Abstract
Problem
Diabetes confers an increased risk of preeclampsia, but its pathogenic role in preeclampsia is poorly understood. The objective of this study was to elucidate the effects of excess glucose on trophoblast function and whether any changes could be reversed by metformin.
Method of Study
The human first trimester trophoblast cell line (Sw.71) was treated with glucose at 5mM, 10mM, 25mM and 50mM, in the presence and absence of metformin. Trophoblast migration was quantified and supernatant cytokine, chemokine, and angiogenic factors measured.
Results
Increasing concentrations of glucose significantly increased trophoblast secretion of the inflammatory cytokines/chemokines: IL-1β, IL-6, IL-8, GRO-α, RANTES and G-CSF; significantly increased trophoblast secretion of the anti-angiogenic factors sFlt-1 and sEndoglin; and significantly decreased trophoblast migration. Excess glucose-induced trophoblast IL-1β production was inhibited by disabling the Nalp3/ASC inflammasome. Metformin partially reduced the glucose-induced inflammatory response, but had no effect on the anti-angiogenic or anti-migratory response.
Conclusion
Excess glucose induced a pro-inflammatory, anti-angiogenic, and anti-migratory state in first trimester trophoblast cells. Glucose-induced trophoblast IL-1β secretion was mediated by the inflammasome. Glucose-induced inflammation was partially reversed by metformin. These findings demonstrate the pleiotropic effects of hyperglycemia on the trophoblast, providing potential explanations for the strong link between diabetes and preeclampsia.
Keywords: Diabetes, Glucose, Metformin, Preeclampsia, Trophoblast
Introduction
More than 8 million women in the United States have pre-gestational diabetes, and it is currently observed in 1% of all pregnancies1. The rate of diabetes in pregnancy is projected to rise in parallel with the growing burden of diabetes worldwide2. Pregnancies complicated by diabetes are at increased risk of adverse pregnancy outcomes3. In particular, poor glycemic control correlates with an increased risk for preeclampsia in a dose-dependent manner4.
Three prevailing observations exist for the pathogenesis of preeclampsia: shallow implantation of the placenta, disruption of the angiogenic balance, and inflammation at the uteroplacental interface. In early gestation, normal placentation requires the cytotrophoblast to invade beyond the decidua and remodel the maternal vasculature to form low-resistance, high-capacitance vessels5. In preeclampsia, impaired trophoblast migration and invasion, and elevated levels of anti-angiogenic factors, such as soluble fms-like tyrosine kinase (sFlt-1)6, may disrupt vascular remodeling. Lastly, a pro-inflammatory milieu, with increased concentrations of cytokine such as interleukin (IL)-6 and IL-8 has also been noted at the maternal-fetal interface in placenta from patients with preeclampsia7, 8.
In patients with diabetes, hyperglycemia is well-known to play a pathological role in development of microvascular disease9. Diabetic nephropathy, neuropathy and retinopathy are also characterized by increased inflammation and altered angiogenesis10. However, whether a similar mechanism of hyperglycemia-induced inflammation and abnormal angiogenesis occurs in the placenta is unclear. Understanding the cellular effects of excess glucose on the development of the human placenta is important because women with diabetes mellitus often have suboptimal glycemic control during the integral period of placentogenesis.
Metformin (1,1-dimethylbuguanide hydrochloride; Pregnancy category B) is a biguanide agent that is widely used as first-line treatment in type 2 diabetes11, 12. Metformin improves peripheral and liver sensitivity to insulin, reduces basal hepatic glucose production, and increases insulin-stimulated uptake and utilization of glucose by peripheral tissues13-15 While insulin remains the most reliable and commonly used diabetes treatment during pregnancy1, numerous studies have demonstrated the safety of metformin in pregnancy, and metformin is becoming increasingly accepted as an alternative16-18. This oral medication has intriguing potential applications to pregnancy, in relation to preeclampsia and abnormal placentation, as it has also been shown to improve inflammation and alter angiogenesis in other diabetic states10, 19. However, the effects of metformin on the placenta and its long-term effects on fetal physiology have not been well-studied16-18. Therefore, the objective of this study was to characterize the impact of excess glucose and metformin on the normal function of human first trimester trophoblast cells by studying their ability to produce cytokines, chemokines, and angiogenic factors and to migrate spontaneously in vitro.
