Abstract
Many diseases are linked with climate trends and variations. In particular, climate change is expected to alter the spatiotemporal dynamics of allergenic airborne pollen and potentially increase occurrence of allergic airway disease. Understanding the spatiotemporal patterns of changes in pollen season timing and levels is thus important in assessing climate impacts on aerobiology and allergy caused by allergenic airborne pollen. Here we describe the spatiotemporal patterns of changes in the seasonal timing and levels of allergenic airborne pollen for multiple taxa in different climate regions at a continental scale. The allergenic pollen seasons of representative trees, weeds and grass during the past decade (2001–2010) across the contiguous United States have been observed to start 3.0 (95% Confidence Interval (CI), 1.1–4.9) days earlier on average than in the 1990s (1994–2000). The average peak value and annual total of daily counted airborne pollen have increased by 42.4% (95% CI, 21.9%–62.9%) and 46.0% (95% CI, 21.5%–70.5%), respectively. Changes of pollen season timing and airborne levels depend on latitude, and are associated with changes of growing degree days, frost free days, and precipitation. These changes are likely due to recent climate change and particularly the enhanced warming and precipitation at higher latitudes in the contiguous United States.
Keywords: pollen, climate change, allergy, tree, weed, grass
Introduction
Climate change has been shown to cause dramatic changes in natural ecosystems and cultivated agricultural systems, and to increase the occurrence of disease in both (Altizer et al., 2013, Boxall et al., 2009). Climate trends and variations impact many prevalent human diseases such as malaria, asthma and hay fever. These climate-linked diseases have raised increasing concerns related to public health (Epstein, 2005, McMichael et al., 2005, Patz et al., 2005). In particular, climate change is expected to modify the patterns of emission and transport of allergenic pollen from trees, weeds and grasses (Frei & Gassner, 2008, García-Mozo et al., 2010, Kinney, 2008, Wan et al., 2002). Like dust mites and cockroaches in indoor environments (Reid & Gamble, 2009), outdoor allergenic pollen is one of the main triggers of allergic airway disease, affecting up to 30% of the population of industrialized countries (Sofiev & Bergmann, 2013). It acts synergistically with common air pollutants, such as ozone and particulate matter, to exacerbate allergic airway disease (Cakmak et al., 2012), resulting in related high medical costs (Lamb et al., 2006).
Understanding the spatiotemporal patterns of changes in pollen season timing and levels is thus important in assessing climate impacts on allergic airway disease (Beggs, 2004, Bielory et al., 2012, Dapul-Hidalgo & Bielory, 2012). Most studies on assessment of climate change effects on allergenic pollen season have involved individual or a few taxa at a single or limited number of locations (Frei & Gassner, 2008, García-Mozo et al., 2010, Wan et al., 2002). Analyses of multiple taxa at stations spanning different climate regions are needed to elucidate climate impacts on allergenic pollen and potential consequences on public health. Changes in temperature and precipitation have been and will be heterogeneous, and enhanced warming and precipitation are very likely to occur at higher latitudes (IPCC, 2013). Furthermore, even in the vicinity of a single locality, different taxa are observed to respond differently to climate change (Fitter & Fitter, 2002). The recent US National Climate Assessment looks at all the work done today on how allergenic pollen season has changed with climate, and shows studies of some regions for some taxa (Melillo et al., 2014), but here for the first time we present a comprehensive national study to investigate the allergenic pollen season variations in the past two decades under a changing climate.
In the current study, allergenic pollen season variations of birch (Betula), oak (Quercus), ragweed (Ambrosia), mugwort (Artemisia) and grass (Poaceae) were investigated using the observed data of daily counted airborne pollen and meteorology factors during the period of 1994–2010 across the contiguous US (CONUS). We sought to examine the spatiotemporal patterns of change of allergenic pollen season timing and levels for multiple taxa in multiple climate regions, and their relationships with recent climate change at a continental scale.
Materials and Methods
Data source
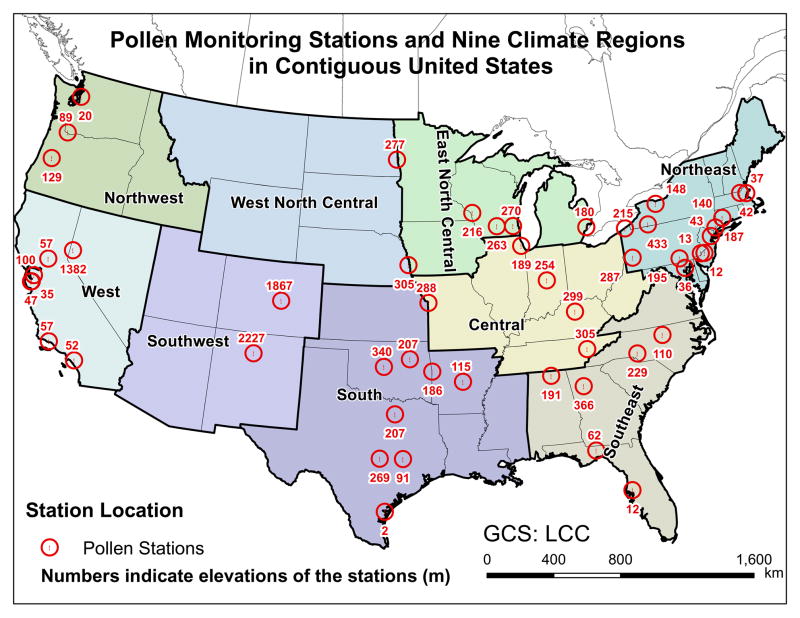
Daily counts of airborne pollen were retrieved from all available stations of the National Allergy Bureau (NAB) of the American Academy of Allergy, Asthma and Immunology (AAAAI) across the CONUS during the period of 1994–2010. The daily counts of airborne pollen are expressed as average atmospheric concentrations with the unit of pollen grains per cubic meter. Fifty out of 82 stations were selected by examining the retrieved airborne pollen counts based on data availability in the nine climate regions of the CONUS (Fig. 1, Table S1, Table S2). For each of the pollen stations, the sampling frequency and period in each pollen season are approximately the same. The sampling frequency is five days per week for most of NAB pollen stations. Observed daily temperature and precipitation were obtained from the National Climatic Data Center (NCDC) meteorological stations nearest to the corresponding NAB pollen stations. Fig. S1 shows the schematic diagram summarizing the materials and methods in the current study.
