Abstract
Background and Purpose
We investigated the impact of focal and diffuse corticospinal tracts damage on sensory-motor disability in multiple sclerosis (MS) patients.
Methods
Twenty-five MS patients underwent 3.0 Tesla (3T) magnetic resonance imaging with diffusion tensor imaging (DTI). The Expanded Disability Status Scale (EDSS) and the Timed 25-Foot Walk test (T25FW) quantified patient physical disability. Fractional anisotropy (FA) and mean diffusivity (MD) of the corticospinal tracts, whole brain and corticospinal tracts lesion volume were also computed. Spearman rank correlation analyses measured the associations between DTI-derived metrics and other measures of disease. Partial correlation analyses between DTI and disability measures were performed and corrected for lesion volumes as appropriate.
Results
Significant associations were seen between FA of the corticospinal tracts and EDSS (r =−0.500, p =0.0011), motor-EDSS (r = −0.519, p=0.008), and T25WF (r=−0.637, p =0.001) scores and MD of the corticospinal tracts and motor-EDSS (r=0.469, p=0.018) and T25WF (r=0.428, p=0.033) scores. When correcting for lesion volumes, only the association between FA of the corticospinal tracts and EDSS (p≤0.01, r≤ −0.516) or motor-EDSS score (p=0.03, r=−0.468,) persisted.
Conclusions
DTI at 3T shows that the impact of diffuse corticospinal tracts disease on sensory-motor disability is greatly mediated by focal lesions in MS.
Keywords: Multiple sclerosis, diffusion tensor imaging, black holes, white matter damage
BACKGROUND AND PURPOSE
In patients with multiple sclerosis (MS) magnetic resonance imaging (MRI) is a powerful paraclinical tool for identifying disease and monitoring its evolution over time [1]. However, brain MRI indices derived from conventional techniques (i.e., lesion volume and atrophy) only moderately explain physical disability of MS patients [2]. To fill the gap in the knowledge of the substrate of clinical disability of MS patients, researchers have oriented their focus towards the use of non-conventional MRI techniques coupled with high field scanners such as 3.0 Tesla (3T).
Among these non-conventional MRI techniques, diffusion tensor imaging (DTI) with tractography is a unique tool for identifying white matter fibers trajectory and providing information about the integrity of specific tracts in vivo [3]. By focusing on specific white matter tracts, DTI-tractography has the unique capability to study regional white matter damage, its relationship with disease in other defined brain regions and its impact on disability. Studies performed in patients with various neurological diseases have proven that DTI may be a useful approach to investigate functional system-specific hypotheses (see references [4, 5, 6, 7, 8] as examples). In MS, topographic analysis of white matter damage has been shown to add specific information regarding the disability substrate (see references [9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27] as examples).
In the present study, we employed DTI-tractography at 3T in patients with MS to isolate and quantify focal and diffuse disease pathology of the corticospinal tracts. The relationship between DTI derived metrics of the corticospinal tracts and measures of sensory-motor disability were investigated. Previous studies have focused on investigating the relationship between isolated damage in the corticospinal tracts and motor disability in patients with MS [10, 14, 15, 16, 19, 20, 25].
With respect to these previously reported studies, our investigation carries two novel aspects. First, regional T1-hypointense lesions appearing as chronic black holes (cBHs) [28] in the corticospinal tracts were identified and their volume quantified. When significant associations between disability and corticospinal tracts disease were disclosed, the regional volumes of the cBHs lying in the corticospinal tracts were used as correcting factors in subsequent partial correlation analyses. Second, we used measures of motor and sensory disability as outcome measures, since the corticospinal tract is known to carry predominantly motor but also sensory fibers.
We aimed at assessing the relative impact of focal and diffuse corticospinal tracts disease on sensory-motor disability of MS patients. Ultimately, the study should provide insight towards the understanding of the complex interplay between focal lesions and diffuse neurodegeneration as source of disability in MS.
MATERIALS AND METHODS
Study design and subjects
The study was performed at the National Institute of Neurological Disorders and Stroke (NINDS), NIH, Bethesda, MD USA. The Institutional Research Board of the NINDS approved the study (NINDS protocol # 05-N-39) and each patient provided informed written consent prior to participation. Twenty-five MS patients [29] classified as relapsing-remitting or secondary progressive according to standard criteria [30] were consecutively enrolled. Patients were not included for the following reasons: (i) inability to provide a written consent form; (ii) contraindication(s) to undergo a 3T MRI; (iii) presence of a clinical relapse or steroid treatment within one month of the study, which could potentially bias the results.
The same day of the MRI scanning, each patient underwent a clinical assessment performed by two examiners blinded to the MRI characteristics of the patient. Physical disability was rated using the Expanded Disability Status Scale (EDSS) [31] and the Timed 25-Foot Walk test (T25FW) [32]. Sensory/motor disability was further characterized using the sensory and motor sub-scores of the EDSS. Clinical, demographic and MRI characteristics of the patients are summarized in Table 1. None of the patients was found to have active lesions, but one patient with relapsing remitting MS presented one contrast-enhancing lesion.