Materials and Methods
Reagents
Sterile D-glucose and L-glucose in 45% solution was purchased from Sigma-Aldrich (St. Louis, MO, USA) and stored at room temperature. Metformin powder was purchased from Enzo Life Sciences (Farmingdale, NY, USA), reconstituted in distilled deionized water, re-filtered with 0.22μm filters, and stored at −20°C. The caspase −1 inhibitor (Z-WEHD-FMK) was purchased from R&D Systems (Minneapolis, MN).
Trophoblast Cell Lines
The immortalized human first trimester trophoblast cell line, Sw.71, was used in these studies. Sw.71 cells were derived by telomerase-mediated transformation of 7-week gestational age trophoblasts20. Sw.71 cells were maintained in DMEM (Life technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (Hyclone, South Logan, UT), 10mM Hepes, 0.1mM minimal essential medium (MEM) non-essential amino acids, 1mM sodium pyruvate, and 100nM penicillin/streptomycin (Life technologies) and maintained at 37°C with 5% CO2. These cells exhibit characteristics of an extravillous trophoblast and function similarly to primary cultures20-22. This study also used Sw.71 cells stably transfected with either the pLKO.1 expression plasmids containing the ASC-shRNA construct, NM_013258.3-718s1c1 (sh-ASC), or a non-target shRNA control, SHC002 (sh-control); or stably transfected with a specific shRNA Nalp3-shRNA (sh-Nalp3) or a Nalp3 mutated targeting sequence as a negative control (Nalp3-mut), as previously described22-24. For all treatment experiments, glucose-free, serum-free RPMI media (Life technologies) was used with sterile glucose added back. L-glucose was used as an osmotic control. Viability of the Sw.71 cells was confirmed using the CellTiter 96TM viability assay (Promega, Madison, WI, USA) at all doses of glucose and metformin used.
Cytokine, Chemokine and Angiogenic Factor Studies
Sw.71 trophoblast cells were treated with RPMI containing glucose at various concentrations representing physiological euglycemia to severe hyperglycemia: 5mM (equivalent to normoglycemia), 10mM (borderline hyperglycemia), 25mM (hyperglycemia), and 50mM (severe hyperglycemia), with or without metformin (0.5mM). This dose of metformin was found experimentally to not affect the viability of trophoblast cells (data not shown), and is commonly used for in vitro studies of metformin25. Glucose concentrations of 25mM and 50mM may be seen in the clinical conditions of diabetic ketoacidosis26 and hyperosmolar hyperglycemia state27, 28. Following a treatment for 72 hours, cell-free culture supernatants were collected, centrifuged at 1500 × g for 10 min, and stored at −80°C until analysis was performed. The concentrations of IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-17, monocyte chemoattractant protein (MCP-1), growth-regulated oncogene-alpha (GRO-α), RANTES (regulated on activation, normal T cell expressed and secreted), granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony stimulating factor (GM-CSF), interferon gamma (IFN-γ), Macrophage inflammatory protein 1 alpha (MIP-1α), MIP-1β, tumor necrosis factor alpha (TNF-α), sFlt-1, sEndoglin, vascular endothelial growth factor (VEGF), and placental growth factor (PlGF) in supernatants were evaluated by ELISA (Assay Designs, Ann Arbor, MI & R&D Systems; Minneapolis, MN) or using the Bioplex multiplex assay (Bio-Rad, Hercules, CA). Uric acid secretion was measured using the QuantiChrom assay kit from BioAssay Systems (Hayward, CA).