Fig. 1.
Distribution of the studied pollen stations (n = 50) across the nine climate regions in the contiguous US. The climate regions are classified according to National Climatic Data Center of the National Oceanic and Atmospheric Administration (Karl & Koss, 1984).
Pollen indices
Start Date, Season Length, Peak Value and Annual Total Production of daily counted airborne pollen were selected as four pollen indices to assess climate change impacts on allergenic pollen season timing and levels (Fig. S2). With day 1 being January 1st, the start date (days from January 1st) is the day when the cumulative pollen count reaches 5% and end date when it reaches 95% of annual total count. This method was used to exclude long-range-transport pollen grains from the local pollen season. These long-range-transport pollen grains from surrounding regions may influence pollen counts at the beginning and end of local pollen seasons (D’Amato et al., 2007, Smith et al., 2008). Season length (day) is defined as the duration between start and end dates. Peak value (pollen grains/m3) is the maximum daily count recorded during a pollen season. Annual production (pollen grains/m3) is defined as the sum of daily counts during a pollen season. Additional description of the derived pollen indices is presented in the Supporting Information.
Climatic factors
Allergenic pollen season timing and levels have been widely reported to be associated with Growing Degree Days (GDD), Frost Free Days (FFD) and accumulated precipitation (García-Mozo et al., 2008, Zhang et al., 2013, Ziska et al., 2011). The fixed-period GDD value was calculated for each taxon in each year at each NAB station. FFD is defined as the interval between the last frost day during spring and the first frost day (daily minimum temperature below 0°C) during fall. FFD has been associated with pollen season length, especially for weed taxa (Ziska et al., 2011). Pollen levels were affected by precipitation preceding and during the pollen seasons (Makra et al., 2012, Zhang et al., 2013). Accumulated precipitation in fixed periods was used in the current study to investigate the climate change impacts on allergenic pollen levels. These fixed periods were selected to approximately cover the allergenic pollen seasons and the time right before the seasons at the studied NAB stations (Table S3).
Mean pollen indices
To reduce the effects of the natural climate and plant-growth variability on pollen indices (Deser et al., 2012, Masaka & Maguchi, 2001), mean pollen indices were calculated for the past decade (2001–2010) and the 1990s (1994–2000) at each station. Pollen data before 1994 were scarce and usually reported on a weekly basis, and thus not adequate for deriving start date and duration of pollen season. Because of proprietary issues, airborne pollen data in 2001 and 2002 are not available to us for most of the studied stations. This makes seven years of airborne pollen data available for period 1994–2000 and approximately eight years of data available for period 2001–2010. For calculating the changes in mean pollen indices between the past decade and the 1990s, at least three years of pollen data in each of the two periods are required. Student’s t tests were performed to check the significance of changes in pollen indices during periods of 1994–2000 and 2001–2010 for each of the five pollen taxa. Since hypotheses tests on four pollen indices based on the same group of observed pollen data may potentially cause spurious significant findings, the Benjamini Hochberg procedure were used to guarantee a false discovery rate of less than 5% (Benjamini & Hochberg, 1995).
Trend and correlation analysis
Regression analysis was performed to identify trends of start date, season length, peak value and annual production of allergenic pollen during 1994–2010 at each of the NAB stations. At least six years of pollen data are required for conducting trend analyses of pollen indices at a NAB pollen monitoring station. Correlation analyses were conducted to examine the relationships between changes in mean pollen indices and changes in mean climatic factors.
The Supporting Information explains, in detail, the equations and parameters for calculating GDD, FFD, accumulated precipitation and mean pollen indices. It also contains information regarding calculations of the changes in mean pollen indices and climatic factors between the periods of 2001–2010 and 1994–2000 at each station, and their corresponding regional and nationwide averages.
