Abstract
Objectives
Until recently, reports of physical activity in rheumatoid arthritis (RA) were limited to self-report methods and/or leisure-time physical activity. Our objectives were to assess, determine correlates of, and compare to well-matched controls both exercise and sedentary time in a typical clinical cohort of RA.
Methods
Persons with established RA (seropositive or radiographic erosions; n=41) without diabetes or cardiovascular disease underwent assessments of traditional and disease-specific correlates of physical activity and seven days of tri-axial accelerometry. Twenty-seven age, gender, and body mass index-matched controls were assessed.
Results
For persons with RA, objectively-measured exercise time was only 3 (1, 11) min/day; only 10% (n=4) of participants exercised 30+ min/day. Median (25th, 75th %) time spent in sedentary activities was 92% (89%, 95%). Exercise time was not related to pain, but was inversely related to disease activity (r=−0.3, P<0.05) and disability (r=−0.3, P<0.05) and positively related to self-efficacy for endurance activity (r=0.4, P<0.05). Sedentary activity was related only to self-efficacy for endurance activity (r=−0.4, P<0.05). When compared to matched controls, persons with RA exhibited poorer self-efficacy for physical activity but similar amounts of exercise and sedentary time.
Conclusions
For persons with RA and without diabetes or cardiovascular disease, time spent in exercise was well below established guidelines and activity patterns were predominantly sedentary. For optimal care in RA, in addition to promoting exercise, clinicians should consider assessing sedentary behavior and self-efficacy for exercise. Future interventions might determine whether increased self-efficacy can increase physical activity in RA.
Keywords: Accelerometer, Self-efficacy, Matched control, Inactivity
Until recently, knowledge of physical activity in rheumatoid arthritis (RA) was based on questionnaires and targeted only leisure-time physical activity. However, self-reported physical activity measures show only moderate validity, are susceptible to over-reporting and rarely assess sedentary behavior, an independent predictor of mortality [1-3] As alternative tools for relating physical activity patterns to health outcomes, accelerometers objectively measure physical activity duration, frequency, intensity and sedentary activity. In this study, our aims were to 1) determine whether exercise and sedentary time as measured by an accelerometer were related to RA disease activity, disability, pain, self-efficacy and motivation for exercise, and 2) compare exercise and sedentary time to those of age, gender, race, and BMI-matched controls.
Methods
Recruited from Duke and Durham VA Rheumatology clinics, 51 participants met RA criteria,[4] had erosive or seropositive (positive rheumatoid factor or anti-cyclic citrullinated peptide) disease, and had no medication changes in the prior three months. Prednisone 5 mg/day or less was allowed. Controls were matched 1:1 by gender, race, age and BMI. Persons with diabetes and cardiovascular disease were excluded. The study was in compliance with the Helsinki Declaration of 1975, as revised in 1983, and approved by the Duke University Institutional Review Board.
Assessments included anthropometrics, joints exams, fasting blood collection, the Health Assessment Questionnaire-Disability Index (HAQ-DI)[5], a pain (prior week) visual analog scale, and a modified (eliminating arthritis) co-morbidity scale[6]. Disease Activity Scores-28 (DAS-28) were computed using the on-line calculator: http://www.das-score.nl/. The Stanford Brief Activity Survey (SBAS) classified self-reported physical activity into five categories: inactive, light, moderate, hard or very hard activity; the latter three are considered meeting US guidelines.[8]
Physical activity was monitored over seven days (waking hours) with RT3 tri-axial accelerometers(RT3, Stayhealthy, Inc., Monrovia, CA). Activity was categorized by metabolic equivalents (METs): Sedentary<1MET; 1≥Low<3METs; 3≤Moderate<5METs; 5≤High<10METs; 10≤Very high. Exercise was defined as the sum of moderate, high and very high activity. Exercise bouts were computed as numbers of periods where moderate, high and very high intensity was recorded for at least 8 of 10 consecutive minutes.[2] As previously validated in RA,[7] 90 consecutive minutes of zero activity data constituted nonwear time, which was excluded from analysis. Ten hours of data were required for a valid day. As established for assessing habitual physical activity,[2] analyses included only participants with four valid days of data (n=41); three participants did not return the device, two had device malfunctions, and five had insufficient data.