Migration Assay
A two-chamber assay was used for the migration studies and has been previously described by our group29. The lower chamber for this assay consisted of 24-well tissue culture plates (BD Falcon, Franklin Lakes, NJ, USA), which contained 800μL of treatment media. Trophoblast cells (1×105 cells in 200μL of respective treatment media) were then seeded into a cell culture well insert with 8-μm pore size membrane (BD Biosciences), which served as the upper chamber. Similar to supernatant experiments, cells were treated with various concentrations of glucose, with and without metformin (0.5mM). Following a 48 hour incubation, the inserts were removed and spontaneous trophoblast cell migration across the membranes were determined using the QCM 24-Well Colorimetric Cell Migration Assay (Chemicon International, Temecula, CA, USA). Migrated cells were then stained, collected, and lysed according to the manufacturer’s instruction. The resulting colored mixture were read in triplicate at 560 nm using a BioRad plate reader (Hercules, CA, USA), and compared to a 100% cell control to determine the relative percent migration.
Statistical Analysis
Experiments were performed at least three times and assayed in duplicate. Data are expressed as mean ± standard error of the mean (SEM) of pooled experiments. Statistical significance was set to p<0.05 and determined using Prism software (GraphPad Software, Inc, La Jolla, CA). For multiple comparisons, significance was determined using either one-way ANOVA or the Kruskal-Wallis test. Otherwise data was analyzed using either the Wilcoxon matched-pairs signed rank test or the Mann-Whitney test.
Results
Excess glucose induces an inflammatory trophoblast cytokine/chemokine profile
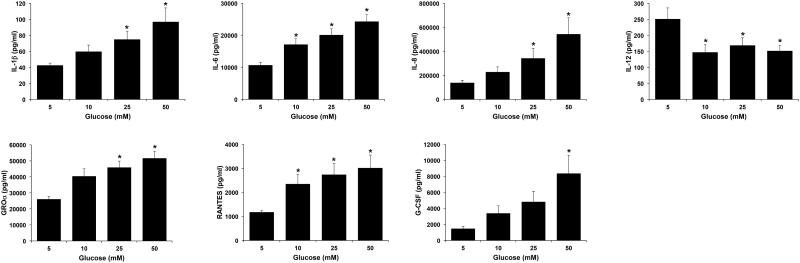
The first objective of this study was to determine the effect of excess glucose on the secretion of cytokines and chemokines by first trimester trophoblast cells. As shown in Figure 1, exposure of Sw.71 cells to increasing levels of glucose significantly increased the secretion of inflammatory cytokines and chemokines in a dose-dependent manner when compared to euglycemia levels of glucose (5mM). IL-6 and RANTES levels were significantly elevated at all glucose levels (10, 25 and 50mM) when compared to 5mM glucose; IL-1β, IL-8, and GRO-α levels were significantly elevated at the hyperglycemic levels of 25 and 50mM glucose; while G-CSF secretion was only significantly elevated at 50mM glucose (Figure 1). In parallel, basal secretion of immunomodulatory IL-12 was significantly downregulated at all glucose concentrations when compared to normoglycemic glucose levels of 5mM (Figure 1). Exposure of Sw.71 cells to increasing levels of glucose had no significant effect on the basal trophoblast secretion of IL-2, IL-4, IL-10, IL-17, IFNγ, MCP-1, TNFα, MIP-1α, MIP-1β or GM-CSF (data not shown).
Figure 1. Effect of glucose on trophoblast cytokine/chemokine secretion.
Sw.71 cells were treated with glucose at 5mM, 10mM, 25mM, and 50mM. Supernatants were analyzed for cytokines/chemokines by ELISA and multiplex assays. Barcharts show changes in IL-1β, IL-6 IL-8, IL-12, GRO-α, RANTES and G-CSF. *p<0.05 compared to 5mM glucose (n=6).