Results
Trends of pollen indices
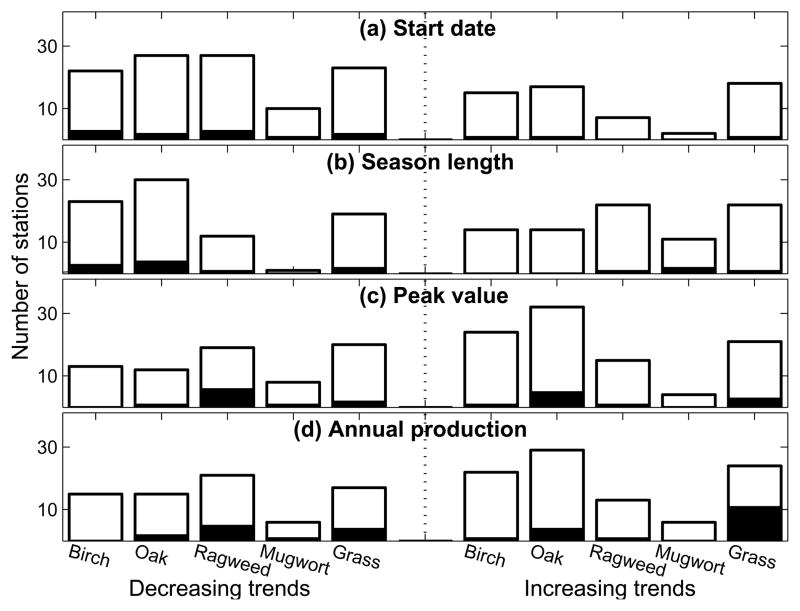
Fig. 2 summarizes the results of trend analyses of start date, season length, peak value and annual production of allergenic pollen season during 1994–2010 for each taxon at each NAB station. For example, for start date of birch pollen, trend analyses on start date were performed at each station based on available pollen data from 1994 to 2010. The number of stations where decreasing trends (i.e., negative slope) of birch pollen start date have been observed, was plotted as the first bar in the left side of Fig. 2a; the section of solid bar gives the number of stations where significant decreasing trends have been observed (p <0.05, Student’s t test). Likewise, the first bar in the right side of Fig. 2a indicates the number of stations where increasing trends of birch pollen start date have been observed.
Fig. 2.
Number of stations where decreasing or increasing trends of pollen indices have been observed from 1994 to 2010. (a) Start Date, (b) Season Length, (b) Peak Value, and (d) Annual Production. The black bar indicates the number of stations at which the observed trends are significant at 5% level based on the Student’s t test. Decreasing trends indicate that pollen season tends to start earlier, season length tends to be shorter, and peak value and annual production tend to decrease.
Decreasing trends during 1994–2010 indicate that the pollen season tends to start earlier, season length tends to be shorter, and peak value and annual production tend to decrease. The allergenic pollen season during the period of 1994–2010 across the CONUS showed early start trends at 59%, 61%, 79%, 83% and 56% of the 50 studied stations for birch, oak, ragweed, mugwort and grass, respectively. Around 7% of the studied stations showed trends of significantly earlier start dates (p <0.05, Student’s t test). Season lengths tended to be shorter at 62% and 68% of the studied stations for birch and oak, respectively, but appeared to be longer at 65%, 92% and 54% of the studied stations for ragweed, mugwort and grass, respectively. The number of stations with significantly different start dates and season lengths in general are proportional to the number of stations with increasing or decreasing trends of start date and season length.
The peak value and annual production of daily counted airborne pollen tended to increase for spring-flowering taxa at most of the studied stations: around 62% of the observations showing increasing trends in peak value and annual production during the period of 1994–2010 (one observation corresponds to airborne pollen count for one taxon at one station during 1994–2010). For the peak value and annual production of the summer-flowering taxa, decreasing trend and significant decreasing trend are more common than increasing.
Changes of mean pollen indices
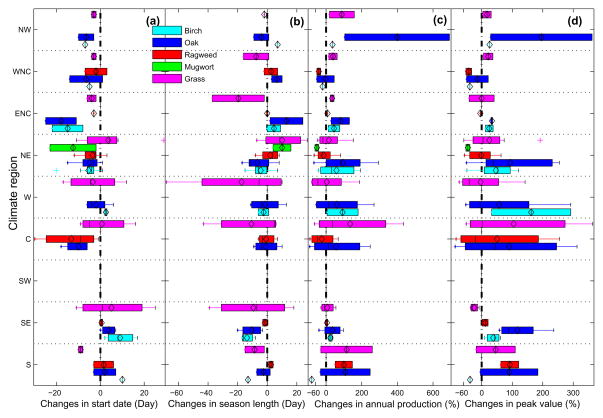
Fig. 3 displays the changes of mean pollen indices between the periods of 1994–2000 and 2001–2010 in nine climate regions across the CONUS. The relative change in peak value was calculated by dividing the changes in mean peak value from two periods by the mean peak value in the period of 1994–2000, i.e. (likewise for annual production). The box plot was generated using changes in mean pollen indices at different stations within the same climate region.
Fig. 3.
Changes in mean pollen indices during period of 2001–2010 from the means during 1994–2000 across the contiguous US. The nine climate regions: South (S), Southeast (SE), Southwest (SW), Central (C), West (W), Northeast (NE), East North Central (ENC), West North Central (WNC), and Northwest (NW). (a) Start Date, (b) Season Length, (c) Annual Production, and (d) Peak Value. In each box plot the central black line is the median; the black diamond is the mean; two sides are the 25th (q1) and 75th (q3) percentiles; the whiskers represent q3+1.5(q3−q1) and q1−1.5(q3−q1), respectively. “Outliers” were plotted as plus (‘+’). A negative number indicates earlier pollen season start date, shorter season length, and decreasing pollen levels.