Self-efficacy and motivation for exercise were assessed with instruments previously used in older populations with arthritis.[9] Questions for self-efficacy (and motivation) for endurance and strength training were as follows: “How sure are you that you could (How much do you want to) walk or do another type of endurance exercise for 30 minutes or more on five or more days per week? The 30 minutes does not have to be done all at the same time;” and, “How sure are you that you could (How much do you want to) do exercises for 15 minutes, three days a week to make your legs stronger? The strength training exercises could be as easy as using elastic exercise bands.” Data were measured with an ordinal scale with responses from 1 to 5. Responses ‘very’ (4) or ‘extremely’ (5) [confident or motivated] were dichotomized as high self-efficacy or motivation, and ‘somewhat’ (3), ‘a little’ (2), or ‘not at all’ (1) were considered low self-efficacy or motivation.
Statistical Analyses
For each participant, daily accelerometer measures were averaged over the number of valid days. Relationships between measures were assessed using Spearman correlations. Mixed models, which accounted for the repeated measure of matched participants, were used to perform comparisons between persons with RA and controls. A P<0.05 was accepted as significant without correction for multiple tests. Power: With 27 matched pairs and at an alpha of 0.05, we had 80% power to detect a standardized difference of 0.55, typically considered a medium difference.
Results
While 41% of RA participants reported regular exercise, objectively-assessed activity was much less. The median (25th, 75th percentile) exercise time was 3 (1, 11) min/day, and only 10% (n=4) engaged in 30 or more min/day (Table 1; Figure 1A). Activity patterns were predominantly sedentary with a median of 92% (89%, 95%) of sedentary activity (Figure 1B; Table 1).
Table 1. Demographic, clinical, and accelerometer data.
| Variable | Rheumatoid Arthritis: Original Cohort (n=51) |
Rheumatoid Arthritis: Valid Accelerometer Data (n=41) |
Rheumatoid Arthritis: Valid Accelerometer and Matched to Controls (n=27) |
Matched Controls (n=27) |
|---|---|---|---|---|
| Demographic and clinical data | ||||
| Age | 55 (46,64) | 55 (48,64) | 55 (48,67) | 55 (50, 62) |
| Gender - Women (% Women) | 36 (71%) | 29 (71%) | 17(63%) | 17(63%) |
| Race – (%) | ||||
| African American | 14 (27%) | 9 (22%) | 2 (7.4%)* | 2 (7.4%) |
| Asian | 1 (2%) | 1 (2%) | 0 | 0 |
| Caucasian | 36 (71%) | 31 (76%) | 25 (93%) | 25 (93%) |
| BMI (kg/m2) | 30.6 (24.8, 35.2) | 31.1 (25.2, 35.2) | 31.1 (23.2, 34.5) | 30.9 (25.7, 33.5) |
| Disease Duration (mo.) | 80.5 (21.0, 264.0) | 80.5 (18.0, 252.0) | 80.5 (21.0, 217.5) | N/a |
| HAQ-DI | 0.50 (0, 1.3) | 0.38 (0, 1.1) | 0.38 (0, 1.0)† | 0 (0,0) |
| DAS-28-ESR median (SD) | 2.9 (2.1, 3.9) | 2.8 (1.9, 3.8) | 2.6(1.2, 3.8) | N/a |
| Remission (DAS-28 <2.6) n (%) | 19 (40%) | 17(43%) | 17(43%) | N/a |
| Low activity (DAS-28 2.6-3.2) n (%) | 8 (17%) | 6(15%) | 6(15%) | N/a |
| Moderate activity (DAS-28 3.2-5.1) n (%) | 16 (33%) | 13 (32%) | 13 (32%) | N/a |
| High activity (DAS-28 >5.1) n (%) | 5 (10%) | 4 (10%) | 4 (10%) | N/a |
| Pain (VAS 100mm) | 30 (17, 66) | 25 (17, 66) | 20 (12, 65)† | 10(3, 14) |
| ESR (mm/h) | 9(1,17) | 9(1,17) | 6.5 (1, 18) | 4(1,5) |
| Co-morbidity Index | 1(1,2) | 1(1,2) | 1(1,2)† | 0 (0, 2) |
| High Self-Efficacy for Endurance PA** | 31 (61%) | 26 (63%) | 10/15 (67%)† | 15/15 (100%) |
