hibbeln JR, Johnson JE, Lin YH, Hyun DY, Loewke JD. Suicide deaths of active-duty US military and omega-3 fatty-acid status: a case-control comparison. J Clin Psychiatry. 2011;72:1585–1590. doi: 10.4088/JCP.11m06879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Roberts RC, Conley RR, Pandey GN. Fatty acid composition of the postmortem prefrontal cortex of adolescent male and female suicide victims. Prostaglandins Leukot Essent Fatty Acids. 2009;80:19–26. doi: 10.1016/j.plefa.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 68.Lalovic A, Levy E, Canetti L, Sequeira A, Montoudis A, Turecki G. Fatty acid composition in postmortem brains of people who completed suicide. J Psychiatry Neurosci. 2007;32:363–370. [PMC free article] [PubMed] [Google Scholar]
- 69.Poudel-Tandukar K, Nanri A, Iwasaki M, Mizoue T, Matsushita Y, Takahashi Y, Noda M, Inoue M, Tsugane S, Japan Public Health Center-based Prospective Study Group Long chain n-3 fatty acids intake, fish consumption, and suicide in a cohort of Japanese men and women: the Japan Public Health Center-based (JPHC) prospective study. J Affect Disord. 2011;129:282–288. doi: 10.1016/j.jad.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 70.Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:729–741. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- 71.Hishimoto A, Cui H, Mouri K, Nushida H, Ueno Y, Maeda K, Shirakawa O. A functional polymorphism of the m-opioid receptor gene is associated with completed suicides. J Neural Transm. 2008;115:531–536. doi: 10.1007/s00702-007-0853-y. [DOI] [PubMed] [Google Scholar]
- 72.Gabilondo AM, Meana JJ, García-Sevilla JA. Increased density of mu-opioid receptors in the postmortem brain of suicide victims. Brain Res. 1995;682:245–250. doi: 10.1016/0006-8993(95)00333-l. [DOI] [PubMed] [Google Scholar]
- 73.González-Maeso J, Rodríguez-Puertas R, Meana JJ, García-Sevilla JA, Guimón J. Neurotransmitter receptor-mediated activation ofG-proteins in brains of suicide victims with mood disorders: selective supersensitivity of alpha(2A)-adrenoceptors. Mol Psychiatry. 2002;7:755–767. doi: 10.1038/sj.mp.4001067. [DOI] [PubMed] [Google Scholar]
- 74.Zalsman G, Molcho A, Huang Y, Dwork A, Li S, Mann JJ. Post-mortem mu-opioid receptor binding in suicide victims and controls. J Neural Transm. 2005;112:949–954. doi: 10.1007/s00702-004-0239-3. [DOI] [PubMed] [Google Scholar]
- 75.Gross-Isseroff R, Dillon KA, Israeli M, Biegon A. Regionally selective increases in mu opioid receptor density in the brains of suicide victims. Brain Res. 1990;530:312–316. doi: 10.1016/0006-8993(90)91301-v. [DOI] [PubMed] [Google Scholar]
- 76.Escribá PV, Ozaita A, García-Sevilla JA. Increased mRNA expression of alpha2A-adrenoceptors, serotonin receptors, and mu-opioid receptors in the brains of suicide victims. Neuropsychopharmacology. 2004;29:1512–1521. doi: 10.1038/sj.npp.1300459. [DOI] [PubMed] [Google Scholar]
- 77.Hurd YL, Herman MM, Hyde TM, Bigelow LB, Weinberger DR, Kleinman JE. Prodynorphin mRNA expression is increased in the patch vs matrix compartment of the caudate nucleus in suicide subjects. Mol Psychiatry. 1997;2:495–500. doi: 10.1038/sj.mp.4000319. [DOI] [PubMed] [Google Scholar]
- 78.Scarone S, Gambini O, Calabrese G, Sacerdote P, Bruni M, Carucci M, Panerai AE. Asymmetrical distribution of beta-endorphin in cerebral hemispheres of suicides: preliminary data. Psychiatry Res. 1990;32:159–166. doi: 10.1016/0165-1781(90)90082-g. [DOI] [PubMed] [Google Scholar]
- 79.Frazer A, Hensler JG. In: Serotonin receptors, in Basic Neurochemistry: Molecular, Cellular, and Medical Aspects. Siegel GJ, Agranoff BW, Albers RW, Price DL, editors. Lippincott-Raven; Philadelphia: 1999. [Google Scholar]
- 80.Fitzpatrick PF. Tetrahydropterin-dependent amino acid hydroxylases. Annu Rev Biochem. 1999;68:355–381. doi: 10.1146/annurev.biochem.68.1.355. [DOI] [PubMed] [Google Scholar]