TABLE 1.
Demographic, clinical and MRI characteristics of the study cohort
| Relapsing remitting (n=16) | Secondary Progressive (n=9) | |
|---|---|---|
| Age | 42.06 ±6.58 | 45.56 ±10.11 |
| Sex (females/males) | 9/7 | 9/0 |
| Years of MS | 10.69 ± 5.60 | 17.44 ± 5.22 |
| EDSS | 2.25 (0–6.0) | 6.0 (1.5–7.5) |
| T25WF | 4.78 ± 2.03 | 30.41 ± 56.45 |
| EDSS-motor | 2.0 (0–4.0) | 3.0 (0–5.0) |
| EDSS-sensory | 1.5 (0–3.0) | 2.0 (1.0–3.0) |
| Brain parenchyma fraction | 0.80 ± 0.05 | 0.76 ± 0.04 |
| Lesion Volumes (cm3) | ||
| cBHs (whole brain) | 3.58 ±3.50 | 6.62 ± 3.64 |
| T2-lesions (whole brain) | 15.47 ±10.11 | 16.33 ±16.39 |
| cBHs (corticospinal tracts) | 1.10 ±1.43 | 2.38 ± 0.97 |
Values are expressed in mean ± standard deviation but for the EDSS score and sub-scores for which median (minimum – maximum value) are reported. cBHs=chronic black holes; EDSS=Expanded Disability Status Scale; MS=multiple sclerosis; MRI=magnetic resonance imaging; T25FW=Timed 25-Foot Walk test.
MRI Acquisition
MRI was performed on a 3T scanner (Signa Excite HDx, GE Healthcare, Milwaukee, WI) using an 8-channel head coil (Invivo Corp, Gainesville, FL). The following sequences were acquired in the axial plane: i) Spin echo (SE) T1-weighted MRI with 54 contiguous slices of 2.4 mm thickness, repetition time (TR) /echo time (TE) = 700 ms / 11 ms, matrix = 256 × 256, field of view (FOV) = 240 × 240 mm2, before and within 10 minutes of injection of a single dose of gadopentatedimeglumine (Magnevist, Berlex Labs, Cedar Knolls, NJ); ii) Fast SE (FSE) T2-weighted with 54 contiguous slices of 2.4 mm thickness, TR / TE = 5100 ms / 120 ms, matrix = 256 × 256, and FOV = 240 × 240 mm2; iii) T1-weighted 3D Inversion Recovery Fast Spoiled Gradient Echo (IR-FSPGR) with TR / TE = 7.5 ms / 3 ms, matrix = 256 × 256, FOV = 240 × 240 mm2, TI = 750ms, flip angle = 16°, bandwidth = 31.25kHz with 1.0 mm slice thickness; and iv) two DTI acquisitions using a single-shot, spin-echo echoplanar imaging (SS-SE-EPI) sequence with 54 contiguous slices of 2.4 mm thickness, TR / TE = 13000 ms / 76 ms, matrix = 96 × 96 (reconstructed to 256 × 256), FOV = 240 × 240 mm2, with ASSET acceleration factor of 2. The DTI acquisition consisted of 3 volumes with no diffusion gradients applied (b = 0) and 33 volumes with diffusion gradients applied in non-collinear directions (b = 1000 s/mm2). All image volumes from all sequences were registered and resampled to the quantitative DTI space using a 12-parameter affine registration algorithm [33, 34].
Image post-processing and analysis
• Whole brain lesion volume computation
Two independent observers marked hyperintense lesions in T2-weighted images and hypointense lesions meeting the definition of cBHs [28] in T1-weighted images. Lesion volume was computed using a semi-automated method in MEDx [35]. Two investigators counted contrast-enhancing lesions. Lesion volume was not computed.
• Brain Parenchymal Fraction computation
The 3D-IR-FSPGR images were used to automatically calculate brain parenchymal fraction using the (structural image evaluation, using normalization, of atrophy (SIENAx) [36] software by one investigator.
• DTI Processing and Fiber Tracking
After correction for movement and EPI induced distortion artifact [34, 37], image data were inspected for any apparent artifacts. Then, the diffusion tensor was calculated on a voxelwise basis and decomposed into eigenvalues and eigenvectors using multivariate fitting. Mean Diffusivity (MD) and Fractional Anisotropy (FA) maps were then produced in DTI-Studio software version 6_9 (Johns Hopkins University, Baltimore, MD, USA) [38].
The FA and primary eigenvector maps were used to obtain 3D reconstruction of the corticospinal tracts. Fiber tracking was performed by a single investigator using the fiber assignment and continuous tractography (FACT) method, [38, 39] implemented in DTI-Studio. Briefly, every voxel in the brain is used as a seed point for fiber tracking. For each voxel, a vector is propagated in the direction of the primary eigenvector of the DT, and the process is repeated iteratively. Fiber-tracking was initiated at an FA value = 0.2 and terminated at an FA value < 0.2 or when the angle between 2 adjacent eigenvectors was >40 degrees [40].