Changes in pollen indices vary by climate region and taxon. The allergenic pollen season in most of the climate regions tended to start earlier in the past decade than the 1990s, but it tended to start later in the South and Southeast climate regions. In general, the allergenic pollen season for the north-eastern CONUS (e.g., Northeast and East North Central climate regions) in the past decade appeared to last longer than in the 1990s; while for the southern CONUS (e.g., South and Southeast climate regions) it appeared to be shorter (Fig. 3, Tables S4). Allergenic pollen levels across the CONUS were observed to increase substantially across different geographic areas in the past decade compared to the 1990s.
Overall, the allergenic pollen seasons for five representative taxa started on average 3.0 (95% CI, 1.1–4.9) days earlier during the past decade than during the 1990s across the CONUS (Table 1, Fig. S3). Significantly earlier start dates (p value <0.05, Student’s t test with Benjamini Hochberg control procedure) are shown for 6.3% of the observations, with an average advancement of 17.0 (95% CI, 8.3–25.7) days in a decade; and 2.1% of the observations showed significantly later start dates than previously. Pollen seasons for spring-flowering allergenic taxa (birch, oak and grass) in the past decade appeared to be on average 3.1–4.8 days shorter than in the 1990s; and pollen seasons of summer-flowering taxa (ragweed and mugwort) appeared to be 1.3–10 days longer than previously.
Table 1.
Differences of mean pollen indices between periods of 2001–2010 and 1994–2000 in the contiguous US. 95% confidence intervals are included in the parentheses.
| Start Date (Days) | Season Length (Days) | Peak Value a (%) | Annual Production a (%) | # of stations | |
|---|---|---|---|---|---|
| Birch | −2.3 (−7.0, 1.9) | −4.4* (−8.8, −0.6) | +44.9* (7.9, 82.0) | +42.8* (4.6, 81.1) | 19 |
| Oak | −4.4* (−7.4, −1.5) | −3.1 (−7.0, 0.8) | +86.4* (37.9, 134.8) | +92.5* (29.4, 155.7) | 28 |
| Ragweed | −4.0 (−7.6, −0.4) | +1.3 (−1.1, 3.6) | +12.4 (−22.9, 47.7) | −3.1 (−30.0, 23.8) | 20 |
| Mugwort | −12.5 (−145.9, 120.9) | +10 (−66.2, 86.2) | −45.4 (−127.4, 36.5) | −51.5 (−179.0, 76.1) | 2 |
| Grass | −0.2 (−4.7, 4.3) | −4.8 (−13.7, 4.2) | +23.0 (−15.0, 61.0) | 43.4 (−3.4, 90.3) | 26 |
| Average | −3.0* (−4.9,−1.1) | −2.6 (−5.4, 0.2) | +42.4* (21.9, 62.9) | +46.0* (21.5, 70.5) | 31 |
The relative change in peak value was calculated by dividing the changes in mean peak value from two periods by the mean peak value in the period of 1994–2000 (i.e., ), and likewise for annual production.
The changes in a mean pollen index for a given taxa during two periods at all available stations were used to calculate the nationwide average and the 95% confidence intervals.
Asterisk (*) indicates statistically significant difference at 5% level based on Student’s t test and Benjamini-Hochber control procedure (false discovery rate < 5%).
The average allergenic airborne pollen levels have increased by 42.4% (95% CI, 21.9%–62.9%) and 46.0% (95% CI, 21.5%–70.5%) based on peak values and annual production, respectively (Table 1, Fig. S3). For allergenic airborne pollen levels, 16.8% of the observations showed significant increase in annual production with an average increase of 179.9% (95% CI, 96.6%–263.2%); and 6.3% of the observations showed significant increase in peak value with an average increase of 283.6% (95% CI, 231.9%–335.4%).
Spatiotemporal patterns of changes of mean pollen indices
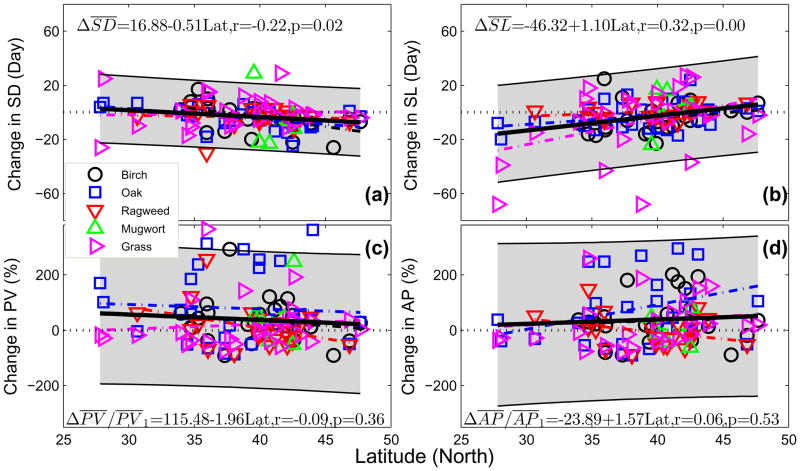
Changes in average allergenic pollen season timing and airborne levels between the past decade and the 1990s were identified as functions of latitude (Fig. 4). Changes in mean start date were found to decrease from later start to earlier start with increasing latitude; changes in mean season length increased from shorter season to longer season with increasing latitude. The latitudinal effects on average allergenic airborne pollen levels varied for different taxa. Overall, changes in average annual production appear to be large at higher latitudes and small at lower latitudes; while changes in average peak value appear to be small at higher latitudes and large at lower latitudes.