- 81.Martínez A, Knappskog PM, Haavik J. A structural approach into human tryptophan hydroxylase and its implications for the regulation of serotonin biosynthesis. Curr Med Chem. 2001;8:1077–1091. doi: 10.2174/0929867013372616. [DOI] [PubMed] [Google Scholar]
- 82.Walther DJ, Peter JU, Bashammakh S, Hörtnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 83.Dubé F, Amireault P. Local serotonergic signaling in mammalian follicles, oocytes, and early embryos. Life Sci. 2007;81:1627–1637. doi: 10.1016/j.lfs.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 84.Perroud N, Neidhart E, Petit B, Vessaz M, Laforge T, Relecom C, La Harpe R, Malafosse A, Guipponi M. Simultaneous analysis of serotonin transporter, tryptophan hydroxylase 1 and 2 gene expression in the ventral prefrontal cortex of suicide victims. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:909–918. doi: 10.1002/ajmg.b.31059. [DOI] [PubMed] [Google Scholar]
- 85.Bach-Mizrachi H, Underwood MD, Kassir SA, Bakalian MJ, Sibille E, Tamir H, Mann JJ, Arango V. Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: major depression and suicide. Neuropsychopharmacology. 2006;31:814–824. doi: 10.1038/sj.npp.1300897. [DOI] [PubMed] [Google Scholar]
- 86.Boldrini M, Underwood MD, Mann JJ, Arango V. More tryptophan hydroxylase in the brainstem dorsal raphe nucleus in depressed suicides. Brain Res. 2005;1041:19–28. doi: 10.1016/j.brainres.2005.01.083. [DOI] [PubMed] [Google Scholar]
- 87.De Luca V, Likhodi O, Van Tol HH, Kennedy JL, Wong AH. Gene expression of tryptophan hydroxylase 2 in post-mortem brain of suicide subjects. Int J Neuropsychopharmacol. 2006;9:21–25. doi: 10.1017/S1461145705005572. [DOI] [PubMed] [Google Scholar]
- 88.Bach-Mizrachi H, Underwood MD, Tin A, Ellis SP, Mann JJ, Arango V. Elevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol Psychiatry. 2008;13:507–513. doi: 10.1038/sj.mp.4002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matthews PR, Harrison PJ. A morphometric, immunohistochemical, and in situ hybridization study of the dorsal raphe nucleus in major depression, bipolar disorder, schizophrenia, and suicide. J Affect Disord. 2012;137:125–134. doi: 10.1016/j.jad.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bach H, Huang YY, Underwood MD, Dwork AJ, Mann JJ, Arango V. Elevated serotonin and 5-HIAA in the brainstem and lower serotonin turnover in the prefrontal cortex of suicides. Synapse. 2014;68:127–130. doi: 10.1002/syn.21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zill P, Büttner A, Eisenmenger W, Möller HJ, Bondy B, Ackenheil M. Single nucleotide polymorphism and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene in suicide victims. Biol Psychiatry. 2004;56:581–586. doi: 10.1016/j.biopsych.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 92.Lopez de Lara C, Brezo J, Rouleau G, Lesage A, Dumont M, Alda M, Benkelfat C, Turecki G. Effect of tryptophan hydroxylase-2 gene variants on suicide risk in major depression. Biol Psychiatry. 2007;62:72–80. doi: 10.1016/j.biopsych.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 93.Stefulj J, Mokrovic G, Hranilovic D, Bordukalo-Niksic T, Bakula M, Kubat M, Jernej B. Functional promoter polymorphism of the neuronal isoform of tryptophan hydroxylase (Tph2) in suicide. Psychiatry Res. 2011;186:446–447. doi: 10.1016/j.psychres.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 94.Mouri K, Hishimoto A, Fukutake M, Shiroiwa K, Asano M, Nagasaki Y, Ueno Y, Shirakawa O, Nishiguchi N, Maeda K. TPH2 is not a susceptibility gene for suicide in Japanese population. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1546–1550. doi: 10.1016/j.pnpbp.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 95.Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- 96.Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry. 2000;57:729–738. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- 97.Stanley M, Virgilio J, Gershon S. Tritiated imipramine binding sites are decreased in the frontal cortex of suicides. Science. 1982;216:1337–1339. doi: 10.1126/science.7079769. [DOI] [PubMed] [Google Scholar]
