Abstract
Background
In tamoxifen-treated patients, breast cancer recurrence differs according to CYP2D6 genotype and endoxifen steady state concentrations (Endx Css). The 13Cdextromethorphan breath test (DM-BT), labeled with 13C at the O-CH3 moiety, measures CYP2D6 enzyme activity. We sought to examine the ability of the DM-BT to identify known CYP2D6 genotypic poor metabolizers and examine the correlation between DMBT and Endx Css.
Methods
DM-BT and tamoxifen pharmacokinetics were obtained at baseline (b), 3 month (3m) and 6 months (6m) following tamoxifen initiation. Potent CYP2D6 inhibitors were prohibited. The correlation between bDM-BT with CYP2D6 genotype and Endx Css was determined. The association between bDM-BT (where values ≤0.9 is an indicator of poor in vivo CYP2D6 metabolism) and Endx Css (using values ≤ 11.2 known to be associated with poorer recurrence free survival) was explored.
Results
91 patients were enrolled and 77 were eligible. CYP2D6 genotype was positively correlated with b, 3m and 6m DMBT (r ranging from 0.457-0. 60 p < 0.001). Both CYP2D6 genotype (r = 0.47; 0.56, p <.0001), and bDM-BT (r=0.60; 0.54; p<.001) were associated with 3m and 6m Endx Css respectively. Seven of 9 patients (78%) with low (≤11.2 nM) 3m Endx Css also had low DM-BT (≤0.9) including 2/2 CYP2D6 PM/PM and 5/5 IM/PM. In contrast, 1 of 48 pts (2%) with a low DM-BT had Endx Css > 11.2 nM.
Conclusions
In patients not taking potent CYP2D6 inhibitors, DM-BT was associated with CYP2D6 genotype and 3m and 6 m Endx Css but did not provide better discrimination of Endx Css compared to CYP2D6 genotype alone. Further studies are needed to identify additional factors which alter Endx Css.
Keywords: Tamoxifen, CYP2D6, 13C-dextromethorphan breath test (DM-BT)
Introduction
Tamoxifen, a selective estrogen receptor modulator (SERM) has been studied and utilized in breast cancer for the last forty years. When administered to women with ER-positive breast cancer for 5 years after surgery, tamoxifen almost halves the annual recurrence rate and reduces the breast cancer mortality rate by one-third in both pre- and post-menopausal women [1]. Tamoxifen is also effective as a preventive treatment for women at high risk for development of breast cancer [2].
Tamoxifen is a prodrug metabolized by the liver cytochrome P450 enzymes CYP3A and CYP2D6 to N-desmethyltamoxifen (NDMT), 4-hydroxytamoxifen (4HT) and endoxifen (Endx). Both 4HT and Endx have substantially greater anti-estrogen and anti-proliferative effects compared to tamoxifen and its primary metabolite, NDMT [3–5]. Endx results from the CYP2D6 mediated oxidation of NDMT and its steady-state plasma concentrations are 5 to 10 fold higher than 4HT [3, 6]. While Endx is similar to 4HT in its ER binding affinity and ability to suppress estradiol (E2)-stimulated cell proliferation [3], studies have demonstrated that these two SERMs result in different gene regulation [7]. In humans, the most important factor contributing to the variability in Endx Css is genetic variation in CYP2D6, the rate limiting enzyme responsible for the formation of this metabolite. Tamoxifen-treated women with CY2D6 genetic variants associated with reduced or absent CYP2D6 activity or who concomitantly take medications which inhibit CYP2D6 activity have significantly lower concentrations of Endx [3, 6].
While the published data demonstrate a consistent relationship between CYP2D6 enzyme activity and Endx concentrations, there has been controversy with regard to the association between CYP2D6 genotype and/or Endx Css and breast cancer recurrence (reviewed in [8]). Given CYP2D6 genotype accounts for only a portion of the variability in Endx concentrations, non-genetic factors that alter CYP2D6 enzyme activity and thus Endx concentrations may impact the pharmacokinetics of tamoxifen and potentially, tamoxifen efficacy.