| High Self-Efficacy for Strengthening PA** | 36 (71%) | 28 (68%) | 10/15 (67%)† | 15/15 (100%) |
| High Motivation for Endurance PA** | 31 (61%) | 25 (61%) | 8/15 (53%) | 11/15 (73%) |
| High Motivation for Strengthening PA** | 33 (65%) | 26 (63%) | 8/15 (53%)† | 13/15 (87%) |
| Self-reported PA (SBAS)- (%) | ||||
| Inactive | 11 (22%) | 7(17%) | 3 (11%) | 2 (13%) |
| Light Intensity | 18 (35%) | 17(41%) | 10 (37%) | 4 (27%) |
| Moderate Intensity | 15 (29%) | 13 (32%) | 10 (37%) | 6 (40%) |
| Hard Intensity | 4 (8%) | 3 (7%) | 3 (11%) | 2 (13%) |
| Very Hard Intensity | 3 (6%) | 1 (2%) | 1 (4%) | 1 (7%) |
| Accelerometer Data | ||||
| Total recording time (min./day) | 918.9 (864.2, 976.3) | 918.9 (864.2, 976.3) | 931.7 (865.8, 1008.1) | |
| Daily exercise‡ (min./day) | 3.3 (1.0, 11.3) | 5.6 (1.1, 16.5) | 10.7 (3.8, 7.2) | |
| Daily exercise‡ bouts | 0 (0, 0.2) | 0 (0, 0.3) | 0 (0, 0.38) | |
| Sedentary Activity | ||||
| Daily Minutes | 854.4 (759.6, 906.9) | 837.0 (759.6, 886.7) | 829.3 (765.6, 887.0) | |
| % Total Daily Minutes | 92.1 (89.2, 95.3) | 91.4 (87.2, 94.2) | 91.1 (86.3, 93.7) | |
| Low Activity | ||||
| Minutes | 64.0 (38.7, 84.7) | 72.4 (56.3, 93.7) | 83.5 (47.5, 105.3) | |
| % Total Daily Minutes | 7.3 (4.6, 9.2) | 7.5 (5.6, 11.1) | 7.8 (5.1, 12.1) | |
| Moderate Activity | ||||
| Minutes | 3.0 (0.9, 11.0) | 5.0 (1.1, 13.3) | 8.3 (3.5, 15.6) | |
| % Total Daily Minutes | 0.3 (0.1, 1.3) | 0.5 (0.1, 1.36) | 0.8 (0.4, 1.8) | |
| High Activity | ||||
| Minutes | 0.25 (0, 0.80) | 0.3 (0, 1.1) | 1.0 (0.1, 3.3) | |
| % Total Daily Minutes | 0.03 (0, 0.09) | 0.0 (0, 0.1) | 0.1 (0.0, 0.4) | |
| Very High Activity | ||||
| Minutes | 0 (0, 0) | 0 (0, 0) | 0 (0.0, 0.1) | |
| % Total Daily Minutes | 0 (0, 0) | 0 (0, 0) | 0 (0.0, 0.0) |
P < 0.05 for nonparametric t-test comparison to original RA cohort
P < 0.05 for nonparametric t-test or Mantel-Haenszel chi-squared comparison to matched controls
1=not at all, 2 = a little, 3 = somewhat, 4= very, and 5 = extremely [confident or motivated].
Exercise = moderate + high + very high activity.
Data are reported as medians (25th and 75th percentiles for continuous variables and as number (percent total) for categorical variables. BMI=body mass index, HAQ-DI=Health assessment questionnaire-disability index, VAS=visual analog scale, DAS-28 (Disease activity score - 28 joint count), ESR=Sedimentation rate, PA=Physical Activity, SBAS=Stanford Brief Activity Survey,
Figure 1. Physical activity in rheumatoid arthritis (RA).
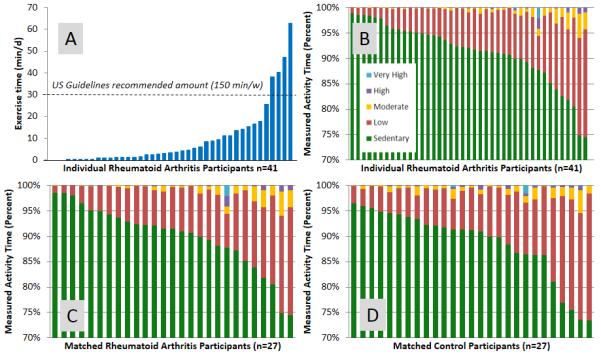
A. Patterns of physical activity for all participants with RA (n=41)
B. Time spent in exercise (moderate, high, very high intensity activity) for all participants with RA (n=41)
C. Patterns of physical activity in only age, gender, race, and body mass index matched participants with RA (n=27)
D. Patterns of physical activity in only age, gender, race, and body mass index matched participants without RA (n=27)
Persons that exercised more had less disease activity, less disability, and fewer comorbidities (R=0.3-0.4, P<0.05 for all). Also, less sedentary persons had fewer co-morbidities (R=0.3, P<0.05). Of the correlates examined, the most consistently related to physical activity was self-efficacy (Table 2). Self-efficacy for endurance training was related to more exercise time and less sedentary time (R=0.4 and −0.4; Ps<0.05). All of the individuals engaging in 30 or more min/day of exercise reported high self-efficacy.
