Abstract
Inhibition of endogenous dentin matrix metalloproteinases (MMPs) within incompletely infiltrated hybrid layers can contribute to the preservation of resin-dentin bonds. This study evaluated the bond stability of interfaces treated with benzalkonium chloride (BAC) and benzalkonium methacrylate (MBAC), and its inhibitory properties in dentin MMP activity. Single-component adhesive ALL-BOND UNIVERSAL, modified with BAC or MBAC in concentrations of 0, 0.5, 1.0 and 2.0% was used for microtensile bond strength (μTBS) evaluation after 24 h, 6 months and 1 yr. Human dentin beams were treated with 37% phosphoric acid, dipped either in 0.5% BAC, 1.0% BAC or water (control) for 60 s and then incubated in SensoLyte generic MMP substrate to determine MMP activity. A significant decrease in μTBS after 6 months and 1 yr was observed for the control group only. No significant differences among groups were shown at 24 h. After 6 months and 1 yr, the control group demonstrated significantly lower μTBS than all treatment groups. Both 0.5% and 1.0%, BAC applied for 60 s inhibited total MMP activity by 31% and 54%, respectively. Both BAC and MBAC contributed to the preservation of resin-dentin bonds likely due to its inhibitory properties of endogenous dentin proteinases.
Keywords: Benzalkonium chloride, Bond strength, Dental adhesive, Dentin, Matrix metalloproteinase
Significant recent research has been devoted to the inhibition of endogenous matrix metalloproteinases of dentin, as a means to improve the durability of the hybrid layer. Host derived matrix metalloproteinases (MMPs) are present in dentin matrices and are known to contribute to the degradation of hybrid layers once they become activated. It is well accepted that dentin treatment with acidic agents such as phosphoric acid or self-etching primers, uncovers and activates endogenous MMPs (1, 2). Inhibition or inactivation of collagenolytic activity of endogenous proteases has gained considerable attention in the last few years. Chlorhexidine (CHX), a cationic antimicrobial agent, has been extensively investigated for its anti-MMP properties. Several studies have shown that CHX is a potent inhibitor of MMP-2, -8 and -9 (3), and can reduce degradation of the resin-dentin bonds overtime (4–7).
Benzalkonium chloride (BAC), a mixture of alkylbenzyl-dimethylammonium chlorides of various lengths alkyl chains, is a nitrogenous cationic surface-acting agent containing a quaternary ammonium group with broad antimicrobial activity. Recent studies have shown effective dentin MMP inhibition with such quaternary ammonium compounds (QACs) (8). A phosphoric acid etchant containing 1.0%wt BAC (Etch-37 w/BAC, Bisco, Schaumburg, IL, USA) has been commercially available for its anti-bacterial properties for many years. Studies have shown that the use of BAC, either topically as a cavity disinfectant (9, 10) or incorporated into a phosphoric acid etchant (11) did not have any adverse effect on the immediate bond strength to dentin. In addition to the effective MMP inhibiting properties (8), recent studies have also shown that BAC, in concentrations of 0.5 or 1.0 wt.% can preserve resin-dentin bonds over time (12, 13). As in bisguanides, positively charged +NH3 binds to negatively charged hydroxyapatite phosphate or collagen carboxylic groups in enamel and dentin, respectively. The resultant electrostatic interaction is, however, weak. Both bisguanides and QACs are also water-soluble and may leach out of the hybrid layer compromising its long-term anti-MMP effectiveness.
More recently, the focus has shifted to the use of QAC agents that may be able to provide sustained anti-proteolytic benefits. The use of polymerizable agents offers promise since they co-polymerize in-situ with adhesive monomers and their MMP-inhibiting effect can presumably be maintained for years, with consequent improvement in the durability of resin-dentin bonds (14). Such an assumption requires validation with long-term studies. Therefore, the aims of this study were to evaluate the bond strength stability of resin-dentin interfaces bonded with conventional benzalkonium chloride (BAC) or with polymerizable benzalkonium methacrylate (MBAC) after 6 months and 1 yr of storage by microtensile bond strength (μTBS), and to determine if BAC can inhibit endogenous MMPs in acid-etched human dentin within 60 s. of topical treatment. The null hypotheses were that BAC, either in conventional or polymerizable forms, would have no effect on the bond strength degradation relative to the control group with no inhibitor, and that BAC could not inhibit MMPs in acid-etched dentin in 60 s.