Dextromethorphan (DM) is an antitussive commonly found in over-the-counter cough and cold medicines that is metabolized in part by CYP2D6 and CYP3A and has been described extensively as a CYP2D6 phenotyping probe [9]. A small study evaluating DM demonstrated a strong correlation between DM AUC and Endx AUC, with the suggestion that DM exposure may be a better predictor of Endx AUC than CYP2D6 alone [10]. However, the standard DM test is cumbersome, requiring blood sampling hours after DM ingestion. A breath test, based on the ingestion of 13C labeled dextromethorphan hydrobromide (13C-DM breath test) (DMBT), has been developed to determine CYP2D6 activity [11] and the13C-DM-BT was significantly associated with both CYP2D6 genotype as well as Endx concentrations in tamoxifen treated patients [12]. However, it is unclear whether the DM-BT test is a better predictor of Endx Css than CYP2D6 genotype alone.
We conducted a prospective study in individuals who were recommended to receive tamoxifen therapy for at least 6 months to examine the ability of the 13C DM-BT to identify known CYP2D6 genotypic poor metabolizers; to assess changes in CYP2D6 enzyme activity during treatment; and to examine the correlation between CYP2D6 enzyme activity and plasma Endx levels.
Methods
Subjects
This study enrolled individuals 18 years of age and older, who were about to begin tamoxifen therapy (20 mg daily by mouth) for either the prevention or treatment of non-invasive or invasive breast cancer with the intent of continuing treatment for at least 6 months. Additional inclusion criteria were: ECOG performance status 0–2 and known CYP2D6 genotype results (determined clinically in a CLIA certified laboratory). Patients who were known CYP2D6 poor metabolizers were eligible if their physician recommended tamoxifen.
Exclusion criteria included: prior exposure to tamoxifen, use of potent CYP2D6 inhibitor or monoamine oxidase inhibitors within 4 weeks of study registration, pulmonary disease, uncontrolled metabolic disease, impaired hepatic activity; history of chronic liver disease; previous adverse reaction to dextromethorphan; inability or unwillingness to fast for 4 hours. Informed consent was obtained prior to study entry. This study was approved by Mayo Clinic institutional review board and is registered at ClincalTrials.gov as NCT00873366.
Patients provided blood samples and underwent a 13C-DM-BT on day 1; once during week 8–10 following initiation of tamoxifen, and once during either month 5–6 post tamoxifen initiation. Patient visits that did not occur within a range of 50–110 days and 160–210 days following tamoxifen initiation, respectively, were not analyzed for that time point. In preparation for the 13C-DM-BT, patients were to fast at least 8 hours prior to scheduled test, abstain from alcohol for at least 24 hours prior to testing, and not take tamoxifen the morning of testing. The 13C-DM-BT began with the collection of a breath sample in a 1.3 liter breath bag. The patient then ingested one Alka Seltzer Gold (ASG) tablet dissolved in 50 mL of water as a means to increase gastrointestinal motility and the absorption of DM. A second ASG solution was prepared containing 13C-DM (0.5mg/kg with a maximum of 60 mg) was ingested 15 minutes after the first ASG solution. The patient rested for 50 minutes and a 2nd breath bag sample was obtained.
Genotyping
CYP2D6 genotype was derived from a peripheral blood specimen. Genotyping was performed in the CLIA certified Mayo Clinic Genotyping facility using the Luminex platform. The specific alleles and their associated activity score (AS) assessed were as follows: UM or AS = 2.0 (*1XN or *2 XN), EM or AS=1.0 (*1, *2, and *2A), IM or AS= 0.5 (*9, *10, *17 and *41), and PM or AS 0.0 (3, *4, *5, *6, *7, *8, *11, and *12). When needed, TaqMan assay and Sanger sequencing were additionally performed. The CYP2D6 activity score was determined for each patient according to the method introduced by Gaedigk et al [13].