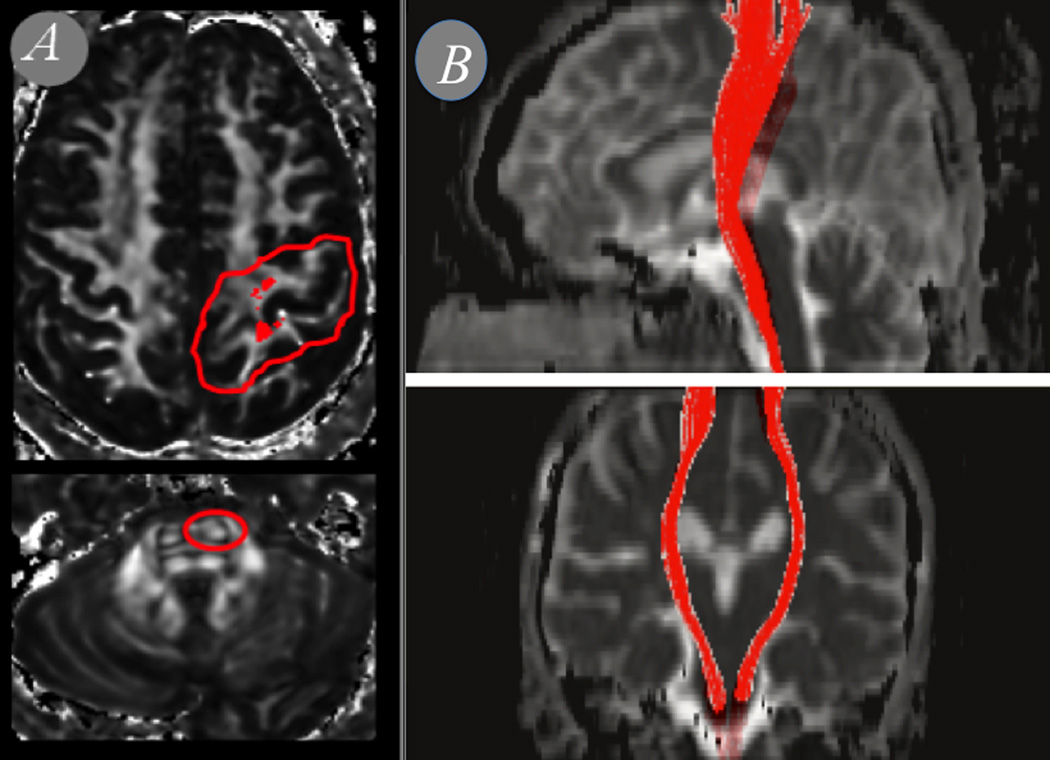
In order to reconstruct the trajectory of the right and left corticospinal tracts for each subject, we selected two primary regions of interest (ROIs) on axial slices as seen on the FA-and color coded-FA maps, based on multi-ROI approach described previously [40]. First, an ROI in the bundle of fibers that runs in the rostrocaudal axis in the anterior pons (anterior to the transverse fibers of the pons); and second, a large ROI encompassing the ipsilateral pre- and postcentral gyri. We included all reconstructed fibers that passed through both ROIs, truncated the fibers at the two ROIs, and excluded fibers that fell outside the main body of the tracts (Figure 1). FA and MD derived from the DTI acquisitions.
Figure 1. ROI placement and reconstruction of the corticospinal tracts.
(a) Primary ROIs on axial slices as seen on the DTI maps and (b) reconstructed fibers that passed through both ROIs.
• Corticospinal tracts lesion (cBHs) volume computation
For each patient, the T1-weighted images were registered to the first (b = 0) image and the corresponded FA map using a 12-parameter affine registration algorithm [34]. cBHs were marked by one observer, and a mask for each lesion was created in MEDx [35]. For each subject, lesion masks were loaded to the corresponded FA map and, based on the anatomical tracts based atlas [36], only the cBH lesion volume of the corticospinal tracts was computed using a semi-automated local thresholding method in MEDx [35]. All lesion masks were visually inspected to confirm their locations, to ensure that partial volume effects were minimized and that each lesion mask contained homogeneous fiber populations of the cortical spinal tracts through examination of slices one dorsal and one caudal from the mask.
Statistical Analysis
All data are indicated in mean and standard deviation unless otherwise specified. For the purpose of the study, although presented separately, DTI data obtained from the left hemisphere were averaged with those obtained from the right hemisphere. The rationale for doing it was based upon the fact that data on physical disability and some of the remaining MRI metrics did not provide information on the lateralization of symptoms or lesions. Group-difference were tested using an unpaired t-test.