MATERIALS AND METHODS
Micro-tensile bond strength
Thirty-five recently extracted, non-carious human molars were used to obtain dentin for bonding. The teeth were obtained under a protocol approved by the State University of New York’s Institutional Review Board. A flat, transversely cut surface of superficial/middle dentin was obtained by means of a water-cooled slow speed diamond saw (Isomet, Buehler, Lake Bluff, IL, USA), and a standardized smear layer was created with 600-grit silicon carbide abrasive paper (SiC paper, Buehler).
A light-cured single-component adhesive (ALL-BOND UNIVERSAL, ABU, Bisco, Schaumburg, IL, USA) was used in this study. ABU is an ethanol/water-based MDP-containing adhesive of ultra-mild acidity (pH>3). As per the manufacturer description, ABU combines etching, priming and bonding in one bottle and can be used with or without phosphoric acid etchant for an etch-and-rinse or self-etch technique, respectively. Experimental adhesive blends of ABU were prepared with conventional benzalkonium chloride (BAC) or with benzalkonium methacrylate (MBAC) in concentrations of 0, 0.5, 1.0 and 2.0 wt.%. The teeth were equally and randomly assigned to seven study groups (Table 1), with five teeth in each group, as follows: 1) ABU with no MMP-inhibitor (control); 2) 0.5% BAC-containing adhesive (0.5% BAC); 3) 1.0% BAC-containing adhesive (1.0% BAC); 4) 2.0% BAC-containing adhesive (2.0% BAC); 5) 0.5% MBAC-containing adhesive (0.5% MBAC); 6) 1.0% MBAC-containing adhesive (1.0% MBAC); and 7) 2.0% MBAC-containing adhesive (2.0% MBAC). The adhesive composition and application protocol, as described by the manufacturer, is summarized in Table 1. All groups were treated with 35% phosphoric acid (Ultra-Etch, Ultradent, South Jordan, UT, USA) for 15 s, rinsed and blot-dried prior to application of the adhesive. The adhesive was applied to the moist dentin surfaces according to the wet-bonding technique and polymerized according to manufacturer’s instructions with LED light-curing unit (Bluephase 16i, Ivoclar-Vivadent, Amherst, NY, USA) for 10 s. with a power density of 1,600 mW/cm2. Composite build-ups were fabricated with resin composite (Filtek Z100, 3M ESPE, Lot# N372074) in shade A2 by application of two increments no greater than 2 mm with each increment polymerized for 40 s. The restored teeth were stored in distilled water and placed in an incubator at 37°C for 24 h to ensure adequate polymerization. After 24 h, all teeth were sectioned and dentin beams with cross-sectional area of 0.9 ± 0.1 mm2 were obtained according to the non-trimming technique (15). The beams were divided in three equal groups for micro-tensile bond strength evaluation at 24 h, 6 months and 1 yr of storage at 37°C in artificial saliva solution (AS, 12.9 mM KCl, 1.9 mM KSCN, 2.4 mM Na2SO4· 10 H2O, 3.3 mM NH4Cl, 1.5 mM CaCl2· 2H2O, 7.5 mM NaHCO3, 0.02 mM ZnCl2, 0.02% sodium azide and 5 mM HEPES buffer pH 7.4). The storage media was replaced with fresh solution once a month to ensure fresh preservatives. After the designated storage time, the beams were stressed to failure with a universal testing machine at a crosshead speed of 1 mm/min (Bisco).
Table 1.