Fig. 4.
Changes in mean pollen indices between the periods of 2001–2010 and 1994–2000 as a function of latitude. (a) Start Date, (b) Season Length, (b) Peak Value, and (d) Annual Production. Heavy black lines represent the overall trends; dashed lines give trends for individual taxa; shaded gray area is the 95% CI of overall trend. Horizontal dotted lines are zero lines.
Allergenic pollen seasons for spring-flowering birch and oak start from the south and shift gradually towards the north, and their season lengths at lower latitudes are generally longer than those at higher latitudes. The enhanced warming at higher latitudes (IPCC, 2013) leads to larger increases in GDD and FFD than at lower latitudes, and thus drives the allergenic plants at higher latitudes to flower earlier and last for a longer duration. This makes the start dates from north to south more synchronous and the season length more uniform during the past decade than previously.
Relationship with recent climate variation
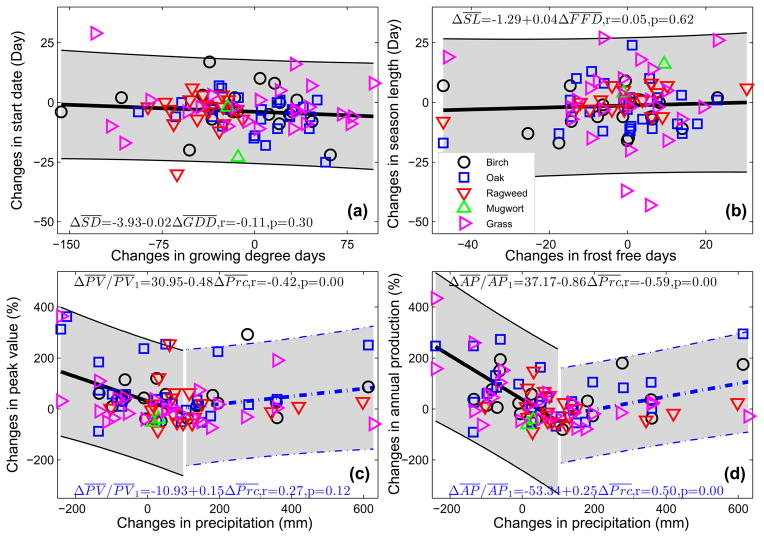
Fig. 5 presents the relationships between changes of mean pollen indices and changes of mean climatic factors during the periods of 2001–2010 and 1994–2000 across CONUS. The trend lines for changes in peak value (Fig. 5c) and annual production (Fig. 5d) are divided into two stages at ΔPrc = 100 mm. This precipitation change of 100 mm was roughly the “valley point” of the curves describing the relationships between change of airborne pollen level and change of precipitation.
Fig. 5.
Changes in mean pollen indices and changes in mean climatic factors between periods of 2001–2010 and 1994–2000. (a) Start Date and GDD, (b) Season Length and FFD, (b) Peak Value and precipitation, and (d) Annual Production and precipitation. Heavy lines represent the trends; shaded gray areas are the 95% CIs. The initial and last dates and base temperature used to calculate GDD, FFD and accumulated precipitation are listed in Table S3. In panels (c) and (d), the trend line is divided into two stages at ΔPrc = 100 mm to show the dual effects of precipitation on airborne pollen levels.
The changes in mean start date are negatively related to changes in GDD between the past decade and the 1990s while the changes in season lengths are positively related to changes in FFD (Fig. 5a and b). Accumulated precipitation during pollen season exerts dual effects on airborne pollen levels (Fig. 5c and d). When the change of precipitation is less than 100 mm, increase of precipitation tends to reduce the airborne pollen levels. Conversely, when the change of precipitation is greater than 100 mm, increase of precipitation tends to increase the airborne pollen levels.
Discussion
Comparisons with previous studies
The widely increasing trends of peak value and annual production of spring flowering taxa are consistent with a European study focused on the trends of observed annual airborne pollen counts from multiple taxa across Europe (Ziello et al., 2012b) (Fig. 2c and d). The study reported that 59% of the observed trends of annual airborne pollen counts increased during various periods from 1977 to 2009 at different European pollen monitoring stations. The later onset and shorter duration of the allergenic pollen season in South and Southeast regions are consistent with the decreasing trends of temperature in these regions (IPCC, 2013) (Fig. 3, Tables S4).