- 98.Rosel P, Arranz B, Vallejo J, Oros M, Menchón JM, Alvarez P, Navarro MA. High affinity [3H]imipramine and [3H]paroxetine binding sites in suicide brains. J Neural Transm. 1997;104:921–929. doi: 10.1007/BF01285560. [DOI] [PubMed] [Google Scholar]
- 99.Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Chen JJ, Mann JJ. Serotonin 1A receptors, serotonin transporter binding, and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- 100.Gross-Isseroff R, Israeli M, Biegon A. Autoradiographic analysis of tritiated imipramine binding in the human brain post mortem: effects of suicide. Arch Gen Psychiatry. 1989;46:237–241. doi: 10.1001/archpsyc.1989.01810030043006. [DOI] [PubMed] [Google Scholar]
- 101.Lopez de Lara C, Dumais A, Rouleau G, Lesage A, Dumont M, Chawky N, Alda M, Benkelfat C, Turecki G. STin2 variant and family history of suicide as significant predictors of suicide completion in major depression. Biol Psychiatry. 2006;59:114–120. doi: 10.1016/j.biopsych.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 102.Buttenschøn HN, Flint TJ, Foldager L, Qin P, Christoffersen S, Hansen NF, Kristensen IB, Mortensen PB, Børglum AD, Mors O. An association study of suicide and candidate genes in the serotonergic system. J Affect Disord. 2013;148:291–298. doi: 10.1016/j.jad.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 103.Jernej B, Stefulj J, Hranilovic D, Balija M, Skavic J, Kubat M. Intronic polymorphism of tryptophan hydroxylase and serotonin transporter: indication for combined effect in pre-disposition to suicide. J Neural Transm. 2004;111:733–738. doi: 10.1007/s00702-003-0114-7. [DOI] [PubMed] [Google Scholar]
- 104.Zupanc T, Pregelj P, Tomori M, Komel R, Paska AV. No association between polymorphisms in four serotonin receptor genes, serotonin transporter gene, and alcohol-related suicide. Psychiatr Danub. 2010;22:522–527. [PubMed] [Google Scholar]
- 105.Hranilovic D, Stefulj J, Furac I, Kubat M, Balija M, Jernej B. Serotonin transporter gene promoter (5-HTTLPR) and intron 2 (VNTR) polymorphisms in Croatian suicide victims. Biol Psychiatry. 2003;54:884–889. doi: 10.1016/s0006-3223(03)00179-3. [DOI] [PubMed] [Google Scholar]
- 106.Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression: postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boldrini M, Underwood MD, Mann JJ, Arango V. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J Psychiatr Res. 2008;42:433–442. doi: 10.1016/j.jpsychires.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Underwood MD, Kassir SA, Bakalian MJ, Galfalvy H, Mann JJ, Arango V. Neuron density and serotonin receptor binding in prefrontal cortex in suicide. Int J Neuropsychopharmacol. 2012;15:435–447. doi: 10.1017/S1461145711000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arora RC, Meltzer HY. Serotonergic measures in the brains of suicide victims: 5-HT2 binding sites in the frontal cortex of suicide victims and control subjects. Am J Psychiatry. 1989;146:730–736. doi: 10.1176/ajp.146.6.730. [DOI] [PubMed] [Google Scholar]
- 110.Stanley M, Mann JJ. Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet. 1983;1:214–216. doi: 10.1016/s0140-6736(83)92590-4. [DOI] [PubMed] [Google Scholar]
- 111.Turecki G, Brière R, Dewar K, Antonetti T, Lesage AD, Séguin M, Chawky N, Vanier C, Alda M, Joober R, Benkelfat C, Rouleau GA. Prediction of level of serotonin 2A receptor binding by serotonin receptor 2A genetic variation in postmortem brain samples from subjects who did or did not commit suicide. Am J Psychiatry. 1999;156:1456–1458. doi: 10.1176/ajp.156.9.1456. [DOI] [PubMed] [Google Scholar]