Glucose-induced trophoblast IL-1β secretion is mediated by the inflammasome
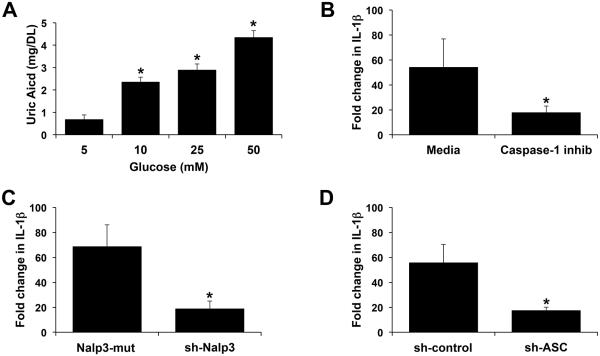
Having established that hyperglycemic levels of glucose induces a pro-inflammatory response in the trophoblast, including the upregulation of IL-1β secretion, we next sought to investigate some of the mechanisms involved. IL-1β secretion occurs after pro-IL-1β is processed into its active form; this is commonly mediated by the Nalp3 inflammasome, a complex of Nalp3 and ASC that subsequently activates caspase-130. In previous studies we demonstrated that human first trimester trophoblast IL-1β production can be mediated by the Nalp3/ASC inflammasome through the endogenous production of uric acid, a specific Nalp3 agonist22, 23. As shown in Figure 2A, glucose at all concentrations (10, 25 and 50mM) significantly increased trophoblast uric acid production when compared to the normoglycemic 5mM glucose control. Excess glucose-induced IL-1β secretion was significantly inhibited by the presence of a caspase 1 inhibitor by 67.1% (Figure 1B). Furthermore, 50mM glucose-induced IL-1β secretion was significantly inhibited by 72.8% when Nalp3 expression was knocked down (Figure 1C), and by 68.8% when ASC expression was knocked down (Figure 1D).
Figure 2. Glucose-induced trophoblast IL-1β secretion is mediated by the inflammasome.
(A) Sw.71 cells were treated with glucose at 5mM, 10mM, 25mM, and 50mM. Supernatants were measured for uric acid. *p<0.05 compared to 5mM glucose (n=3). (B) Sw.71 cells were treated with glucose at 5mM or 50mM in the presence of media or a caspase-1 inhibitor (caspase-1 inhib; 5μM) after which supernatants were measured for IL-1β by ELISA. (C-D) Sw.71 cells transfected to express either (C) shRNA for Nalp3 (sh-Nalp3) or a Nalp3 mutated targeting sequence (Nalp3-mut); or (D) shRNA for ASC (sh-ASC) or a control sequence (sh-control) were treated with glucose at 5mM or 50mM after which supernatants were measured for IL-1β by ELISA. (B-D) Bar charts show fold change in IL-1β in response to 50mM glucose relative to 5mM glucose (*p<0.05; n=3).
Metformin decreases trophoblast secretion of cytokines and chemokines in both normal and excess glucose conditions
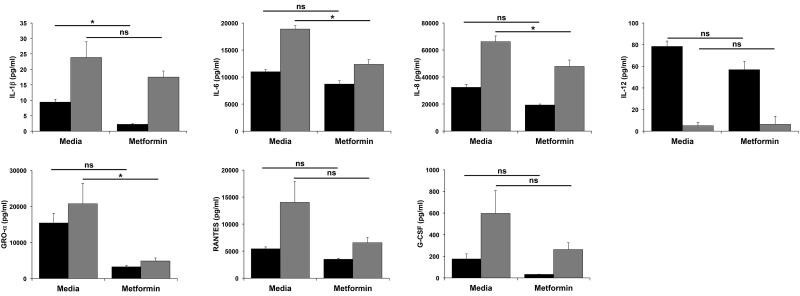
Having determined the effect of excess glucose levels on basal trophoblast cytokine and chemokine secretion, we next sought to evaluate the ability of metformin to modulate these glucose-induced alterations in trophoblast function. Since all the affected cytokines/chemokines were significantly altered at the glucose dose equivalent to severe hyperglycemia, Sw.71 cells were treated with media containing 5mM or 50mM glucose, with or without metformin (0.5mM) for 72 hours. As shown in Figure 3, under normal glucose conditions (5mM), metformin significantly reduced the basal secretion of IL-1β, but had no effect on the basal levels of IL-6, IL-8, IL-12, GRO-α, RANTES or G-CSF. Under conditions of excess glucose (50mM) where we observed a change in cytokine/chemokine secretion, metformin significantly reduced trophoblast production of IL-6, IL-8, and GRO-α, but had no effect on the excess glucose modulation of IL-1β, IL-12, RANTES or G-CSF secretion (Figure 3). While excess glucose had no effect on trophoblast secretion of IL-17, IFNγ, TNFα and MIP-1β, metformin was able to reduce the basal levels of these cytokines/chemokines (Supplemental Figure 1).