The average advancement of allergenic pollen season onset in the past decade is consistent with the reported decadal advancements of phenology events (e.g., flowering) of trees, weeds and grasses (Bock et al., 2014, Parmesan & Yohe, 2003, Root et al., 2003, Ziello et al., 2012a) (Table 1, Fig. S3). Similar latitudinal effects on altered ragweed pollen season length in North America have been reported by Ziska et al. (Ziska et al., 2011) (Fig. 4). Similar synchrony of onset of birch pollen season in different regions has been observed in Finland during 1989–2006 (Ranta et al., 2008) (Fig. 4).
Impacts of temperature and precipitation
Over the past two decades, temperature and precipitation changes over North America have been larger at higher latitudes and altitudes (IPCC, 2013). This enhanced warming and precipitation at higher latitudes and altitudes has caused poleward and upward shifts of distribution ranges of plants and animals across different ecosystems (Inouye et al., 2000, Walther et al., 2002). The spatiotemporal patterns of changes in allergenic pollen season timing and airborne levels are likely due to the latitudinal patterns of temperature and precipitation in the Northern Hemisphere. The larger increase of temperature and precipitation at higher latitudes (IPCC, 2013) caused larger changes in start date and annual production of allergenic pollen at higher latitudes (Fig. 4a and d). Change of peak value and season length may be dominated by changes in precipitation. Larger increase of precipitation and its frequency at higher latitudes washes out more airborne pollen during the pollen season, and thus reduces the peak value of airborne pollen at higher latitudes (Fig. 4c). The reduced season length of allergenic pollen at lower latitude is most likely caused by the decreasing temperatures in the South and Southeast regions in the CONUS (IPCC, 2013), and those at middle latitudes are likely due to the increased precipitation and rainy days.
On one hand, increasing precipitation can directly wash out more airborne pollen, and therefore decrease the peak values and annual total counts of airborne pollen. On the other hand, climate change, even on the scale of years to decades, can change the distributions and abundances of plants and animals (Blois et al., 2013, Overpeck et al., 1990, Pastor & Post, 1988); a large increase in precipitation may favor the growth and expansion of habitat of allergenic plants at higher latitudes, at locations that have not been favorable for plant growth because of dry and cold conditions, thus increasing the production of airborne pollen.
The dual effect of precipitation on airborne allergenic pollen levels is particularly prominent at higher latitudes. If similar trends of enhanced warming and precipitation at higher latitudes continue, earlier exposure times and higher exposure levels to allergenic pollens may occur with potentially substantial consequences to public health. This will likely increase the prevalence (number of individuals becoming allergic) and the morbidity (severity and duration) of the population suffering from allergies and asthma.
Uncertainties in the current study
The variable number of NAB stations in nine climate regions could potentially cause bias when we compare the allergenic pollen season variations among different climate regions (Fig. 3). Specifically, since there are only three NAB stations in each of the Northwest, West North Central and Southwest regions there is a scarcity of data for these regions. To reduce this bias, Fig. 4 was generated to account for allergenic pollen season variations across latitudes without confining the data in climate regions. Fig. 3 and Fig. 4 should be considered together for comparison of allergenic pollen season variations among different regions and locations. Furthermore, incorporation of the airborne pollen data during the missing years and more recent years (e.g., 2011–2013) into the analyses could improve the results of the current study.
The causal attribution of changes in allergenic pollen season timing and levels to variation and trend of a single climatic factor in Fig. 5 is substantially compounded by multiple other factors and their combinations (Makra et al., 2012, Walther et al., 2002, Zhang et al., 2013). The distances between NAB pollen stations and corresponding closest NOAA meteorology stations vary from a few kilometers to tens of kilometers depending on the stations. The mismatch of locations between pollen and meteorology stations may play a role in the weak relationships found in Fig. 5.
Factors affecting pollen season timing and airborne levels interact in complex ways, and it may not be surprising to find a weak correlation with temperature or precipitation changes (Walther et al., 2002, Ziello et al., 2012b). Population shifts and changes of land use in the proximity of the NAB counting stations may play an important role in determining the amount of airborne pollen collected at the corresponding stations (Haberle et al., 2014, Reid & Gamble, 2009, Ziska & Beggs, 2012). Because of the fertilizer effect of CO2 in the atmosphere, increase of CO2 level itself or combined with rising temperature has been reported to substantially influence pollen and spore production (English et al., 2009, Rogers et al., 2010, Ziska et al., 2009, Ziska et al., 2003, Ziska et al., 2007). Data describing these compounding factors (e.g., CO2 level and land changes) are generally not available or very limited during the period of 1994–2010 for most of the NAB pollen stations. Further study to quantify the influence of these compounding factors and their combinations is needed to improve our understanding of climate impact on spatiotemporal distributions of aeroallergens. Extrapolation of these results into the future based on projections of future climate, assessing the impacts of climate change on future allergic airway disease, is also an obvious next step.
Supplementary Material
Acknowledgments
This research was funded in part by USEPA under STAR Grant EPA-RD-83454701-0 (PI: L. Bielory, MD) to Rutgers University, and by the NIEHS sponsored Center for Environmental Exposures and Disease at EOHSI (P30ES005022). We thank AAAAI-National Allergy Bureau and NOAA for providing airborne pollen data and climate data, respectively. We also thank Dr. Estelle Levetin and Dr. Richard Lankow for thoughtful comments and Ms. Linda Everett for editorial assistance.
Footnotes
Supporting Information Legends
Additional Supporting Information may be found in the online version of this article:
Table S1. Coordinates, elevations and main climate characteristics for the studied pollen stations.