Table 2. Relations for accelerometer-derived physical activity measures and demographic and clinical data (n=41).
| Time spent in exercise (min.) |
Time spent in sedentary activity (% min.) |
|
|---|---|---|
| Age (y) | −0.04 | −0.06 |
| Gender (Men=0; Women=1) | −0.39 | 0.37 |
| Race (Caucasian=0; NonCaucasian=1) | −0.05 | 0.05 |
| BMI (kg/m2) | −0.02 | −0.12 |
| Waist circumference (cm) | 0.08 | −0.22 |
| Biologic Use (No=0; Yes=1) | −0.04 | −0.03 |
| DMARD use (No=0; Yes=1) | −0.22 | 0.19 |
| NSAID use daily (No=0; Yes=1) | −0.22 | 0.25 |
| Prednisone use daily (No=0; Yes=1) | 0.02 | −0.11 |
| Disease duration (mo.) | −0.01 | −0.01 |
| HAQ-DI | −0.32 | 0.30 |
| Pain VAS | −0.23 | 0.25 |
| DAS-28(ESR) | −0.31 | 0.27 |
| ESR | −0.23 | 0.18 |
| Co-morbidity Index | −0.40 | 0.31 |
| End PA Self-Efficacy | 0.42 | −0.38 |
| Strength PA Self-Efficacy | 0.36 | −0.24 |
| End PA Motivation | 0.34 | −0.25 |
| Strength PA Motivation | 0.13 | 0.01 |
| Self-reported PA | 0.46 | −0.39 |
Data are presented as Spearman correlation coefficients. Bolded coefficients indicate P<0.05.
Comparison to match controls
Analyses comparing RA to controls were limited to 27 dyads with complete accelerometer data. While persons with RA reported increased amounts of disability, pain, comorbidities, and poorer self-efficacy for physical activity, (P<0.05 for all, Table 1), there were no statistically significant differences between the two groups for self-reported or accelerometer-measured physical activity (Figure 1C/D).
Discussion
This report highlights the on-going problem of physical inactivity in persons with RA, emphasizing a pattern predominated by sedentary behavior and reduced self-efficacy for exercise. Prior reports suggest that when objectively assessed, 42% of persons with RA are completely inactive, performing no exercise bouts in a week [10]; accordingly, we found 61% were inactive and only 10% met physical activity recommendations [11]. Most remarkably, sedentary behavior constituted over 90% of recorded time (~14 h/d), nearly one and a half times US older adults (~9 h/d sedentary).[12] Independent of amounts of physical activity, sedentary activity has been associated with a number of poor outcomes including mortality, diabetes, and cardiovascular events.[3] These associations suggest that reducing sedentary activity – as well as promoting exercise – should become a focus of recommendations for persons with RA.
One potential area of intervention that emerged was self-efficacy for exercise. Exercise self-efficacy was the strongest, most consistent correlate of both physical activity and sedentary behavior even above disease-related factors. While self-efficacy for exercise has been associated with physical activity [13], to our knowledge, our study is the first demonstrating an association in RA between self-efficacy for exercise and sedentary behavior. Using questions such as those described here or other validated questionnaires (ie. Self-Efficacy for Physical Activity scale [14]), routine assessment of self-efficacy for exercise might identify patients who need more intensive behavioral counselling. Evidence-based strategies include goal setting, education of physical activity benefits, and mastery experiences to increase exercise self-efficacy.
Interestingly, when matched for age, gender, race, and BMI, persons with RA self-report and demonstrate levels of physical activity similar to controls despite barriers such as increased pain, disability, and co-morbidities. A recent report using accelerometers (Actical) compared physical activity in persons with RA to gender and BMI-matched controls, but the controls were significantly younger than the persons with RA, calling into question the conclusion that persons with RA engage in more sedentary and less total physical activity than healthy people.[15]
While sample size limited our ability to detect small group differences, we had 80% power to detect significant differences as small as 10 minutes per day of exercise time and 5% of time spent in sedentary activity, thresholds below which differences are unlikely to be clinically relevant. Also, the geographical location of our study, the southeastern United States, introduced a bias towards overall lower activity levels potentially creating a “floor effect” and thereby diminishing the observed differences between the two groups. Thus, in areas of the country or communities whose residents engage in higher levels of exercise, it is possible that the observed differences in physical activity between persons with RA and controls might be larger. Additional study limitations included a sampling bias, as participants were from an academic setting with potentially increased disease burden; however, we observed a wide range of disease activity and disability. Nonetheless, it is important to recognize that the findings in this study are generalizable only over included activity ranges, co-morbidities, and demographics. For example, as noted in Table 1, as compared to the original cohort, the cohort with accelerometer data and healthy matches under-presented African-Americans. Additionally, this analysis describes relationships which are relatively small (R=0.3-0.4), such that there are likely other important, unidentified determinants of exercise and sedentary time. Similarly, this cross-sectional investigation cannot determine causality, e.g., whether lower amounts of objectively-measured physical activity contribute to, precede, or follow the progression of disability, arthritis severity, co-morbidities, or self-efficacy.