The associations between imaging and clinical measures were first assessed using a Spearman rank correlation analysis. Thereafter, partial correlation analyses using lesion volume of cBHs (both whole brain and corticospinal tracts cBHs) as controlling variables were employed to assess the relationship between DTI-derived metrics and measures of physical disability, as appropriate.
All reported p-values were based on two-tailed statistical tests, with a significance level of 0.05. The statistical analyses were performed using Statistical Package for Social Science (SPSS, Inc. Chicago, IL, USA) version 17.0.
RESULTS
Associations between DTI-derived metrics and clinical measures
In Table 2, DTI-derived indices, FA and MD, of patients are reported. Spearman rank correlation analyses disclosed associations between MD or FA and EDSS score and sub-scores, as well as the T25WF. We report details of these associations in Table 3. There were no significant associations between the mean FA and MD of the corticospinal tracts and either age or disease duration of patients (data not shown).
TABLE 2.
DTI-derived metrics of the study cohort
| Patients (n=25) |
Relapsing remitting (n=16) |
Secondary Progressive (n=9) |
P=value | |
|---|---|---|---|---|
| MD of the corticospinal tracts (mm2/sec) | ||||
| Left hemisphere | 0.83 ± 0.11 | 0.80 ± 0.12 | 0.88 ± 0.14 | NS |
| Right hemisphere | 0.83 ± 0.11 | 0.81 ± 0.13 | 0.88 ± 0.15 | NS |
| FA of the corticospinal tracts | ||||
| Left hemisphere | 0.57 ± 0.03 | 0.59 ± 0.12 | 0.55 ± 0.02 | 0.0001 |
| Right hemisphere | 0.57 ± 0.02 | 0.57 ± 0.12 | 0.55 ± 0.03 | 0.04 |
Values are expressed in mean ± standard deviation. DTI=diffusion tensor imaging; FA=fractional anisotropy; MD=mean diffusivity. P-value relates to differences between patients with relapsing remitting MS and those with secondary progressive MS.
TABLE 3.
Associations - r values (p values) - between physical disability and DTI-derived metrics
| Total-EDSS | Motor-EDSS | Sensory-EDSS | T25WF | |
|---|---|---|---|---|
| MD | NS | 0.469 (0.018) | NS | 0.428 (0.033) |
| FA | −0.500 (0.011) | −0.519 (0.008) | NS | −0.637 (0.001) |
NS: p > 0.05; EDSS=Expanded Disability Status Scale; FA=fractional anisotropy; MD=mean diffusivity; T25FW=Timed 25- Foot Walk test.
Associations between non-DTI related MRI measures and clinical measures
Spearman rank correlation analyses disclosed no significant associations between age and any of the examined non-DTI MRI variables (data not shown). Similarly, both lesion volume on T2-weighted images and brain parenchymal fraction were not significantly associated with any of the clinical variables of interest (data not shown). Conversely, both global (i.e., entire brain) and regional (i.e., in the corticospinal tracts) cBHs lesion volumes were associated with each of the disability scores used as outcome measures. These scores included: total-EDSS, motor-EDSS, sensory-EDSS and T25WF. We report details of these significant associations in Table 4.
TABLE 4.
Associations - r values (p values) - between physical disability and cBHs lesion volume
| cBHs lesion volume | ||
|---|---|---|
| Entire brain | Corticospinal tracts | |
| Total-EDSS | 0.599 (0.003) | 0.570 (0.001) |
| Motor-EDSS | 0.650 (0.001) | 0.674 (0.001) |
| Sensory-EDSS | 0.624 (0.002) | 0.683 (0.001) |
| T25WF | 0.501 (0.018) | 0.615 (0.003) |
cBHs=chronic black holes; EDSS=Expanded Disability Status Scale; T25FW=Timed 25- Foot Walk test.
Associations between DTI-derived metrics and clinical measures while controlling for cBHs lesion volume
To rule out the possibility that the significant relations seen between DTI-derived measures and physical disability could reflect the effect of the association between measures of disability and regional and global cBHs lesion volumes, partial correlation analyses were performed. In these analyses, MD and FA of the corticospinal tracts were used as independent variables, EDSS-total, EDSS-motor score and the T25WF were used as dependent variables, and lesions volume of global and regional were used as controlling variables. Upon such an analysis, only some of the associations previously disclosed persisted, which we report in Table 5.
TABLE 5.
Partial correlation analyses - r values (p values) - between scores of physical disability and DTI-derived metrics
| Total-EDSS | Motor-EDSS | Sensory-EDSS | T25WF | |
|---|---|---|---|---|
| Controlling factor: global (i.e., entire brain) cBHs lesion volume | ||||
| MD | NS | NS | NS | NS |
| FA | −0.587 (0.005) | −0.468 (p=0.03) | NS | NS |
| Controlling factor: regional (i.e., corticospinal tracts) cBHs lesion volume | ||||
| MD | NS | NS | NS | NS |
| FA | −0.516 (p=0.01) | NS | NS | NS |
NS: p > 0.05; cBHs=chronic black holes; DTI=diffusion tensor imaging; FA=fractional anisotropy; EDSS=Expanded Disability Status Scale; MD=mean diffusivity; T25FW=Timed 25- Foot Walk test.