Table S2. Pollen monitoring stations and years with valid pollen data for five studied pollen taxa.
Table S3. Initial and Last Dates and temperature for calculating GDD, FFD and accumulated Precipitation.
Table S4. Changes of allergenic pollen seasons between the last decade and the 1990s in the nine climate regions across the CONUS.
Fig. S1. Schematic diagram for assessment of allergenic pollen season variations under the changing climate in the CONUS.
Fig. S2. Daily pollen curves observed at the Springfield, NJ station, 1994-2010.
Fig. S3. Frequency distribution of changes in mean pollen indices between the last decade and 1990s.
References
- Altizer S, Ostfeld RS, Johnson PTJ, Kutz S, Harvell CD. Climate change and infectious diseases: from evidence to a predictive framework. Science. 2013;341:514–519. doi: 10.1126/science.1239401. [DOI] [PubMed] [Google Scholar]
- Beggs PJ. Impacts of climate change on aeroallergens: past and future. Clinical & Experimental Allergy. 2004;34:1507–1513. doi: 10.1111/j.1365-2222.2004.02061.x. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995:289–300. [Google Scholar]
- Bielory L, Lyons K, Goldberg R. Climate change and allergic disease. Current Allergy and Asthma Reports. 2012;12:485–494. doi: 10.1007/s11882-012-0314-z. [DOI] [PubMed] [Google Scholar]
- Blois JL, Zarnetske PL, Fitzpatrick MC, Finnegan S. Climate change and the past, present, and future of biotic interactions. Science. 2013;341:499–504. doi: 10.1126/science.1237184. [DOI] [PubMed] [Google Scholar]
- Bock A, Sparks TH, Estrella N, et al. Changes in first flowering dates and flowering duration of 232 plant species on the island of Guernsey. Global Change Biology. 2014 doi: 10.1111/gcb.12579. [DOI] [PubMed] [Google Scholar]
- Boxall AB, Hardy A, Beulke S, et al. Impacts of climate change on indirect human exposure to pathogens and chemicals from agriculture. Environmental Health Perspectives. 2009;117:508. doi: 10.1289/ehp.0800084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak S, Dales RE, Coates F. Does air pollution increase the effect of aeroallergens on hospitalization for asthma? Journal of Allergy and Clinical Immunology. 2012;129:228–231. doi: 10.1016/j.jaci.2011.09.025. [DOI] [PubMed] [Google Scholar]
- D’Amato G, Cecchi L, Bonini S, et al. Allergenic pollen and pollen allergy in Europe. Allergy. 2007;62:976–990. doi: 10.1111/j.1398-9995.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- Dapul-Hidalgo G, Bielory L. Climate change and allergic diseases. Annals of Allergy, Asthma & Immunology. 2012;109:166–172. doi: 10.1016/j.anai.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Deser C, Knutti R, Solomon S, Phillips AS. Communication of the role of natural variability in future North American climate. Nature Clim Change. 2012;2:775–779. [Google Scholar]
- English PB, Sinclair AH, Ross Z, et al. Environmental health indicators of climate change for the United States: findings from the State Environmental Health Indicator Collaborative. Environmental Health Perspectives. 2009;117:1673. doi: 10.1289/ehp.0900708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein PR. Climate change and human health. New England Journal of Medicine. 2005;353:1433–1436. doi: 10.1056/NEJMp058079. [DOI] [PubMed] [Google Scholar]
- Fitter AH, Fitter RSR. Rapid changes in flowering time in British plants. Science. 2002;296:1689–1691. doi: 10.1126/science.1071617. [DOI] [PubMed] [Google Scholar]
- Frei T, Gassner E. Climate change and its impact on birch pollen quantities and the start of the pollen season an example from Switzerland for the period 1969–2006. International Journal of Biometeorology. 2008;52:667–674. doi: 10.1007/s00484-008-0159-2. [DOI] [PubMed] [Google Scholar]
- García-Mozo H, Chuine I, Aira MJ, et al. Regional phenological models for forecasting the start and peak of the Quercus pollen season in Spain. Agricultural and Forest Meteorology. 2008;148:372–380. [Google Scholar]
- García-Mozo H, Galán C, Alcázar P, et al. Trends in grass pollen season in southern Spain. Aerobiologia. 2010;26:157–169. [Google Scholar]
- Haberle SG, Bowman DMJS, Newnham RM, et al. The macroecology of airborne pollen in Australian and New Zealand urban areas. PLoS One. 2014;9:e97925. doi: 10.1371/journal.pone.0097925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye DW, Barr B, Armitage KB, Inouye BD. Climate change is affecting altitudinal migrants and hibernating species. Proceedings of the National Academy of Sciences. 2000;97:1630–1633. doi: 10.1073/pnas.97.4.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC. Climate change 2013: the physical science basis. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, editors. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, United Kingdom: Cambridge University Press; 2013. [DOI] [Google Scholar]
- Karl T, Koss WJ. Regional and national monthly, seasonal, and annual temperature weighted by area, 1895–1983. Asheville, NC: National Climatic Data Center; 1984. [Google Scholar]