Figure 3. Effect of metformin on glucose-mediated modulation of trophoblast cytokine/chemokine secretion.
Sw.71 cells were treated with glucose at 5mM (black bars) or 50mM (gray bars) in the presence of media or metformin (0.5mM) (n=3). Supernatants were measured for cytokines/chemokines by ELISA and multiplex assays. Barcharts show changes in IL-1β, IL-6, IL-8, IL-12, GRO-α, RANTES and G-CSF (*p<0.05; ns=not significant).
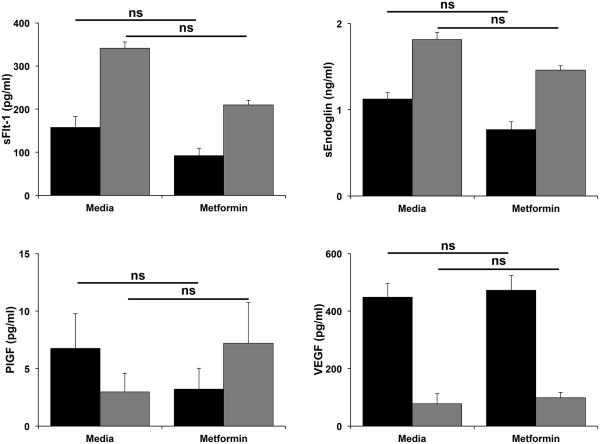
Excess glucose alters basal trophoblast angiogenic factor secretion and this is unaffected by metformin
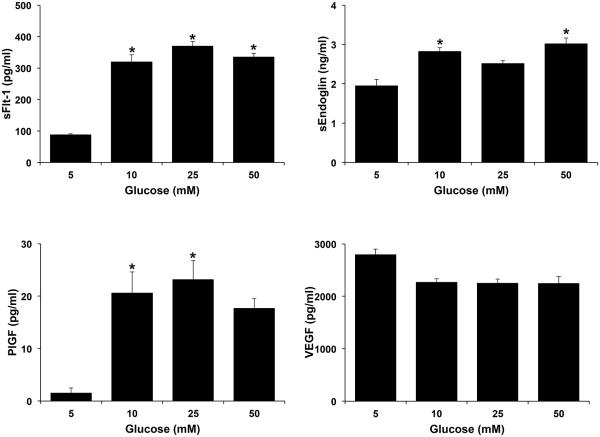
The next objective of this study was to determine the effects of excess glucose on the secretion of the anti-angiogenic factors, sFlt-1 and sEndoglin, and pro-angiogenic factors, VEGF and PlGF, by first trimester trophoblast cells. As shown in Figure 4, exposure of Sw.71 cells to increasing levels of glucose (10, 25 and 50mM) significantly increased the secretion of sFlt-1 when compared to the 5mM normoglycemic control. Glucose at 10 and 50mM also significantly increased trophoblast secretion of sEndoglin when compared to the 5mM normoglycemic control (Figure 4). While trophoblast secretion of PlGF was significantly upregulated by borderline and hyperglycemic levels of glucose (10 and 25mM) when compared to the 5mM normoglycemic control, the 50mM concentration had no significant effect (Figure 4). Increasing levels of glucose at all doses had no significant effect on trophoblast secretion of VEGF (Figure 4). As shown in Figure 5, metformin had no significant effect on trophoblast secretion of sFlt-1, sEndoglin, PlGF or VEGF under either normoglycemic (5mM glucose) or severe hyperglycemia (50mM glucose) conditions.