Tam and Metabolite Assay
Tam and its metabolites were measured in plasma using a modification of the sensitive, specific HPLC assay with fluorescence detection published by Lee et al [13]. In brief, analytes were extracted in a hexane, isopropanol solution and the supernatant dried under a stream of nitrogen before reconstitution in mobile phase for HPLC analysis. E-Endx, Z-Endx, 4HT, NDMT, tamoxifen and toremifene (the internal standard) were separated with a validated assay using a C18 reverse phase column with a high pressure concave gradient starting at 20% A (35% acetonitrile and 65% 20 mM KH2PO4 buffer pH 3.0) and ending at 100% B (75% acetonitrile and 25% 20 mM KH2PO4 buffer pH 3.0) after 30 minutes. Post column photo activation of tamoxifen and its metabolites was done with a PHRED system (Aura Industries, New York, NY) followed by fluorescent detection (Ex 250 nm, Em 370nm).
DM-BT
Clinical trial material grade 13C-DM (API) was synthesized by Cambridge Isotope Laboratories (Andover, Massachusetts, USA) as a powder meeting USP standards. Production of the drug substance meets good manufacturing practice (GMP) guidelines. The oral liquid formulation (2.08 mg/mL) was manufactured under GMP conditions in the GMP facility of Confab Laboratories Inc. in Montreal, Canada.
13CO2 and 12CO2 in exhaled breath samples was measured by IR spectrometry using the POCone™ spectrophotometer manufactured by Photal Electronics, Japan. The amount of 13CO2 present in breath samples is expressed as a delta over baseline ratio that represents a change in the 13CO2/12CO2 ratio of breath samples collected before and after 13C-DM ingestion.
Statistical Methods
Spearman rank order correlation coefficients, ρ, were used to assess the strength of the association between two continuous variables. The probability values for ρ were computed transforming r and using a t-distribution with n-2 degrees of freedom.
Results
Patient Characteristics
Ninety one women and one man were enrolled between May 2009 and September 2011. Six women withdrew consent prior to the start of testing. Another 8 patients were excluded from the analysis cohort for the following reasons: non-adherence to tamoxifen (n=2), use of a CYP2D6 inhibitor (n=1), and lack of sufficient samples for DM analysis (n=5). The characteristics for the evaluable patients (n=77) are listed in Table 1.
Table 1.
Patient characteristics
| Age | |
| N | 77 |
| Median | 52.0 |
| Range | (28.0–86.0) |
| Race | |
| White | 74 (96.1%) |
| Black or African American | 1 (1.3%) |
| American Indian or Alaska Native | 2 (2.6%) |
| Ethnicity | |
| Hispanic or Latino | 2 (2.6%) |
| Not Hispanic or Latino | 75 (97.4%) |
| ECOG Performance Score | |
| 0 | 73 (94.8%) |
| 1 | 4 (5.2%) |
| BMI | |
| N | 77 |
| Median | 26.8 |
| Range | (18.9–47.8) |
| Disease Status | |
| Noninvasive Breast Cancer (DCIS) | 4 (5.2%) |
| Invasive Breast Cancer in Adjuvant | 72 (93.5%) |
| Invasive Breast Cancer in Metastatic | 1 (1.3%) |
Genotyping
The CYP2D6 genotypes were within Hardy-Weinberg equilibrium. The allele frequencies of CYP2D6 *4, *41, *9 and *10 in this cohort were as follows 16.2%, 11.0%, 2.6% and 2.6%, respectively. The *3, *5 and *6 alleles occurred less than 2%. The genotypes, activity scores (AS) and corresponding phenotypes for entire cohort are provided in Table 2.
Table 2.