- 112.Sequeira A, Morgan L, Walsh DM, Cartagena PM, Choudary P, Li J, Schatzberg AF, Watson SJ, Akil H, Myers RM, Jones EG, Bunney WE, Vawter MP. Gene expression changes in the prefrontal cortex, anterior cingulate cortex, and nucleus accumbens of mood disorders subjects that committed suicide. PLoS ONE. 2012;7:e35367. doi: 10.1371/journal.pone.0035367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Faludi G, Sarosi A, Palkovits M. Regional distribution and relative abundance of serotonin(2c) receptors in human brain: effect of suicide. Neurochem Res. 2006;31:167–176. doi: 10.1007/s11064-005-9006-6. [DOI] [PubMed] [Google Scholar]
- 114.Zouk H, McGirr A, Lebel V, Benkelfat C, Rouleau G, Turecki G. The effect of genetic variation of the serotonin 1B receptor gene on impulsive aggressive behavior and suicide. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:996–1002. doi: 10.1002/ajmg.b.30521. [DOI] [PubMed] [Google Scholar]
- 115.Azenha D, Alves M, Matos R, Santa JF, Silva B, Cordeiro C, Vieira DN, Ambrósio AM. Male specific association between the 5-HTR6 gene 267C/T SNP and suicide in the Portuguese population. Neurosci Lett. 2009;466:128–130. doi: 10.1016/j.neulet.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 116.Videtic A, Peternelj TT, Zupanc T, Balazic J, Komel R. Promoter and functional polymorphisms of HTR2C and suicide victims. Genes Brain Behav. 2009;8:541–545. doi: 10.1111/j.1601-183X.2009.00505.x. [DOI] [PubMed] [Google Scholar]
- 117.Turecki G, Sequeira A, Gingras Y, Séguin M, Lesage A, Tousignant M, Chawky N, Vanier C, Lipp O, Benkelfat C, Rouleau GA. Suicide and serotonin: study of variation at seven serotonin receptor genes in suicide completers. Am J Med Genet B Neuropsychiatr Genet. 2003;118B:36–40. doi: 10.1002/ajmg.b.10006. [DOI] [PubMed] [Google Scholar]
- 118.Crawford J, Sutherland GR, Goldney RD. No evidence for association of 5-HT2A receptor polymorphism with suicide. Am J Med Genet. 2000;96:879–880. doi: 10.1002/1096-8628(20001204)96:6<879::aid-ajmg39>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 119.Ono H, Shirakawa O, Nishiguchi N, Nishimura A, Nushida H, Ueno Y, Maeda K. Serotonin 2A receptor gene polymorphism is not associated with completed suicide. J Psychiatr Res. 2001;35:173–176. doi: 10.1016/s0022-3956(01)00015-2. [DOI] [PubMed] [Google Scholar]
- 120.Galfalvy H, Zalsman G, Huang YY, Murphy L, Rosoklija G, Dwork AJ, Haghighi F, Arango V, Mann JJ. A pilot genome wide association and gene expression array study of suicide with and without major depression. World J Biol Psychiatry. 2013;14:574–582. doi: 10.3109/15622975.2011.597875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jokinen J, Nordström AL, Nordström P. Cerebrospinal fluid monoamine metabolites and suicide. Nord J Psychiatry. 2009;63:276–279. doi: 10.1080/08039480802571077. [DOI] [PubMed] [Google Scholar]
- 122.Asberg M, Träskman L, Thorén P. 5-HIAA in the cerebrospinal fluid. A biochemical suicide predictor? Arch Gen Psychiatry. 1976;33:1193–1197. doi: 10.1001/archpsyc.1976.01770100055005. [DOI] [PubMed] [Google Scholar]
- 123.Träskman L, Asberg M, Bertilsson L, Sjöstrand L. Monoamine metabolites in CSF and suicidal behavior. Arch Gen Psychiatry. 1981;38:631–636. doi: 10.1001/archpsyc.1981.01780310031002. [DOI] [PubMed] [Google Scholar]
- 124.Jokinen J, Nordström AL, Nordström P. CSF 5-HIAA and DST non-suppression: orthogonal biologic risk factors for suicide in male mood disorder inpatients. Psychiatry Res. 2009;165:96–102. doi: 10.1016/j.psychres.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 125.Owen F, Chambers DR, Cooper SJ, Crow TJ, Johnson JA, Lofthouse R, Poulter M. Serotonergic mechanisms in brains of suicide victims. Brain Res. 1986;362:185–188. doi: 10.1016/0006-8993(86)91415-0. [DOI] [PubMed] [Google Scholar]