Associations between DTI-derived metrics and standard MRI measures of disease
Several significant associations were seen among all the conventional MRI metrics. Mean FA of the corticospinal tracts was not associated with any other imaging measures of disease, but MD of the corticospinal tracts. MD of the corticospinal tracts was instead associated with both global and focal cBHs lesion volumes. We report details of the associations seen in Table 6.
TABLE 6.
Associations - r values (p values) - among imaging measures
| Corticospinal tracts disease | Global lesion volume | Brain parenchyma fraction |
|||||
|---|---|---|---|---|---|---|---|
| MD | FA | cBHs # | cBHs | T2-Lesion | |||
| Corticospinal tracts disease | MD | 0.510*(0.009) | 0.574 (0.006) | 0.617 (0.002) | 0.619 (0.001) | −.524*(0.01) | |
| FA | 0.510*(0.009) | NS | NS | NS | NS | ||
| cBHs# | 0.574 (0.006) | NS | 0.900 (<0.0001) | 0.532*(0.013) | −.506*(0.094) | ||
| Global lesion volume | cBHs | 0.617 (0.002) | NS | 0.900 (<0.0001) | 0.715 (<0.0001) | NS | |
| T2-Lesion | 0.619 (0.001) | NS | 0.532*(0.013) | 0.715 (<0.0001) | NS | ||
| Brain parenchyma fraction | −0.524 (0.01) | NS | −0.506 (0.023) | −0.506*(0.094) | NS | ||
NS: p> 0.05.
lesion volume cBHs=chronic black holes; DTI=diffusion tensor imaging; FA=fractional anisotropy.
DISCUSSION
Our study provides the novel demonstration that the relation between FA of the corticospinal tracts and patients’ physical disability depends on the presence of focal lesions encompassing the tract. In the present discussion we will first comment on the similarities between our findings and previously reported ones. We will then highlight the novel aspects of our work and conclude by addressing our study limitations and possible future directions of research.
Similarity between our findings and the ones reported by other authors
In our study, we first confirm previous authors demonstration that DTI changes in the corticospinal tracts occur and are associated with measures of motor disability in MS. By applying an automated technique to map the corticospinal tracts on brain MRIs of a cohort of 25 MS patients (similar to ours in terms of demographic and clinical characteristics), Wilson and collaborators demonstrated that in patients, FA changes were correlated to the pyramidal score of the EDSS and that such an association was greater than the one seen with the global EDSS score [10].
Several authors confirmed and expanded these initial demonstrations towards different directions of investigations later on. For instance, Reich and collaborators found that MS-induced disease in the corticospinal tracts could explain 30% to 40% of the variance in ankle and hip strength of MS patients [16]. In a small cohort of MS patients, Gorgoraptis and collaborators [19] also investigated the relationship between connectivity measure and FA of the corticospinal tracts and motor disability of MS patients. Although absolute FA values did not differ between patients and controls, likely because of the relatively small sample size (n=10), the authors showed a significant association between connectivity measure and FA of the corticospinal tracts and global EDSS score. Giorgio and collaborators [20] found that also in early relapsing remitting MS patients, changes related to minor clinical disability were localized to several specific white matter tracts, inclusive of the corticospinal tracts, and were driven by changes in radial diffusivity within both lesions and white matter regions outside lesions, appearing as normal. More recently Naismith and collaborators [25] examined the relationships between MD-derived metrics and FA of the corticospinal tracts and motor disability in a cohort of 37 patients with MS and neuromyelitis optica, known to have remote spinal cord disease. The authors found that the radial diffusivity of the corticospinal tracts was related to the 25FTW score, and similarly to our findings, they observed that both radial diffusivity and FA of the corticospinal tracts explained part of the variance of the EDSS score.
On note, Lin and collaborators [14] computed four DTI-derived indices of the pyramidal tracts of 29 relapsing remitting MS patients. These indices included FA, directionally averaged diffusion coefficient, axial and radial diffusion coefficient. The relations between clinical and imaging measures of disease were not analyzed in this study. However, the results of Lin and collaborators are certainly worth highlighting in the context of our work since the authors found that all the DTI indices derived from the normal-appearing white matter of the corticospinal tracts were significantly associated with the regional lesion volume. In that study, regional lesion volume was assessed in a different manner than in ours. Such a difference carries a potential biological and technical explanation of the inter-studies variability. First, the authors focused on hypertense lesions in T2-weighted MRI rather than cBHs. Lesions in T2-weighted images represent a wider spectrum of disease pathology than the more advanced one reflected by the presence of cBHs. Second, regional lesion volume was extrapolated by registering the whole brain lesion volume into the MNI space. The latter might have created a more selective extrapolation of the corticospinal tract, since it was based upon an averaged skeleton of common voxels rather than individualized tractography. Such a difference in lesion classification and computation may certainly explain why, while we confirm the association between lesion volume and corticospinal tracts MD, we rather failed to find any significant association between corticospinal tracts FA and lesion volumes.