Distribution of CYP2D6 genotypes grouped by CYP2D6 metabolism phenotype and activity scores (n=77)
| CYP2D6 Phenotype Group | Genotype | Activity score | n | Total for Phenotype group |
|---|---|---|---|---|
| EM/UM | *1/*2AXN | 3 | 1 | 2 |
| *1/*1XN | 3 | 1 | ||
| IM/UM | *41/*2AXN | 2.5 | 1 | 1 |
| EM/EM | *1/*1 | 2 | 12 | 30 |
| *1/*2A | 2 | 13 | ||
| *1/*2 | 2 | 2 | ||
| *2/*2 | 2 | 1 | ||
| *2A/*2A | 2 | 2 | ||
| EM/IM | *1/*9 | 1.5 | 2 | 15 |
| *1/*10 | 1.5 | 3 | ||
| *1/*41 | 1.5 | 7 | ||
| *2A/*9 | 1.5 | 1 | ||
| *2A/*41 | 1.5 | 2 | ||
| EM/PM | *1/*3 | 1 | 1 | 19 |
| *1/*4 | 1 | 8 | ||
| *2/*4 | 1 | 1 | ||
| *2/*4XN | 1 | 1 | ||
| *2A/*4 | 1 | 6 | ||
| *2A/*5 | 1 | 1 | ||
| *2A/*6 | 1 | 1 | ||
| IM/IM | *41/*41 | 1 | 1 | 1 |
| IM/PM | *3/*41 | 0.5 | 1 | 7 |
| *4/*9 | 0.5 | 1 | ||
| *4/*10 | 0.5 | 1 | ||
| *4/*41 | 0.5 | 4 | ||
| PM/PM | *3/*4 | 0 | 1 | 2 |
| *4/*4 | 0 | 1 |
CYP2D6 Genotype and Tamoxifen pharmacokinetics
Of the 77 eligible patients who provided baseline blood and DM-BT samples, DM-BT values and pharmacokinetic data were available for 60 and 57 patients, respectively, at the 3 month visit and 55 and 54 patients, respectively at the 6 month visit. Neither tamoxifen, NDMT, or 4HT pharmacokinetics were found to differ by CYP2D6 genotype. The median and range for each of these metabolites are included in the supplementary Table.
CYP2D6 Genotype and Endoxifen Pharmacokinetics
CYP2D6 genotype was positively correlated with the 3m Endx steady state concentrations (Endx Css) (r = 0.47, p < 0.0001), n=57) (Figure 1a); 6 month Endx Css (r= 0.56, p<0.0001, n=54); 3m Endx/NDMT ratio (r = 0.60, p < 0.0001, n=57)(Figure 1b); and the 6m Endx/NDMT ratio (r=0.61, p < 0.0001, n=54)
Figure 1.
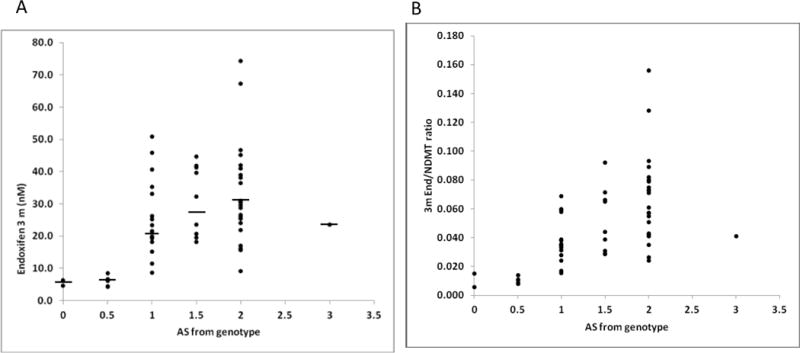
a): Correlation between CYP2D6 activity score with a) 3 month Endoxifen Css (r = 0.47, p< 0.0001, n = 57) and b) 3 month Endx/NDMT ratio (r = 0.59, p <0.0001, n = 57). Median Endoxifen concentrations are indicated by a solid line
CYP2D6 Genotype and DM-BT
DM-BT values obtained at baseline, 3m and 6m were positively correlated with CYP2D6 genotype as follows: b (r = 0.55, p < 0.0001, n=77) (Figure 2); 3m (r = 0.58, p< 0.0001, n=60); and 6m (r = 0.55, p < 0.001, n=55).