Study groups, composition and application procedures for the two adhesives evaluated as per manufacturer recommendations.
| Study Groups and Materials
| ||||
|---|---|---|---|---|
| Group | Code | Lot | Description | Composition / Application Protocol |
| 1 | ABU (control) | 1200013871 | 35% H3PO4 followed by ABU with no inhibitor | ULTRA-ETCH (Ultradent, South Jordan, UT, USA) |
|
|
||||
| 2 | 0.5% BAC | 722-130c | 35% H3PO4 followed by 0.5% BAC-adhesive |
Composition: 35% phosphoric acid |
|
|
||||
| 3 | 1.0% BAC | 722-130b | 35% H3PO4 followed by 1.0% BAC-adhesive |
Application protocol:
|
|
|
||||
| 4 | 2.0% BAC | 722-130a | 35% H3PO4 followed by 2.0% BAC-adhesive | ALL-BOND UNIVERSAL (ABU/ Bisco Inc, Schaumburg, IL, USA) |
|
|
||||
| 5 | 0.5% MBAC | 722-130f | 35% H3PO4 followed by 0.5% MBAC-adhesive |
Composition: Ethanol (>20%), Bis-GMA (>20%) |
|
|
||||
| 6 | 1.0% MBAC | 722-130e | 35% H3PO4 followed by 1.0% MBAC-adhesive |
Application protocol:
|
|
| ||||
| 7 | 2.0% MBAC | 722-130d | 35% H3PO4 followed by 2.0% MBAC-adhesive | |
ABU, ALL-BOND UNIVERSAL; BAC, benzalkonium chloride; H3PO4, phosphoric acid; MBAC, benzalkonium methacrylate.
Since the data met the requirements of normal distribution (Kolmogorov-Smirnov test) and equal variance (Levene’s test), a two-way analysis of variance (ANOVA) was used to analyze the effect of the variables, treatment group and incubation time, on μTBS. Post-hoc Tukey’s test was used for pairwise multiple comparisons among group means. A significance level of p<0.05 was used for all tests. All statistical analysis was performed with SigmaStat version 3.5 (San Jose, CA, USA).
Dentin MMP Activity
Fifteen extracted non-carious human third molars were obtained from 18–21 year-old patients with informed consent under a protocol approved by the Georgia Regents University and stored frozen until required. After removal of the roots, mid-coronal dentin was exposed by removing enamel and superficial dentin using an Isomet saw (Isomet, Buehler, Lake Bluff, IL, USA) under water cooling. One 1 mm-thick dentin disk was obtained from each tooth. Thirty dentin beams of 2×1×6 mm were obtained from fifteen dentin disks.
Because soluble MMPs extracted from dentin matrices or purchased rhMMPs are more susceptible to inhibitors or inactivators than matrix-bound MMPs (16), we elected to study dentin MMP activity while they were matrix-bound. To determine the total MMP activity of demineralized dentin, the beams were etched with 37% phosphoric acid for 15 s. and then rinsed in deionized water (DW) for another 15 s. The etched-beams were then treated either with DW or aqueous solutions of BAC in concentrations of 0.5 or 1.0% w/v for 60 s. We elected to study inhibition of dentin MMP activity with 0.5 and 1.0% BAC since these concentrations have shown improved performance in previous studies (8, 13). Each group contained ten specimens. The beams were immediately placed in 200 μl of a generic MMP substrate (SensoLyte Generic MMP colorimetric assay kit - catalog No. 72095, AnaSpec Inc., Lot# 131-029, Fremont, CA, USA) for 1 h at 25°C in a 96-well plate. After 1 h, the total MMP activity was spectrophotometrically determined by measuring the absorbance of each of the wells at 412 nm in a plate reader (Synergy HT microplate reader, BioTek Instruments, Winooski, VT, USA) against blanks. Ten individual values were averaged to obtain a mean value for each subgroup. All chemicals were purchased from Sigma/Aldrich Chemical Co. and used as received.