Figure 4. Effect of glucose on trophoblast angiogenic factor secretion.
Sw.71 cells were treated with glucose at 5mM, 10mM, 25mM, and 50mM. Supernatants were measured for angiogenic factors by ELISA and multiplex assays. Barcharts show changes in sFlt-1, sEndoglin, PlGF and VEGF. *p<0.05 compared to 5mM glucose (n=3).
Figure 5. Effect of metformin on glucose-mediated modulation of trophoblast angiogenic factor secretion.
Sw.71 cells were treatment with glucose at 5mM (black bars) or 50mM (gray bars) in the presence of media or metformin (0.5mM) (n=3). Supernatants were measured for angiogenic factors by ELISA and multiplex assays. Barcharts show changes in sFlt-1, sEndoglin, PlGF and VEGF (ns=not significant).
Excess glucose decreases basal trophoblast migration
The final objective of this study was to determine the effect of excess glucose on the ability of the trophoblast to spontaneously migrate, and the ability of metformin to modulate this response. As shown in Fig. 6A, exposure of Sw.71 cells to hyperglycemic levels of glucose (25 and 50mM) significantly decreased basal trophoblast migration. However, metformin did not affect basal trophoblast migration, nor was it able to reverse the excess glucose-mediated decrease in cell migration (Fig 6B).
Figure 6. Effect of glucose and metformin on trophoblast cell migration.
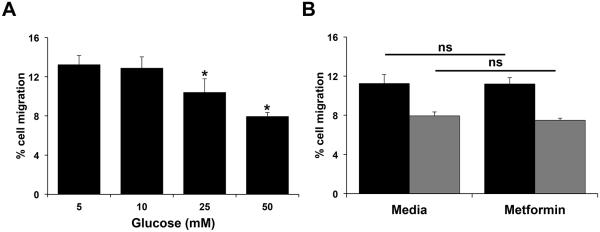
Sw.71 cells were incubated with: (A) glucose at 5mM, 10mM, 25mM, and 50mM (n=7); and (B) glucose at 5mM (black bars) and 50mM (gray bars) in the presence of media or metformin (0.5mM) (n=4). After 48 hours, cell migration was measured. *p<0.05 compared to 5mM glucose; ns=not significant.
Discussion
Despite the increasing prevalence of diabetes in the pregnant population1, the effects of hyperglycemia on the placenta remain poorly understood. We focused on the impact excess glucose levels have on human first trimester trophoblast function, given the unique role of these cells in placentation and pathogenesis of adverse pregnancy outcomes, such as preeclampsia. Trophoblast dysfunction is implicated in the shallow implantation and poor vascular remodeling seen in states of uteroplacental insufficiency6. Moreover, these alterations associated with preeclampsia are thought to occur early in gestation31.
The effects of hyperglycemia on biochemical networks in BeWo cells have been described32, but the implications of this previous work are limited by the use of a choriocarcinoma cell line, with a potential loss of differentiated cell function. Sw.71 cells, on the other hand, are well- characterized and mimic the characteristics of primary extravillous trophoblast cells20-22. The present study describes, for the first time, the broad effects of hyperglycemia on a non-cancerous human trophoblast cell line by investigating the cell’s complete cytokine, angiogenic, and migratory profile as well as the potential role of metformin in reversing these effects.