Figure 2.
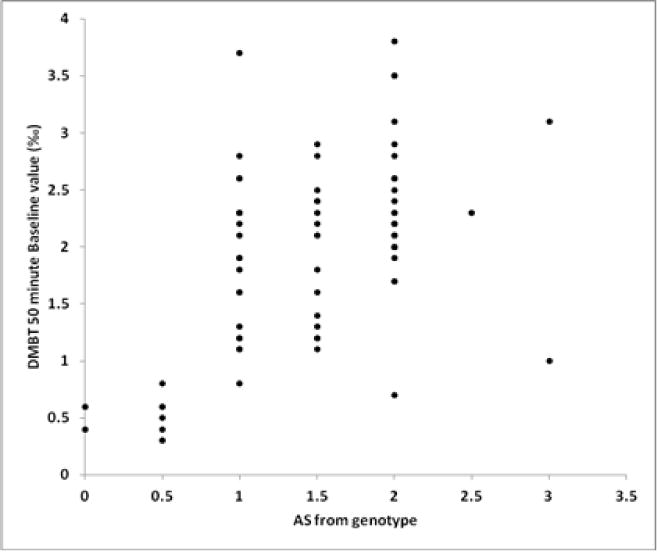
Correlation of Baseline DMBT with Activity score with CYP2D6 Activity Score (r = 0.6, p = <0.0001, n = 77).
DM-BT and Endoxifen Pharmacokinetics
bDM-BT values were significantly associated with both 3m (r = 0.60; p < 0.01, n=57) (Figure 3) and 6m (r = 0.54, p<0.01, n=54) Endx Css. The strength of the association between 3m DMBT and 3m endoxifen Css (r = 0.51, n = 56) was similar to that of the 6m DMBT correlation with 6 m endoxifen Css (r = 0.54, n = 53). For patients with at least one EM allele, there was some evidence for an increase in the DOB over time; however, there was no evidence for a change in DOB values for patients with the following activity scores: 0, 0.5, or 1 (Table 3). bDM-BT values were also found to be correlated with the 3 m End/NDMT ratio (r = 0.56, n = 57) (Figure 3b).
Figure 3.
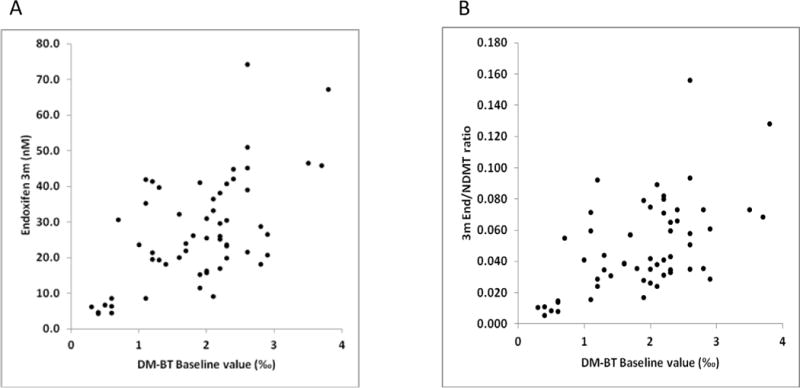
a) Correlation between baseline DMBT values and 3 month Endx Css (r = 0.6, n = 57) and b) baseline DMBT values and 3 month End/NDMT ratio Css (r = 0.56, n = 57).
Table 3.