- 126.Cheetham SC, Crompton MR, Czudek C, Horton RW, Katona CL, Reynolds GP. Serotonin concentrations and turnover in brains of depressed suicides. Brain Res. 1989;502:332–340. doi: 10.1016/0006-8993(89)90629-x. [DOI] [PubMed] [Google Scholar]
- 127.Nagatsu T. Genes for human catecholamine-synthesizing enzymes. Neurosci Res. 1991;12:315–345. doi: 10.1016/0168-0102(91)90001-f. [DOI] [PubMed] [Google Scholar]
- 128.Sokoloff P, Schwartz JC. Novel dopamine receptors half a decade later. Trends Pharmacol Sci. 1995;16:270–275. doi: 10.1016/s0165-6147(00)89044-6. [DOI] [PubMed] [Google Scholar]
- 129.Pitchot W, Reggers J, Pinto E, Hansenne M, Fuchs S, Pirard S, Ansseau M. Reduced dopaminergic activity in depressed suicides. Psychoneuroendocrinology. 2001;26:331–335. doi: 10.1016/s0306-4530(00)00047-0. [DOI] [PubMed] [Google Scholar]
- 130.Schroeder C, Tank J, Goldstein DS, Stoeter M, Haertter S, Luft FC, Jordan J. Influence of St John’s wort on catecholamine turnover and cardiovascular regulation in humans. Clin Pharmacol Ther. 2004;76:480–489. doi: 10.1016/j.clpt.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 131.Allard P, Norlén M. Unchanged density of caudate nucleus dopamine uptake sites in depressed suicide victims. J Neural Transm. 1997;104:1353–1360. doi: 10.1007/BF01294736. [DOI] [PubMed] [Google Scholar]
- 132.Allard P, Norlén M. Caudate nucleus dopamine D(2) receptors in depressed suicide victims. Neuropsychobiology. 2001;44:70–73. doi: 10.1159/000054918. [DOI] [PubMed] [Google Scholar]
- 133.Bowden C, Theodorou AE, Cheetham SC, Lowther S, Katona CL, Crompton MR, Horton RW. Dopamine D1 and D2 receptor binding sites in brain samples from depressed suicides and controls. Brain Res. 1997;752:227–233. doi: 10.1016/s0006-8993(96)01460-6. [DOI] [PubMed] [Google Scholar]
- 134.Ohmori T, Arora RC, Meltzer HY. Serotonergic measures in suicide brain: the concentration of 5-HIAA, HVA, and tryptophan in frontal cortex of suicide victims. Biol Psychiatry. 1992;32:57–71. doi: 10.1016/0006-3223(92)90142-m. [DOI] [PubMed] [Google Scholar]
- 135.Bowden C, Cheetham SC, Lowther S, Katona CL, Crompton MR, Horton RW. Reduced dopamine turnover in the basal ganglia of depressed suicides. Brain Res. 1997;769:135–140. doi: 10.1016/s0006-8993(97)00692-6. [DOI] [PubMed] [Google Scholar]
- 136.Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H. GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol. 2002;213:1–47. doi: 10.1016/s0074-7696(02)13011-7. [DOI] [PubMed] [Google Scholar]
- 137.Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 138.Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ. International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- 139.Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, Rehal S, Klempan T, Gratton A, Benkelfat C, Rouleau GA, Mechawar N, Turecki G. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS ONE. 2009;4:e6585. doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Poulter MO, Du L, Zhurov V, Palkovits M, Faludi G, Merali Z, Anisman H. Altered Organization of GABA(A) Receptor mRNA Expression in the Depressed Suicide Brain. Front Mol Neurosci. 2010;3:3. doi: 10.3389/neuro.02.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhu H, Karolewicz B, Nail E, Stockmeier CA, Szebeni K, Ordway GA. Normal [3H]flunitrazepam binding to GABAA receptors in the locus coeruleus in major depression and suicide. Brain Res. 2006;1125:138–146. doi: 10.1016/j.brainres.2006.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Poulter MO, Du L, Weaver IC, Palkovits M, Faludi G, Merali Z, Szyf M, Anisman H. GABAA receptor promoter hypermethylation in suicide brain: implications for the involvement of epigenetic processes. Biol Psychiatry. 2008;64:645–652. doi: 10.1016/j.biopsych.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 143.Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, ffrench-Mullen J, Turecki G. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatry. 2009;14:175–189. doi: 10.1038/sj.mp.4002110. [DOI] [PubMed] [Google Scholar]