The generic MMP assay uses a proprietary thiopeptide to assay MMP-1, 2, 3, 7, 8, 9, 12, 13 and 14. Thus, the kit measured the total endogenous MMP activity of dentin. A standard curve of absorbance of the substrate vs. rh MMP-9 activity (ng) was constructed to permit expression of total MMP activity in MMP-9 equivalents. We chose to use rhMMP-9 because the preform can be easily activated by trypsin, unlike rhMMP-2 that requires the use of ADMA, which is not compatible with the SensoLyte assay reagents. The rhMMP-9 was activated using trypsin at a final concentration of 10 μg/ml, pH 7.4 at 37°C for 2 h. Then the trypsin was inactivated by addition of trypsin inhibitor at a final concentration of 100 μg/ml. As the ability of BAC to inactivate MMPs was the same, regardless of whether we used the 412 nm absorbance values or converted it to MMP-9 equivalents, we elected to publish the absorbance values.
A Kruskal-Wallis and Dunn’s multiple comparisons tests were used to evaluate the effect of BAC concentration on the percentage inhibition of MMP activity since the data was not normally distributed (Kolmogorov-Smirnov test). A one-way ANOVA and post-hoc multiple comparison procedures Holm-Sidak method were used to evaluate the effect of BAC concentration on the absorbance values since these values were normally distributed (Kolmogorov-Smirnov test). A significance level of p<0.05 was used for all tests. All statistical analyses were performed with SigmaStat.
RESULTS
Micro-tensile bond strength
Two-way ANOVA revealed a significant effect of the treatment group (p<0.001) and an interaction effect between treatment group and incubation time (p=0.002), but no effect of the incubation time, on the bond strength. Table 2 summarizes the mean micro-tensile bond strength (μTBS) values of BAC and MBAC bonded specimens at 24 h, 6 months and 1 yr of storage in an AS solution. Multiple comparisons revealed no significant differences in μTBS values among the treatment groups at 24 h. After 6 months and 1 yr., the untreated control group demonstrated significantly lower μTBS than all treatment groups (p<0.05). However, there were no differences among the treatment groups.
Table 2.
Mean micro-tensile bond strengths (μTBS) of BAC and MBAC bonded specimens at 24 h, 6 months and 1 yr of storage.
| 24 h | 6 months | 1 yr. | |
|---|---|---|---|
| ABU | 29.4 (4.7) A,a | 16.4 (4.4) A,b | 15.3 (4.6) A,b |
| 0.5% BAC | 30.6 (7.4) A,a | 31.4 (3.9) B,a | 30.1 (9.8) B,a |
| 1.0% BAC | 31.3 (6.9) A,a | 29.9 (5.2) B,a | 33.5 (10.6) B,a |
| 2.0% BAC | 29.1 (8.5) A,a | 27.5 (5.4) B,a | 25.2 (8.4) B,a |
| 0.5% MBAC | 25.8 (6.0) A,a | 28.7 (9.0) B,a | 32.6 (4.6) B,a |
| 1.0% MBAC | 26.0 (4.3) A,a | 27.4 (4.0) B,a | 32.0 (4.8) B,a |
| 2.0% MBAC | 30.5 (5.4) A,a | 28.8 (6.4) B,a | 30.2 (8.5) B,a |
Values are means ± SD (n = 10 specimens per subgroup). Different superscript letters indicate significant differences between groups (p<0.05). Different capital letters designate significant differences between subgroups in vertical columns. Different lower case letters designate significant differences in horizontal rows.
When evaluating the differences in μTBS for each of the individual treatment groups at the different incubation times, only the control group demonstrated a significant decrease in μTBS after 6 months (p<0.001) and 1 yr. (p<0.001) relative to its baseline values. Although some experimental groups reveled an apparent increase in bond strength, because of the high standard deviation values, these differences remained not statistically significant. Neither of the experimental groups demonstrated significant variations from their 24-h μTBS values.