Median (and range) endoxifen steady state concentrations at 3 and 6 months according to CYP2D6 genotype
| CYP2D6 Phenotype Group | CYP2D6 Activity Score | Median Endx concentration (nM) (range) | |||
|---|---|---|---|---|---|
| 3 months (n=57) | 6 months (n= 54) | ||||
| n | Endx Css | n | Endx Css | ||
| EM/UM | 3.0 | 1 | 23.6 | 2 | 38.5 (23.1–53.9) |
| IM/UM | 2.5 | 0 | NA | 0 | NA |
| EM/EM | 2.0 | 22 | 30.5 (9.1 – 74.3) | 21 | 26.7 (10.9 – 72.9) |
| EM/IM | 1.5 | 10 | 27.9 (17.0 – 44.7) | 8 | 30.4 (16.1 – 38.3) |
| IM/IM | 1.0 | 1 | 11.5 | 1 | 11.3 |
| EM/PM | 16 | 21.5 (8.6 – 50.9) | 15 | 19.1 (4.8 – 53.2) | |
| IM/PM | 0.5 | 5 | 6.1 (4.3 – 8.5) | 5 | 4.7 (2.9 – 12.0) |
| PM/PM | 0 | 2 | 5.5 (4.6 – 6.4) | 2 | 6.7 (6.4 – 7.1) |
DM-BT DOB Threshold
Seven of the 9 patients (78%) who had low (≤11.2 nM) 3m endoxifen concentrations also had low DM-BT values (≤0.9) including 2/2 patients with AS of 0 and 5/5 with AS of 0.5. In contrast, only 1 of 48 patients (2%) who had a low bDM-BT had a high 3m endoxifen concentration (Table 3).
Association between age and tamoxifen metabolism
Previous studies have demonstrated a correlation between tamoxifen and its metabolites with age [14]. In this study, we found no evidence that either 3m or 6m steady state concentrations of tamoxifen, NDMT, 4HT, or Endx were correlated with age (p-values > 0.11).
Discussion
This is the first prospective study to evaluate the association between bDM-BT obtained prior to starting tamoxifen with 3 and 6 month steady state Endx concentrations. These data provide further evidence that a dextromethorphan based metabolism assay may be of value as a phenotyping probe to predict tamoxifen pharmacokinetics.
In this study, CYP2D6 genotype was moderately correlated with both 3 and 6m Endx Css as well as the metabolic ratio of Endx/NDMT. Having demonstrated this, we sought to assess the correlation between the DM-BT with CYP2D6 genotype as well as steady state Endx pharmacokinetics. Our findings demonstrate that the DM-BT performed remarkably similar to CYP2D6 genotype in terms of its correlation with 3 and 6m End Css (Figure 3).
Because patients with a reduced Z-Endx Css have been identified to exhibit a higher risk of recurrence when treated with Tam in the adjuvant setting [15], we used a predefined DM-BT cut point of 0.9 (low ≤ 0.9; high > 0.9), previously associated with low Endx concentrations [12]. 78% (7/9) of patients with a low (≤11.2 nM) 3-month endoxifen concentrations had a low DM-BT, including 2/2 patients with AS of 0 and 5/5 with AS of 0.5. In contrast, only 2 % (1/48) with an endoxifen concentration > 11.2nM had a low DM-BT.
While CYP2D6 metabolism is the most important enzyme for the catalysis of NDMT to Endx, CYP3A is important both for the formation of NDMT, but also for the potential conversion of 4HT to Endx [16]. Therefore, it has been hypothesized that dextromethorphan, which shares similar routes of metabolism, might be a better phenotyping tool that CYP2D6 genotype alone for prediction of Endx Css [10]. However, the DMBT is labeled with 13C at the -O-CH3 group, making it a probe for CYP2D6 enzyme activity. In this study, patients who were taking CYP2D6 inhibitors were not eligible and both CYP2D6 genotype and the DM-BT performed similarly in terms of predicting Endx Css. These findingssuggest that the DM-BT, as a CYP2D6 phenotyping tool, may not necessarily provide a better prediction of Endx Css compared to CYP2D6 genotype alone. However, the DM-BT is likely to provide a more accurate estimate of CYP2D6 enzyme activity (and Endx Css) in patients taking CYP2D6 inhibitors, as has been demonstrated anecdotally [12]. Notably, in our study, one patient enrolled in the study took paroxetine prior to the baseline visit and had significantly lower baseline DOB30 values (>50% lower) than at 3 and 6 month visits after paroxetine was discontinued, indicating that the DM-BT is capable of identifying CYP2D6 inhibition. Further studies in much larger cohorts would be necessary to determine whether the DM-BT could replace CYP2D6 genotyping as a tool for predicting Endx and ultimately, whether a pre-defined DM-BT cut-point could be established to identify tamoxifen treated patients at higher risk of recurrence.