- 144.Cross JA, Cheetham SC, Crompton MR, Katona CL, Horton RW. Brain GABAB binding sites in depressed suicide victims. Psychiatry Res. 1988;26:119–129. doi: 10.1016/0165-1781(88)90066-2. [DOI] [PubMed] [Google Scholar]
- 145.Tamminga CA. Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol. 1998;12:21–36. doi: 10.1615/critrevneurobiol.v12.i1-2.20. [DOI] [PubMed] [Google Scholar]
- 146.Korpi ER, Kleinman JE, Wyatt RJ. GABA concentrations in forebrain areas of suicide victims. Biol Psychiatry. 1988;23:109–114. doi: 10.1016/0006-3223(88)90079-0. [DOI] [PubMed] [Google Scholar]
- 147.Noga JT, Hyde TM, Herman MM, Spurney CF, Bigelow LB, Weinberger DR, Kleinman JE. Glutamate receptors in the postmortem striatum of schizophrenic, suicide, and control brains. Synapse. 1997;27:168–176. doi: 10.1002/(SICI)1098-2396(199711)27:3<168::AID-SYN2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 148.Palmer AM, Burns MA, Arango V, Mann JJ. Similar effects of glycine, zinc, and an oxidizing agent on [3H]dizocilpine binding to the N-methyl-d-aspartate receptor in neocortical tissue from suicide victims and controls. J Neural Transm. 1994;96:1–8. doi: 10.1007/BF01277923. [DOI] [PubMed] [Google Scholar]
- 149.Freed WJ, Dillon-Carter O, Kleinman JE. Properties of [3H]AMPA binding in postmortem human brain from psychotic subjects and controls: increases in caudate nucleus associated with suicide. Exp Neurol. 1993;121:48–56. doi: 10.1006/exnr.1993.1070. [DOI] [PubMed] [Google Scholar]
- 150.Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, Myers RM, Bunney WE, Jr, Akil H, Watson SJ, Jones EG. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci USA. 2005;102:15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nowak G, Ordway GA, Paul IA. Alterations in the N-methyl-d-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. 1995;675:157–164. doi: 10.1016/0006-8993(95)00057-w. [DOI] [PubMed] [Google Scholar]
- 152.Holemans S, De Paermentier F, Horton RW, Crompton MR, Katona CL, Maloteaux JM. NMDA glutamatergic receptors, labelled with [3H]MK-801, in brain samples from drug-free depressed suicides. Brain Res. 1993;616:138–143. doi: 10.1016/0006-8993(93)90202-x. [DOI] [PubMed] [Google Scholar]
- 153.Thoenen H. Neurotrophins and activity-dependent plasticity. Prog Brain Res. 2000;128:183–191. doi: 10.1016/S0079-6123(00)28016-3. [DOI] [PubMed] [Google Scholar]
- 154.Cooper JD, Skepper JN, Berzaghi MD, Lindholm D, Sofroniew MV. Delayed death of septal cholinergic neurons after excitotoxic ablation of hippocampal neurons during early postnatal development in the rat. Exp Neurol. 1996;139:143–155. doi: 10.1006/exnr.1996.0089. [DOI] [PubMed] [Google Scholar]
- 155.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 156.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 157.Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol. 2010;70:289–297. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- 158.Zarate C, Jr, Machado-Vieira R, Henter I, Ibrahim L, Diazgranados N, Salvadore G. Glutamatergic modulators: the future of treating mood disorders? Harv Rev Psychiatry. 2010;18:293–303. doi: 10.3109/10673229.2010.511059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rajkowska G. Morphometric methods for studying the prefrontal cortex in suicide victims and psychiatric patients. Ann N Y Acad Sci. 1997;836:253–268. doi: 10.1111/j.1749-6632.1997.tb52364.x. [DOI] [PubMed] [Google Scholar]