Novel findings with respect to previous literature
In analyzing the relationship between corticospinal tracts disease and sensory-motor disability of MS patients, our results permitted assessing the fact that both regional and global cBHs lesion volume in part contribute to the association between DTI-derived metrics of the corticospinal tracts and sensory-motor disability of MS patients. Opposite to Lin and collaborators [14], who focused on lesions in T2-weighted MRI, we focused our analysis on cBHs. Both pathological [41] and imaging [42] studies have suggested that cBHs represent white matter lesions with a more advanced pathological process, including axonal degeneration, and seem to be correlated with MS disability better than (larger) hyperintense white matter lesions in T2-weighted MRI [43].
First, while lesion volume of both regional and global cBHs explained part of the variance of the sensory disability of our patients, none of the DTI-derived metrics indicative of corticospinal tracts disease was significantly associated with sensory impairment of patients. One may postulate several hypotheses to explain the findings. In our opinion, however, the results may depend on the fact that a relatively small amount of sensory fibers is contained in the corticospinal tract. The disease changes measured by FA and MD in this small amount of sensory fibers contained in the corticospinal tracts may be not sufficient to exert a significant effect on sensory disability.
We observed that both MD and FA of the corticospinal tracts were associated with measures of disability. However, when regional and global cBHs lesion volumes were taken into account, none of the associations between MD and measures of disability were retained. Similarly, the association between motor EDSS and FA changes in the corticospinal tracts was lost after the correction for regional cBHs lesion volume. Conversely: (1) the associations between FA of the corticospinal tracts and total EDSS were maintained when corrected for regional and global cBHs lesion volume and (2) the association between FA of the corticospinal tracts and motor EDSS score was retained when corrected for global, but again not regional cBHs lesion volume. The results are interesting and demonstrate that a topographically more stringent relation between DTI-derived metrics of the corticospinal tracts and patients’ physical disability is greatly dependent on the presence of regional cBHs.
Explaining why the disclosed findings held true of FA-measures but not MD-measures remains challenging. However several inferences may be postulated. DTI measures are related to the underling microstructure of the white matter, allowing in vivo inference about the structural organization of the tissue [3]. The specificity of the measures is complex and still limited, since several histological changes can be related to changes in DTI parameters. Nevertheless, it remains known that damage to and / or increased disorganization of white matter tissue are reflected by both increased MD and decreased FA [3]. On the other hand, previous experimental studies have suggested that MD and FA can reflect slightly different microstructural phenomena. FA is mainly related to changes in the arrangement of axonal membranes, while MD seems to reflect a more complex substrate, related to the composition of both intra- and extracellular elements of the tissue [44]. Accordingly, in our study, changes in MD were correlated to white matter focal lesions in the corticospinal tracts, while FA was not. At the same time, being FA probably is a more sensitive measure of axonal dysfunction, it is not surprising that its association with physical disability tends to persist despite correcting for cBHs lesion volume.
Despite these considerations, we believe it is noteworthy to highlight that the topographical association between motor disability and corticospinal tracts disease is in general lost when regional cBHs lesion volume is taken into account. Several inferences may be postulated to explain the findings. These inferences are not necessarily mutually exclusive. First, one may argue that the result of the motor disability is highly dependent on the amount of cBHs in the corticospinal tracts. These lesions clearly create profound fiber damage with axonal cut and interrupt its functionality. Notwithstanding the validity of this argument, one may also postulate that the presence of cBHs is responsible for wallerian degeneration that is clearly dependent on the amount (and size) of lesions. This phenomenon is in part reflected by the measurements of the DTI-derived metrics in the entire corticospinal tract and may be contributing to patient disability to some extent, even if not disclosed in our patients’ cohort.
Study limitations and conclusions
The main limitation of this study is provided by the fact that, although comparable to previous literature, a relative small sample size was examined precluding analysis of data in patients with relapsing remitting and secondary progressive MS, separately. As we present in Table 2, differences were seen in MD and FA values between the two groups of patients. However, likely because of the small number of patients included in the secondary progressive group, these differences were not always statistically significant. The type of disease process one could see by DTI may differ between the two groups of patients. Wallerian degeneration is a significant component of disease pathology in patients with secondary progressive MS, while demyelination predominates in relapsing remitting patients. Differences in these processes would be expressed in different DTI parameters, particularly in transverse vs. longitudinal eigenvalues. Clearly, given the small sample size of patients, these differences were not investigated and shall be part of future studies with larger patient cohorts.
Second, data of FA of the corticospinal tracts tissue outside lesions (i.e, appearing as normal) are not provided. Had this been done, one could further assess the relation between FA of the tract outside lesions and motor disability.