In this study, only 2 CYP2D6 PM (activity score 0) were identified (2.5%), and upwards of 6–8% of patients would be expected to carry this phenotype in a predominantly Caucasian population. The overall low number of poor metabolizers is related to the fact that at the Mayo Clinic, CYP2D6 genotype is offered as a clinical test to postmenopausal women with invasive, estrogen receptor positive breast cancer who are considering tamoxifen as adjuvant treatment. Therefore, patients known to be CYP2D6 poor metabolizers may have opted for an aromatase inhibitor, instead of tamoxifen, thus leading to a smaller than expected number of CYP2D6 PM.
Recent studies have evaluated whether other non-genetic factors are associated with the steady state concentrations of tamoxifen and its metabolites. Lien et al reported that age was positive correlated with serum concentrations of tamoxifen and its metabolites. In our study, we found no evidence that steady state concentrations of tamoxifen and its metabolites are associated with age. However, it should be noted that the concentrations of endoxifen in our study (range 4.6–74 nM) were nearly 8 fold lower than that of Lien et al [14] and mirror those as reported by Murdter et al [6]. The most likely reason for these differences is that like Murdter et al, we individually measured the concentrations of (Z)-endoxifen versus the pharmacologically inactive 4-hydroxy-desmethyltamoxifen.
In summary, in patients about to initiate tamoxifen not taking concurrent CYP2D6 inhibitors, the DM-BT is associated with CYP2D6 genotype and Endx Css, however, the DM-BT did not provide better discrimination of Endx Css compared to CYP2D6 genotype. Further studies are needed to determine whether DM-BT can substitute for CYP2D6 genotype in identifying patients with reduced CYP2D6 enzyme activity and thus Endx concentrations, especially in those patients on concomitant CYP2D6 inhibitors.
Supplementary Material
Table 4.
Median DM-BT (delta over baseline (DOB) values for each genotype group at baseline, 3, and 6 months
| CYP2D6 Activity Score | DM-BT DOB values | |||||
|---|---|---|---|---|---|---|
| Baseline (n=77) | 3 months (n= 60) | 6 months (n=55) | ||||
| n | median | n | median | n | median | |
| 3.0 | 2 | 2.05 (1.0–3.1) | 1 | 2.0 | 2 | 3.2 (3.0–3.4) |
| 2.5 | 1 | 2.3 | 1 | 1.9 | 0 | na |
| 2.0 | 30 | 2.2 (0.7 – 3.8) | 23 | 2.7 (1.5 – 4.2) | 22 | 2.7 (1.2 – 4.0) |
| 1.5 | 15 | 2.1 (1.1 – 2.9) | 9 | 2.4 (1.7 – 3.4) | 8 | 2.25 (1.7 – 2.8) |
| 1.0 | 1 | 1.9 | 1 | 1.5 | 0 | na |
| 19 | 1.9 (0.8 – 3.7) | 17 | 2.2 (−0.02 – 3.3) | 16 | 2.4 (1.6 – 4.1) | |
| 0.5 | 7 | 0.5 (0.3 – 0.8) | 6 | 1.15 (0.3 – 1.9) | 5 | 0.8 (0.7 – 2.4) |
| 0 | 2 | 0.5 (0.4 – 0.6) | 2 | 0.15 (0.0 – 0.3) | 2 | 0.3 (0.2 – 0.4) |
Acknowledgments
Source of Funding: Supported in part by R43CA 110874-3 (Goetz, Reid, Ames, Suman, Rosen, Modak), 1R01CA133049-01 (MPG, MMA, JR), the Mayo Comprehensive Cancer Center Grant (CA15083; MMA, JMR, VS, and MG) and the Mayo Clinic Breast Cancer SPORE (CA 116201; MMA, MPG, VS, and JNI).