Diabetes is known to create a pro-inflammatory microenvironment that can progress to microvascular and macrovascular complications10. Our excess glucose-induced findings in the trophoblast mirror the inflammatory milieu seen in other organ systems affected by hyperglycemia, such as the kidneys, retina and neurons10. We found that increasing levels of glucose elevated trophoblast secretion of the inflammatory cytokines/chemokines: IL-1β, IL-6, IL-8, GRO-α, RANTES, and G-CSF, while the basal secretion of the immunomodulatory cytokine, IL-12, was reduced. Our data on the hyperglycemia-induced trophoblast secretion of IL-6 confirms data found in a recent study using a similar culture system33. Our observation that high levels of glucose trigger IL-1β secretion through the Nalp3/ASC inflammasome is in keeping with models of diabetic neuropathy and nephropathy showing involvement of the Nalp1 and Nalp3 inflammasomes34, 35. Moreover, like other triggers of trophoblast inflammasome activation and subsequent IL-1β production, glucose may be activating this pathway through the endogenous production of the Nalp3 agonist and danger signal, uric acid22, 23. Together, this pro-inflammatory state may contribute to the increased inflammation seen in preeclampsia8. Indeed, similarly to our observed glucose-induced inflammatory profile generated by the trophoblast, in pregnancies with preeclampsia, IL-1β, IL-6, IL-8, GRO-α, RANTES and G-CSF are increased in the placenta and/or the maternal circulation36-38.
The end-organ effects seen in diabetes may also result from alterations in the angiogenic balance, such as excessive angiogenesis in diabetic retinopathy19. In the retina, increased angiogenesis and VEGF result in proliferative retinopathy19. In the trophoblast, interestingly, hyperglycemia paradoxically induces an anti-angiogenic state, similar to that seen in preeclamptic pregnancies. In particular, sFlt-1, a soluble VEGF receptor, and sEndoglin, a co-receptor for TGF-β, have both been well-studied and are known to be elevated in maternal plasma in women with preeclampsia compared to healthy pregnant controls39. Our observations of the excess glucose-induced anti-angiogenic milieu align with our current understanding of the pathogenesis of preeclampsia. Herein we found that increasing levels of glucose elevated trophoblast release of sFlt-1 and sEndoglin, similar to findings by Cawyer et al., at similar glucose concentrations33. However, contrary to the study by Cawyer et al., who reported reduced PlGF and VEGF levels33, we found high levels of glucose elevated trophoblast PlGF secretion and had no effect on the secretion of VEGF. Nonetheless, the net effect may be anti-angiogenic.
Lastly, cell migration may be either promoted or impeded by excess glucose, depending on the cell type. For example, high levels of glucose increase rat brain astrocyte migration40, but impedes human fibroblast migration41. Our study shows for the first time that hyperglycemic conditions may decrease the ability of trophoblast cells to spontaneously migrate. While we have previously found that decreased IL-6 production is associated with reduced trophoblast migration42, in this current study we saw glucose increasing IL-6 production while reducing cell migration. Thus, glucose may be modulating another pro- or anti-migratory factor(s) in the trophoblast. Preeclampsia is associated with failure of cytotrophoblasts to invade and remodel the uterine spiral arteries, thereby compromising blood flow to the maternal-fetal interface5. Thus, the shallow trophoblast migration resulting from exposure to excess glucose may result in uteroplacental insufficiency in patients with diabetes.
Optimal pre-pregnancy control of existing diabetes is most effective in decreasing risks associated with diabetes in pregnancy43. Unfortunately, relatively few women with diabetes receive preconceptional counseling44. Management of diabetes upon identification of a new pregnancy, therefore, requires rapid initiation and titration of an appropriate insulin regimen. Euglycemia is often not achieved until after the period of integral placentation in the first and early second trimesters. Metformin may provide a possible alternative therapy due to its ease of use, its role as the first-line medication in the non-pregnant population, and its beneficial effects in other organ systems. However, the effects of metformin on the human trophoblast had not been studied. Metformin co-treatment was able to reduce both basal and glucose-induced up-regulated secretion of some, but not all, inflammatory cytokines and chemokines by trophoblast. However, metformin was unsuccessful in reversing the anti-angiogenic response and migratory deficit. Although the treatment in vitro reverses some of the findings seen in preeclampsia, the clinical effects of reducing cytokine and chemokine levels are unknown.