Thus, in persons with RA without diabetes or cardiovascular disease, objective assessments of physical activity emphasize a pattern of predominantly sedentary behavior and substantial deficiencies in meeting physical activity recommendations. In addition to promoting exercise, efforts to improve health in persons with RA should target improvements in self-efficacy for endurance activity as well as ways to minimize time spent in sedentary activity.
Acknowledgements
We thank the participants of this investigation as well as the clinical faculty from the Division of Rheumatology and Immunology at Duke University Medical Center who referred patients for this investigation. We appreciate helpful discussions with career award mentors, Drs. Helen Hoenig, Gregory Samsa, and Deborah Muoio. This work was supported by NIH/NIAMS K23AR054904, NIH/NIA NIH/NIA P30AG028716, and an ACR-REF/ASP Junior Career Development Award in Geriatric Medicine funded via Atlantic Philanthropies, ACR-REF, John A. Hartford Foundation and ASP.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Helmerhorst HJ, Brage S, Warren J, Besson H, Ekelund U. A systematic review of reliability and objective criterion-related validity of physical activity questionnaires. Int J Behav Nutr Phys Act. 2012;9:103. doi: 10.1186/1479-5868-9-103. doi: 10.1186/479-5868-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 3.Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55(11):2895–905. doi: 10.1007/s00125-012-2677-z. doi: 10.1007/s00125-012-2677-z. Epub 2012 Aug 14. [DOI] [PubMed] [Google Scholar]
- 4.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 5.Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: Dimensions and Practical Applications. Health Qual Life Outcomes. 2003;1(1):20. doi: 10.1186/1477-7525-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rigler SK, Studenski S, Wallace D, Reker DM, Duncan PW. Co-morbidity adjustment for functional outcomes in community-dwelling older adults. Clin Rehabil. 2002;16(4):420–8. doi: 10.1191/0269215502cr515oa. [DOI] [PubMed] [Google Scholar]
- 7.Semanik P, Song J, Chang RW, Manheim L, Ainsworth B, Dunlop D. Assessing physical activity in persons with rheumatoid arthritis using accelerometry. Med Sci Sports Exerc. 2010;42(8):1493–501. doi: 10.1249/MSS.0b013e3181cfc9da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor-Piliae RE, Norton LC, Haskell WL, Mahbouda MH, Fair JM, Iribarren C, et al. Validation of a new brief physical activity survey among men and women aged 60-69 years. Am J Epidemiol. 2006;164(6):598–606. doi: 10.1093/aje/kwj248. [DOI] [PubMed] [Google Scholar]
- 9.McAuley E. Self-efficacy and the maintenance of exercise participation in older adults. J Behav Med. 1993;16(1):103–13. doi: 10.1007/BF00844757. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Dunlop D, Ehrlich-Jones L, Semanik P, Song J, Manheim L, et al. Public health impact of risk factors for physical inactivity in adults with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64(4):488–93. doi: 10.1002/acr.21582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Services UDoHaH Physical Activity Guidelines for Americans. 2008 www.health.gov/PAGuidelines. 2008.
- 12.Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, et al. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am J Epidemiol. 2008;167(7):875–81. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demmelmaier I, Bergman P, Nordgren B, Jensen I, Opava CH. Current and maintained health-enhancing physical activity in rheumatoid arthritis: a cross-sectional study. Arthritis Care Res (Hoboken) 2013;65(7):1166–76. doi: 10.1002/acr.21951. [DOI] [PubMed] [Google Scholar]
- 14.Mielenz TJ, Kubiak-Rizzone KL, Alvarez KJ, Hlavacek PR, Freburger JK, Giuliani C, et al. Association of self-efficacy and outcome expectations with physical activity in adults with arthritis. Arthritis. 2013;2013:621396. doi: 10.1155/2013/621396. doi: 10.1155/2013/621396. Epub 2013 Oct 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prioreschi A, Hodkinson B, Avidon I, Tikly M, McVeigh JA. The clinical utility of accelerometry in patients with rheumatoid arthritis. Rheumatology (Oxford) 2013;52(9):1721–7. doi: 10.1093/rheumatology/ket216. [DOI] [PubMed] [Google Scholar]