Footnotes
Conflicts of Interest: Anil Modak is an employee at Cambridge Isotope Laboratories Inc. which manufactures the 13C labeled Dextromethorphan used in the study. Commercialization of the dextromethorphan breath test could be financially beneficial to the company. All remaining authors have declared no conflicts of interest.
References
- 1.Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 3.Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 4.Wu X, Hawse JR, Subramaniam M, et al. The Tamoxifen Metabolite, Endoxifen, Is a Potent Antiestrogen that Targets Estrogen Receptor {alpha} for Degradation in Breast Cancer Cells. Cancer Res. 2009;69:1722–1727. doi: 10.1158/0008-5472.CAN-08-3933. [DOI] [PubMed] [Google Scholar]
- 5.Wu X, Subramaniam M, Grygo SB, et al. Estrogen receptor-beta sensitizes breast cancer cells to the anti-estrogenic actions of endoxifen. Br Cancer Res. 2011;13:R27. doi: 10.1186/bcr2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murdter TE, Schroth W, Bacchus-Gerybadze L, et al. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther. 2011;89:708–717. doi: 10.1038/clpt.2011.27. [DOI] [PubMed] [Google Scholar]
- 7.Hawse JR, Subramaniam M, Cicek M, et al. Endoxifen’s molecular mechanisms of action are concentration dependent and different than that of other anti-estrogens. PloS one. 2013;8:e54613. doi: 10.1371/journal.pone.0054613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brauch H, Schroth W, Goetz MP, et al. Tamoxifen use in postmenopausal breast cancer: CYP2D6 matters. J Clin Oncol. 2013;31:176–180. doi: 10.1200/JCO.2012.44.6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu A, Haining RL. Comparative contribution to dextromethorphan metabolism by cytochrome P450 isoforms in vitro: can dextromethorphan be used as a dual probe for both CTP2D6 and CYP3A activities? Drug Metabolism and Disposition. 2001;29:1514–1520. [PubMed] [Google Scholar]
- 10.de Graan AJ, Teunissen SF, de Vos FY, et al. Dextromethorphan as a phenotyping test to predict endoxifen exposure in patients on tamoxifen treatment. J Clin Oncol. 2011;29:3240–3246. doi: 10.1200/JCO.2010.32.9839. [DOI] [PubMed] [Google Scholar]
- 11.Leeder JS, Pearce RE, Gaedigk A, et al. Evaluation of a [13C]-dextromethorphan breath test to assess CYP2D6 phenotype. J Clin Pharmacol. 2008;48:1041–1051. doi: 10.1177/0091270008319709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Opdam FL, Dezentje VO, den Hartigh J, et al. The use of the 13C-dextromethorphan breath test for phenotyping CYP2D6 in breast cancer patients using tamoxifen: association with CYP2D6 genotype and serum endoxifen levels. Cancer Chemo Pharmacol. 2013;71:593–601. doi: 10.1007/s00280-012-2034-4. [DOI] [PubMed] [Google Scholar]
- 13.Lee KH, Ward BA, Desta Z, et al. Quantification of tamoxifen and three metabolites in plasma by high-performance liquid chromatography with fluorescence detection: application to a clinical trial. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;791:245–253. doi: 10.1016/s1570-0232(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 14.Lien EA, Soiland H, Lundgren S, et al. Serum concentrations of tamoxifen and its metabolites increase with age during steady-state treatment. Br Cancer Res Treat. 2013;141:243–248. doi: 10.1007/s10549-013-2677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madlensky L, Natarajan L, Tchu S, et al. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther. 2011;89:718–725. doi: 10.1038/clpt.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310:1062–1075. doi: 10.1124/jpet.104.065607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