Dentin MMP Activity
Benzalkonium chloride, in concentrations of 0.5% and 1.0%, applied for 60 s. inhibited the total MMP activity by 31% and 54%, respectively (Table 3). Both BAC concentrations demonstrated significantly greater MMP inhibition than the control group (p<0.05), and they were also significantly different from each other. The absorbance values are shown in Table 3. The greatest absorbance values, suggestive of more MMP activity, were shown for the control group (0.638). Lower absorbance values were seen for the BAC treatment groups with values of 0.440, and 0.292 for 0.5% and 1.0%, respectively. This indicates that there was a dose-dependent inhibition of total MMP activity by BAC. Absorbance values for both 0.5% and 1.0% were significantly lower than that of the control group (p=0.025 and p=0.017, respectively), with 1.0% BAC showing significantly lower absorbance values than that of 0.5% BAC (p=0.05).
Table 3.
Absorbance values (412 nm) and percent inhibition of total MMP activity after treatment of demineralized beams with benzalkonium chloride in 0.5 % and 1.0 % for 60 s. using the SensoLyte Generic MMP assay kit, relative to an untreated control with 0% inhibition of total MMP activity.
| Control (ABU) | 0.5% BAC | 1.0% BAC | |
|---|---|---|---|
| Absorbance (412 nm) | 0.638 (0.17) A | 0.440 (0.09) B | 0.292 (0.06) C |
| % MMP inhibition | 0.0 (0.0) A | 31.0 (14.1) B | 54.2 (9.0) C |
Values are means ± SD (n = 10). Groups identified by different letters are significantly different (p<0.05).
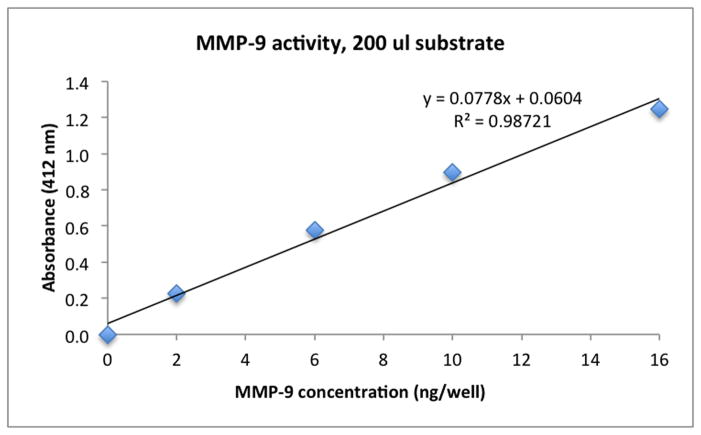
Fig. 1 shows the absorbance vs. amount of rhMMP-9 (ng/well). Increasing amounts of rhMMP-9 were added to the SensoLyte substrate, which gave increasing absorbance of colored product, demonstrating that the colorimertric method is sensitive to changes in enzymatic activity. The control (uninhibited) absorbance value of the endogenous MMPs in a single dentin beam in Table 3 was 0.638 at 412 nm. Insertion of this absorbance value (y) in the regression equation of Fig. 1 shows that absorbance was equivalent to 7.4 ng of MMP-9 per well.
Figure 1.
Increase in absorbance at 412 nm as a function of increasing concentrations of rhMMP-9 when incubated with the SensoLyte Generic MMP assay kit.
DISCUSSION
The present study evaluated whether BAC and MBAC could increase the stability of adhesive interfaces when incorporated into a primer/adhesive blend, in concentrations of 0.5%, 1.0% and 2.0% after 6 months and 1 yr. of storage, and whether BAC could inactivate endogenous MMPs in acid etched human dentin within 60 s. of treatment. Our results require rejection of the first null hypothesis that “BAC or MBAC would have no effect on the bond strength relative to the control” since BAC-containing adhesive blends, either in conventional or polymerizable forms, yielded stable bond strengths after one year. The second part of the null hypothesis that “BAC could not inhibit MMPs in dentin” must also be rejected since treatment with both 0.5% and 1.0% BAC yielded inhibition of the total MMP activity by 31% and 54%, respectively when applied to acid-etched dentin for 60 s. The absorbance values obtained using the SensoLyte Generic MMP assay were used to calculate the percent inhibition of total MMP activity for groups treated with BAC relative to an untreated control with 0% inhibition.