Not with standing the above limitations, we believe that our results provide a respectable demonstration of the fact that the impact that corticospinal tracts disease has in the sensory-motor disability of patients with MS is greatly mediated by regional focal disease, i.e., cBHs. Whether such an association is merely due to the lesion volume per se or to the combination of the lesion presence and the remote damage exerted by lesions in the normal surrounding tissue is an intriguing question that deserves further investigations.
Acknowledgments
The study was supported by the Intramural program of the NINDS-NIH, Bethesda, MD, USA.
Footnotes
CONFLICTS OF INTEREST
None of the authors has any conflicts with the work
REFERENCES
- 1.Tillema JM, Pirko I. Neuroradiological evaluation of demyelinating disease. Ther Adv Neurol Disord. 2013;6:249–268. doi: 10.1177/1756285613478870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkhof F. MRI in multiple sclerosis: correlation with expanded disability status scale (EDSS) Mult Scler. 995;5:283–286. doi: 10.1177/135245859900500415. [DOI] [PubMed] [Google Scholar]
- 3.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 4.O'Sullivan M, Singhal S, Charlton R, Markus HS. Diffusion tensor imaging of thalamus correlates with cognition in CADASIL without dementia. Neurology. 2004;62:702–707. doi: 10.1212/01.wnl.0000113760.72706.d2. [DOI] [PubMed] [Google Scholar]
- 5.Tovar-Moll F, Moll J, Bramati IE, de Souza AS, Andreiuolo PA, de Oliveira-Souza R. The human pyramidal syndrome Redux. Neuroreport. 2007;18:1417–1421. doi: 10.1097/WNR.0b013e3282e9a509. [DOI] [PubMed] [Google Scholar]
- 6.Tir M, Delmaire C, Besson P, Defebvre L. The value of novel MRI techniques in Parkinson-plus syndromes: Diffusion tensor imaging and anatomical connectivity studies. Rev Neurol (Paris) 2014 Mar 20; doi: 10.1016/j.neurol.2013.10.013. pii: S0035-3787(14)00754-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Bonilha L, Rorden C, Fridriksson J. Assessing the clinical effect of residual cortical disconnection after ischemic strokes. Stroke. 2014;45:988–993. doi: 10.1161/STROKEAHA.113.004137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucci M, Mandelli ML, Berman JI, et al. Quantifying diffusion MRI tractography of the corticospinal tract in brain tumors with deterministic and probabilistic methods. Neuroimage Clin. 2013;3:361–368. doi: 10.1016/j.nicl.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rovaris M, Iannucci G, Falautano M, et al. Cognitive dysfunction in patients with mildly disabling relapsing-remitting multiple sclerosis: an exploratory study with diffusion tensor MR imaging. Journal of the Neurological Sciences. 2002;195:103–109. doi: 10.1016/s0022-510x(01)00690-6. [DOI] [PubMed] [Google Scholar]
- 10.Wilson M, Tench CR, Morgan PS, Blumhardt LD. Pyramidal tract mapping by diffusion tensor magnetic resonance imaging in multiple sclerosis: improving correlations with disability. J Neurol Neurosurg Psychiatry. 2003;74:203–207. doi: 10.1136/jnnp.74.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh J, Henry RG, Genain C, et al. Mechanisms of normal appearing corpus callosum injury related to pericallosal T1 lesions in multiple sclerosis using directional diffusion tensor and 1H MRS imaging. Journal of Neurology, Neurosurgery and Psychiatry. 2004;75:1281–1286. doi: 10.1136/jnnp.2004.039032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hickman SJ, Wheeler-Kingshott CAM, Jones SJ, et al. Optic nerve diffusion measurement from diffusion-weighted imaging in optic neuritis. AJNR. American Journal of Neuroradiology. 2005;26:951–956. [PMC free article] [PubMed] [Google Scholar]
- 13.Trip SA, Wheeler-Kingshott C, Jones SJ, et al. Optic nerve diffusion tensor imaging in optic neuritis. NeuroImage. 2006;30:498–505. doi: 10.1016/j.neuroimage.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Lin F, Yu C, Jiang T, Li K, Chanc P. Diffusion tensor tractography-based group mapping of the pyramidal tract in relapsing-remitting multiple sclerosis patients. AJNR. American Journal of Neuroradiology. 2007;28:278–282. [PMC free article] [PubMed] [Google Scholar]
- 15.Reich DS, Smith SA, Zackowski KM, et al. Multiparametric magnetic resonance imaging analysis of the corticospinal tract in multiple sclerosis. NeuroImage. 2007;38:271–272. doi: 10.1016/j.neuroimage.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reich DS, Zackowski KM, Gordon-Lipkin EM. Corticospinal tract abnormalities are associated with weakness in multiple sclerosis. AJNR Am J Neuroradiol. 2008;29:333–239. doi: 10.3174/ajnr.A0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ceccarelli A, Rocca MA, Valsasina P. Structural and functional magnetic resonance imaging correlates of motor network dysfunction in primary progressive multiple sclerosis. Eur J Neurosci. 2010;31:1273–1280. doi: 10.1111/j.1460-9568.2010.07147.x. [DOI] [PubMed] [Google Scholar]