- 160.Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, Arango V, John Mann J. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology. 2013;38:1068–1077. doi: 10.1038/npp.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Altshuler LL, Casanova MF, Goldberg TE, Kleinman JE. The hippocampus and parahippocampus in schizophrenia, suicide, and control brains. Arch Gen Psychiatry. 1990;47:1029–1034. doi: 10.1001/archpsyc.1990.01810230045008. [DOI] [PubMed] [Google Scholar]
- 162.Pandey GN, Ren X, Rizavi HS, Conley RR, Roberts RC, Dwivedi Y. Brain-derived neurotrophic factor and tyrosine kinase B receptor signalling in post-mortem brain of teenage suicide victims. Int J Neuropsychopharmacol. 2008;11:1047–1061. doi: 10.1017/S1461145708009000. [DOI] [PubMed] [Google Scholar]
- 163.Maussion G, Yang J, Yerko V, Barker P, Mechawar N, Ernst C, Turecki G. Regulation of a truncated form of tropomyosin-related kinase B (TrkB) by Hsa-miR-185* in frontal cortex of suicide completers. PLoS ONE. 2012;7:e39301. doi: 10.1371/journal.pone.0039301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 165.Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 2005;136:29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 166.Dwivedi Y, Rizavi HS, Zhang H, Mondal AC, Roberts RC, Conley RR, Pandey GN. Neurotrophin receptor activation and expression in human postmortem brain: effect of suicide. Biol Psychiatry. 2009;65:319–328. doi: 10.1016/j.biopsych.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dwivedi Y, Mondal AC, Rizavi HS, Conley RR. Suicide brain is associated with decreased expression of neurotrophins. Biol Psychiatry. 2005;58:315–324. doi: 10.1016/j.biopsych.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 168.Pregelj P, Nedic G, Paska AV, Zupanc T, Nikolac M, Balažic J, Tomori M, Komel R, Seler DM, Pivac N. The association between brain-derived neurotrophic factor polymorphism (BDNF Val66Met) and suicide. J Affect Disord. 2011;128:287–290. doi: 10.1016/j.jad.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 169.Keller S, Sarchiapone M, Zarrilli F, Tomaiuolo R, Carli V, Angrisano T, Videtic A, Amato F, Pero R, di Giannantonio M, Iosue M, Lembo F, Castaldo G, Chiariotti L. TrkB gene expression and DNA methylation state in Wernicke area does not associate with suicidal behavior. J Affect Disord. 2011;135:400–404. doi: 10.1016/j.jad.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 170.Keller S, Sarchiapone M, Zarrilli F, Videtic A, Ferraro A, Carli V, Sacchetti S, Lembo F, Angiolillo A, Jovanovic N, Pisanti F, Tomaiuolo R, Monticelli A, Balazic J, Roy A, Marusic A, Cocozza S, Fusco A, Bruni CB, Castaldo G, Chiariotti L. Increased BDNF promoter methylation in the Wernicke area of suicide subjects. Arch Gen Psychiatry. 2010;67:258–267. doi: 10.1001/archgenpsychiatry.2010.9. [DOI] [PubMed] [Google Scholar]
- 171.Bondy B, Erfurth A, de Jonge S, Krüger M, Meyer H. Possible association of the short allele of the serotonin transporter promoter gene polymorphism (5-HTTLPR) with violent suicide. Mol Psychiatry. 2000;5:193–195. doi: 10.1038/sj.mp.4000678. [DOI] [PubMed] [Google Scholar]
- 172.Videtic A, Zupanc T, Pregelj P, Balazic J, Tomori M, Komel R. Suicide, stress, and serotonin receptor 1A promoter polymorphism -1019C.G in Slovenian suicide victims. Eur Arch Psychiatry Clin Neurosci. 2009;259:234–238. doi: 10.1007/s00406-008-0861-4. [DOI] [PubMed] [Google Scholar]
- 173.Ratta-apha W, Hishimoto A, Mouri K, Shiroiwa K, Sasada T, Yoshida M, Okazaki S, Supriyanto I, Asano M, Ueno Y, Shirakawa O, Sora I. Haplotype analysis of the DISC1 Ser704Cys variant in Japanese suicide completers. Psychiatry Res. 2014;215:249–251. doi: 10.1016/j.psychres.2013.11.007. [DOI] [PubMed] [Google Scholar]