Relative to the use of protease inhibitors alone, which may slowly leach out of the hybrid layer (17), the use of MMP inhibiting agents that co-polymerize with the adhesive monomers in situ, are expected to provide sustained anti-proteolytic benefits, presumably maintaining their MMP-inhibiting effect for years. In this study, we showed that treatment with conventional BAC or MBAC can prevent bond degradation. The MMP inhibitory properties of 0.5% and 1.0% BAC, also demonstrated in this study, may help explain, at least partially, the stable bond strengths observed after 1 yr. These findings are in agreement with previous results, which have also shown effective MMP inhibition with BAC (8, 13). Benzalkonium methacrylate is similar in structure to conventional BAC except that the former is more hydrophobic and it contains a polymerizable group, which allows MBAC to covalently co-polymerize with adhesive monomers. Once polymerization has taken place, MBAC should not leach out from the hybrid layer allowing sustained anti-proteolytic benefits, less water absorption and hence, less hydrolytic degradation.
Other agents with MMP inhibitory properties, which can copolymerize with adhesive monomers, such as 12-methacryloyloxydodecylpyridinium bromide (MDPB), a component of Clearfil Protect Bond and Clearfil Protect SE, have been shown advantageous in previous studies (18–20). Tezvergil-Mutluay et al. recently demonstrated that MDPB effectively inhibited soluble MMP-9 as or more effectively as Galardin, a potent specific, synthetic MMP inhibitor, and almost completely inhibited the degradation of demineralized dentin collagen (21). Although in vitro and clinical studies have shown that QAMs, specifically MDPB, may inhibit collagenolytic enzymes in the hybrid layer (22–24), reductions in bond strength comparable to other adhesives have also been reported with quaternary ammonium compounds (25–27). Most of the available studies, however, fail to show whether there is a direct correlation between an agent’s anti-MMP properties and its bond strength survival overtime.
In the present study, a direct correlation between improved bond stability and the known anti-MMP properties of BAC was demonstrated. Based on previous results by Tezvergil-Mutluay et al., who showed that 2.0% BAC did not yield additional MMP inhibition relative to 1.0% BAC (8), we elected to test the anti-MMP properties of 0.5% and 1.0% BAC. In that same study, it was shown that increasing the amount of BAC in the incubation medium, increased the amount of BAC bound to demineralized dentin to a plateau point after which, no further binding occurred (8). This point was below 2.0% BAC. In that study, a 55–66% MMP inhibition was shown with the use of 1.0% BAC, which is similar to our results of 54% inhibition with 1.0% BAC. Even if greater MMP inhibition rates were possible with the use of higher concentrations of BAC, if no further binding to demineralized dentin can take place, additional benefits to the properties of the polymerized matrix or bond strength are not possible. This explains why, above certain concentration of a given agent, either no effect or even a negative effect on the properties of the polymerized matrix may be observed, and thus the benefit of greater MMP inhibition does not outweigh the risk of adversely affecting the properties of the polymerized polymer matrix.
The adhesive blend investigated in our study incorporated the therapeutic agent BAC to a blend containing ethanol/water as a solvent, a polymerizable acidic monomer, hydrophobic resin, cross-linking dimethacrylate monomers, hydrophilic monomer, and polymerization initiator. Incorporation of BAC into the primer/adhesive blend yielded no effect on the bond strength at 24 h indicating that the therapeutic agent may be safely incorporated into the adhesive blend without a compromise to its immediate bond strength. Further evaluation of the effect of BAC and other agents, incorporated into adhesive blends, on the properties of the polymerized adhesive and resulting inter-diffusion layer should precede the adoption of such mixtures into clinical practice. Our results are limited to the materials and techniques tested in the present study. The behavior of blends admixed with therapeutic agents but applied in self-etching mode also deserves investigation given the substantial effect of the pH micro-environment on MMP activation and activity (1).