- Kinney PL. Climate change, air quality, and human health. American Journal of Preventive Medicine. 2008;35:459–467. doi: 10.1016/j.amepre.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Lamb CE, Ratner PH, Johnson CE, et al. Economic impact of workplace productivity losses due to allergic rhinitis compared with select medical conditions in the United States from an employer perspective. Current Medical Research and Opinion. 2006;22:1203–1210. doi: 10.1185/030079906X112552. [DOI] [PubMed] [Google Scholar]
- Makra L, Matyasovszky I, Paldy A, Deak AJ. The influence of extreme high and low temperatures and precipitation totals on pollen seasons of Ambrosia, Poaceae and Populus in Szeged, southern Hungary. Grana. 2012;51:215–227. [Google Scholar]
- Masaka K, Maguchi S. Modelling the masting behaviour of Betula platyphylla var. japonica using the resource budget model. Annals of Botany. 2001;88:1049–1055. [Google Scholar]
- McMichael AJ, Woodruff RE, Hales S. Climate change and human health: present and future risks. The Lancet. 2005;367:859–869. doi: 10.1016/S0140-6736(06)68079-3. [DOI] [PubMed] [Google Scholar]
- Melillo JM, Terese R, Yohe GW. Climate change impacts in the United States: the third national climate assessment. Washington, DC, USA: U.S. Global Change Research Program; 2014. [DOI] [Google Scholar]
- Overpeck JT, Rind D, Goldberg R. Climate-induced changes in forest disturbance and vegetation. Nature. 1990;343:51–53. [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- Pastor J, Post WM. Response of northern forests to CO2-induced climate change. Nature. 1988;334:55–58. [Google Scholar]
- Patz JA, Campbell-Lendrum D, Holloway T, Foley JA. Impact of regional climate change on human health. Nature. 2005;438:310–317. doi: 10.1038/nature04188. [DOI] [PubMed] [Google Scholar]
- Ranta H, Hokkanen T, Linkosalo T, Laukkanen L, Bondestam K, Oksanen A. Male flowering of birch: Spatial synchronization, year-to-year variation and relation of catkin numbers and airborne pollen counts. Forest Ecology and Management. 2008;255:643–650. [Google Scholar]
- Reid CE, Gamble JL. Aeroallergens, allergic disease, and climate change: impacts and adaptation. Eco Health. 2009;6:458–470. doi: 10.1007/s10393-009-0261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CA, Wolf J, O’Neill NR, Muilenberg ML, Ziska LH. Elevated atmospheric carbon dioxide concentrations amplify Alternaria alternata sporulation and total antigen production. Environmental Health Perspectives. 2010;118:1223–1228. doi: 10.1289/ehp.0901867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- Smith M, Skjøth CA, Myszkowska D, et al. Long-range transport of Ambrosia pollen to Poland. Agricultural and Forest Meteorology. 2008;148:1402–1411. [Google Scholar]
- Sofiev M, Bergmann K-C. Allergenic Pollen: A Review of the Production, Release, Distribution and Health Impacts. Springer; 2013. [Google Scholar]
- Walther G-R, Post E, Convey P, et al. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- Wan S, Yuan T, Bowdish S, Wallace L, Russell SD, Luo Y. Response of an allergenic species, Ambrosia psilostachya (Asteraceae), to experimental warming and clipping: implications for public health. American Journal of Botany. 2002;89:1843–1846. doi: 10.3732/ajb.89.11.1843. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Isukapalli SS, Bielory L, Georgopoulos PG. Bayesian analysis of climate change effects on observed and projected airborne levels of birch pollen. Atmospheric Environment. 2013;68:64–73. doi: 10.1016/j.atmosenv.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziello C, Böck A, Estrella N, Ankerst D, Menzel A. First flowering of wind pollinated species with the greatest phenological advances in Europe. Ecography. 2012a;35:1017–1023. [Google Scholar]
- Ziello C, Sparks TH, Estrella N, et al. Changes to airborne pollen counts across Europe. PLoS One. 2012b;7:e34076. doi: 10.1371/journal.pone.0034076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziska L, Knowlton K, Rogers C, et al. Recent warming by latitude associated with increased length of ragweed pollen season in central North America. Proceedings of the National Academy of Sciences. 2011;108:4248–4251. doi: 10.1073/pnas.1014107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziska LH, Beggs PJ. Anthropogenic climate change and allergen exposure: The role of plant biology. Journal of Allergy and Clinical Immunology. 2012;129:27–32. doi: 10.1016/j.jaci.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Ziska LH, Epstein PR, Schlesinger WH. Rising CO2, climate change, and public health: exploring the links to plant biology. Environmental Health Perspectives. 2009;117:155. doi: 10.1289/ehp.11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziska LH, Gebhard DE, Frenz DA, Faulkner S, Singer BD, Straka JG. Cities as harbingers of climate change: Common ragweed, urbanization, and public health. Journal of Allergy and Clinical Immunology. 2003;111:290–295. doi: 10.1067/mai.2003.53. [DOI] [PubMed] [Google Scholar]
- Ziska LH, George K, Frenz DA. Establishment and persistence of common ragweed (Ambrosia artemisiifolia L.) in disturbed soil as a function of an urban–rural macro-environment. Global Change Biology. 2007;13:266–274. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.