- 18.Ozturk A, Smith SA, Gordon-Lipkin EM. MRI of the corpus callosum in multiple sclerosis: association with disability. Mult Scler. 2010;16:166–177. doi: 10.1177/1352458509353649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorgoraptis N, Wheeler-Kingshott CA, Jenkins TM. Combining tractography and cortical measures to test system-specific hypotheses in multiple sclerosis. Mult Scler. 2010;16:555–565. doi: 10.1177/1352458510362440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giorgio A, Palace J, Johansen-Berg H. Relationships of brain white matter microstructure with clinical and MR measures in relapsing-remitting multiple sclerosis. J Magn Reson Imaging. 2010;31:309–316. doi: 10.1002/jmri.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu B, Ye B, Yang Y, et al. Quantitative diffusion tensor deterministic and probabilistic fiber tractography in relapsing-remitting multiple sclerosis. European Journal of Radiology. 2011;79:101–107. doi: 10.1016/j.ejrad.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Duan Y, He Y, et al. Whole brain white matter changes revealed by multiple diffusion metrics in multiple sclerosis: a TBSS study. European Journal of Radiology. 2012;81:2826–2832. doi: 10.1016/j.ejrad.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Onu M, Roceanu A, Sboto-Frankenstein U, et al. Diffusion abnormality maps in demyelinating disease: correlations with clinical scores. European Journal of Radiology. 2012;81:e386–e391. doi: 10.1016/j.ejrad.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 24.van der Walt A, Kolbe SC, Wang YE, et al. Optic nerve diffusion tensor imaging after acute optic neuritis predicts axonal and visual outcomes. PLoS One. 2013;8:e83825. doi: 10.1371/journal.pone.0083825. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naismith Xu J, Klawiter EC, et al. Spinal cord tract diffusion tensor imaging reveals disability substrate in demyelinating disease. Neurology. 2013;80:2201–2209. doi: 10.1212/WNL.0b013e318296e8f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bester M, zar M, Petracca M, Babb JS, Herbert J, Grossman RI, Inglese M. Tract-specific white matter correlates of fatigue and cognitive impairment in benign multiple sclerosis. J Neurol Sci. 2013;330:61–66. doi: 10.1016/j.jns.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pardini M, Bergamino M, Bommarito G, Bonzano L, Luigi Mancardi G, Roccatagliata L. Structural correlates of subjective and objective memory performance in multiple sclerosis. Hippocampus. 2014;24:436–445. doi: 10.1002/hipo.22237. [DOI] [PubMed] [Google Scholar]
- 28.Bagnato F, Jeffries N, Richert ND. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782–1789. doi: 10.1093/brain/awg182. [DOI] [PubMed] [Google Scholar]
- 29.Poser CM, Paty DW, Scheinberg L. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 30.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 31.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 32.Goodkin DE, Hertsgaard D, Seminary J. Upper extremity function in multiple sclerosis: improving assessment sensitivity with box-and-block and nine-hole peg tests. Arch Phys Med Rehabil. 1988;69:850–854. [PubMed] [Google Scholar]
- 33.Tovar-Moll F, Evangelou IE, Chiu AW. Thalamic involvement and its impact on clinical disability in patients with multiple sclerosis: a diffusion tensor imaging study at 3T. AJNR Am J Neuroradiol. 2009;30:1380–1386. doi: 10.3174/ajnr.A1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- 35.Gupta S, Solomon JM, Tasciyan TA. Interferon-beta-1b effects on re-enhancing lesions in patients with multiple sclerosis. Mult Scler. 2005;11:658–668. doi: 10.1191/1352458505ms1229oa. [DOI] [PubMed] [Google Scholar]
- 36.Smith SM, Zhang Y, Jenkinson M. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 37.Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34:65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- 38.Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 40.Wakana S, Caprihan A, Panzenboeck MM. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Waesberghe JH, Kamphorst W, De Groot CJ, et al. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol. 1999;46:747–754. doi: 10.1002/1531-8249(199911)46:5<747::aid-ana10>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- 42.Fisher E, Chang A, Fox RJ. Imaging correlates of axonal swelling in chronic multiple sclerosis brains. Ann Neurol. 2007;62:219–228. doi: 10.1002/ana.21113. [DOI] [PubMed] [Google Scholar]
- 43.Truyen L, Van Waesberghe JHTM, Van Walderveen MAA, et al. Accumulation of T1 hypointense lesions (“black holes”) on T1 spin-echo MRI correlates with disease progression in multiple sclerosis. Neurology. 1996;47:1469–1476. doi: 10.1212/wnl.47.6.1469. [DOI] [PubMed] [Google Scholar]
- 44.Weaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]