It is interesting to speculate on the possible therapeutic effects of a mixture of 0.5% BAC and 0.5% MBAC. The unpolymerizable BAC may bind to demineralized dentin and their matrix-bound hydrolases immediately, while the polymerizable MBAC would copolymerize with the adhesive monomers in ABU. If after 1 or 2 yrs., the unpolymerized BAC slowly leaches from the hybrid layer during clinical function and any esterases attack the polymerized MBAC, the BAC portion of MBAC could leach into the hybrid layer to maintain inhibition of endogenous proteases for an extended period of time. This remains to be investigated in future experiments.
Within the limitations of this in vitro study, it may be concluded that BAC inactivates matrix-bound dentin proteinases in demineralized dentin matrices in a dose-dependent manner. Application of BAC, in concentrations of 0.5% and 1.0%, for 60 s. inactivated the total MMP activity by 31 and 54% respectively. Both BAC and MBAC contributed to the preservation of the resin-dentin bonds. All BAC-treated groups yielded stable bond strengths, while the untreated controls showed decreases in bond strength after 6 months and 1 yr.
Acknowledgments
This work was supported, in part, by grant R01 DE015306 from the NIDCR to DHP (PI) and by the King Abdulazziz University Faculty of Dentistry, to DHP as their Highly-Cited Scholar.
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
References
- 1.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Mannello F, Tjäderhane L, Toledano M, Pashley EL, Tay FR. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials. 2006;27:4470–4476. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Tay FR, Pashley DH, Loushine RJ, Weller RN, Monticelli F, Osorio R. Self-etching adhesives increase collagenolytic activity in radicular dentin. J Endod. 2006;32:862–868. doi: 10.1016/j.joen.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clin Diagn Lab Immunol. 1999;6:437–439. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjäderhane L, Ruggeri A, Jr, Tay FR, Dorigo EDS, Pashley DH. Chlorhexidine stabilizes the adhesive interface: A 2-year in vitro study. Dent Mater. 2010;26:320–325. doi: 10.1016/j.dental.2009.11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrilho MRO, Carvalho RM, de Goes MF, di Hipólito V, Geraldeli S, Tay FR, Pashley DH, Tjäderhane L. Chlorhexidine preserves dentin bond in vitro. J Dent Res. 2007;86:90–94. doi: 10.1177/154405910708600115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brackett WW, Tay FR, Brackett MG, Dib A, Sword RJ, Pashley DH. The effect of chlorhexidine on dentin hybrid layers in vivo. Oper Dent. 2007;32:107–111. doi: 10.2341/06-55. [DOI] [PubMed] [Google Scholar]
- 7.Carrilho MRO, Geraldeli S, Tay FR, de Goes MF, Carvalho RM, Tjäderhane L, Reis AF, Hebling J, Mazzoni A, Breschi L, Pashley DH. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007;86:529–533. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 8.Tezvergil-Mutluay A, Mutluay MM, Gu L-s, Zhang K, Agee KA, Carvalho RM, Manso A, Carrilho M, Tay FR, Breschi L, Suh B-I, Pashley DH. The anti-MMP activity of benzalkonium chloride. J Dent. 2011;39:57–64. doi: 10.1016/j.jdent.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Say EC, Koray F, Tarim B, Soyman M, Gulmez T. In vitro effect of cavity disinfectants on the bond strength of dentin bonding systems. Quintessence Int. 2004;35:56–60. [PubMed] [Google Scholar]
- 10.Turkun M, Cal E, Toman M, Toksavul S. Effects of dentin disinfectants on the shear bond strength of all-ceramics to dentin. Oper Dent. 2005;30:453–460. [PubMed] [Google Scholar]
- 11.Kanca J., 3rd One step bond strength to enamel and dentin. Am J Dent. 1997;10:5–8. [PubMed] [Google Scholar]
- 12.Sabatini C, Kim J, Alias PO. In vitro evaluation of benzalkonium chloride in the preservation of adhesive interfaces. Oper Dent. 2013;39:283–290. doi: 10.2341/13-131-LR. [DOI] [PubMed] [Google Scholar]
- 13.Sabatini C, Patel SK. Matrix metalloproteinase inhibitory properties of benzalkonium chloride stabilizes adhesive interfaces. Eur J Oral Sci. 2013;121:610–616. doi: 10.1111/eos.12089. [DOI] [PubMed] [Google Scholar]
- 14.Pashley DH, Tay FR, Imazato S. How to increase the durability of resin-dentin bonds. Compend Contin Educ Dent Suppl. 2011;32:60–66. [PubMed] [Google Scholar]
- 15.Shono Y, Ogawa T, Terashita M, Carvalho RM, Pashley EL, Pashley DH. Regional measurement of resin-dentin bonding as an array. J Dent Res. 1999;78:699–705. doi: 10.1177/00220345990780021001. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Uchiyama T, Carrilho M, Agee KA, Mazzoni A, Breschi L, Carvalho RM, Tjaderhane L, Looney S, Wimmer C, Tezvergil-Mutluay A, Tay FR, Pashley DH. Chlorhexidine binding to mineralized versus demineralized dentin powder. Dent Mater. 2010;26:771–778. doi: 10.1016/j.dental.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricci HA, Sanabe ME, de Souza Costa CA, Pashley DH, Hebling J. Chlorhexidine increases the longevity of in vivo resin-dentin bonds. Eur J Oral Sci. 2010;118:411–416. doi: 10.1111/j.1600-0722.2010.00754.x. [DOI] [PubMed] [Google Scholar]
- 18.Imazato S. Bio-active restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dent Mater J. 2009;28:11–19. doi: 10.4012/dmj.28.11. [DOI] [PubMed] [Google Scholar]
- 19.Imazato S, Kinomoto Y, Tarumi H, Torii M, Russell RR, McCabe JF. Incorporation of antibacterial monomer MDPB into dentin primer. J Dent Res. 1997;76:768–772. doi: 10.1177/00220345970760030901. [DOI] [PubMed] [Google Scholar]
- 20.Imazato S, Tay FR, Kaneshiro AV, Takahashi Y, Ebisu S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dent Mater. 2007;23:170–176. doi: 10.1016/j.dental.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, Breschi L, Nishitani Y, Tay FR, Pashley DH. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J Dent Res. 2011;90:535–540. doi: 10.1177/0022034510389472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donmez N, Belli S, Pashley DH, Tay FR. Ultrastructural correlates of in vivo/in vitro bond degradation in self-etch adhesives. J Dent Res. 2005;84:355–359. doi: 10.1177/154405910508400412. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima M, Okuda M, Ogata M, Pereira PN, Tagami J, Pashley DH. The durability of a fluoride-releasing resin adhesive system to dentin. Oper Dent. 2003;28:186–192. [PubMed] [Google Scholar]
- 24.Van Landuyt KL, De Munck J, Mine A, Cardoso MV, Peumans M, Van Meerbeek B. Filler debonding & subhybrid-layer failures in self-etch adhesives. J Dent Res. 2010;89:1045–1050. doi: 10.1177/0022034510375285. [DOI] [PubMed] [Google Scholar]
- 25.De Munck J, Mine A, Vivan Cardoso M, De Almeida Neves A, Van Landuyt KL, Poitevin A, Van Meerbeek B. Effect of dentin location and long-term water storage on bonding effectiveness of dentin adhesives. Dent Mater J. 2011;30:7–13. doi: 10.4012/dmj.2010-085. [DOI] [PubMed] [Google Scholar]
- 26.De Munck J, Mine A, Van den Steen PE, Van Landuyt KL, Poitevin A, Opdenakker G, Van Meerbeek B. Enzymatic degradation of adhesive-dentin interfaces produced by mild self-etch adhesives. Eur J Oral Sci. 2010;118:494–501. doi: 10.1111/j.1600-0722.2010.00758.x. [DOI] [PubMed] [Google Scholar]
- 27.Sabatini C. Effect of a chlorhexidine-containing adhesive on dentin bond strength stability. Oper Dent. 2013;38:609–617. doi: 10.2341/12-239-L. [DOI] [PubMed] [Google Scholar]