One limitation of our study is the unknown concentrations of metformin in placental tissues in vivo. Plasma levels of metformin may underestimate the local penetration and concentration in tissue45. A dose previously described in in vitro literature was chosen25. A second limitation is the use of a trophoblast cell line for these experiments rather than primary cultures. However, Sw.71 cells have been well-characterized to mimic the properties of extravillous trophoblast cells20-22. The mechanisms by which excess glucose and metformin modulate trophoblast function are mostly unknown, although our findings suggest a role for the Nalp3/ASC inflammasome in glucose-induced IL-1β production; a novel observation. In contrast, the pathogenesis of non-placental microvascular complications of diabetes have been extensively studied. Reactive oxygen species, advanced glycation end-products (AGE), and NF-κB activation have all been implicated in the pathogenesis of diabetic nephropathy, retinopathy, and neuropathy10, and may play a similar role in the placenta; and a recent study in trophoblast cells suggest a role for MAPK p38 and peroxisome proliferator-activated receptor-gamma (PPAR-γ)33. Metformin, on the other hand, is known to activate adenosine-5’-monophosphate (AMP)-activated protein kinase (AMPK) in hepatocytes46 AMPK, a major cellular energy sensor and master regulator of metabolic homeostasis, also participates in cytokine regulation in skeletal muscles47. Activation of AMPK in the trophoblast may be involved in metformin’s ability to modulate cytokine and chemokine production in trophoblast cells. Further studies are needed to explore the mechanisms of glucose and metformin action in the trophoblast.
In summary, this study characterizes the effects of excess glucose on the inflammatory, angiogenic and migratory profile of human first trimester trophoblast cells, providing potential explanations for the strong link between diabetes and preeclampsia. This study also suggests the potential use of metformin as an alternative therapy in the first trimester to reverse the possibly detrimental effects of excess glucose on the developing placenta.
Supplementary Material
Supplemental Figure 1. Effect of metformin on glucose-mediated modulation of trophoblast cytokine/chemokine secretion. Sw.71 cells were with glucose at 5mM (black bars) or 50mM (gray bars) in the presence of media or metformin (0.5mM) (n=3). Supernatants were analyzed for cytokines/chemokines by multiplex assays. Barcharts show changes in IL-17, IFNγ, TNFα and MIP-1β (*p<0.05; ns=not significant).
Acknowledgments
This work was supported in part by the following grants: Albert McKern Award for Perinatal Research (to CSH); Yale Women’s Reproductive Health Research (WRHR) Career Development Center K12HD 047018-07 (to Hugh S. Taylor); and R01HD049446 from NICHD, NIH (to VMA).
References
- 1.American College of Obstetricians and Gynecologist Committee on Practice Bulletins Practice Bulletin No. 60: Pregestational Diabetes Mellitus. 2005;105:675–685. [Google Scholar]
- 2.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes research and clinical practice. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologist Committee on Practice Bulletins Practice Bulletin No. 137: Gestational diabetes mellitus. 2013;122:406–416. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 4.Holmes VA, Young IS, Patterson CC, Pearson DW, Walker JD, Maresh MJ, McCance DR. Diabetes, Pre-eclampsia Intervention Trial Study G: Optimal glycemic control, pre-eclampsia, and gestational hypertension in women with type 1 diabetes in the diabetes and pre-eclampsia intervention trial. Diabetes care. 2011;34:1683–1688. doi: 10.2337/dc11-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? The Journal of clinical investigation. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 7.Bowen RS, Gu Y, Zhang Y, Lewis DF, Wang Y. Hypoxia promotes interleukin-6 and −8 but reduces interleukin-10 production by placental trophoblast cells from preeclamptic pregnancies. J Soc Gynecol Investig. 2005;12:428–432. doi: 10.1016/j.jsgi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Saito S, Nakashima A. A review of the mechanism for poor placentation in early-onset preeclampsia: the role of autophagy in trophoblast invasion and vascular remodeling. Journal of reproductive immunology. 2014;101-102:80–88. doi: 10.1016/j.jri.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Bmj. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen DV, Shaw LC, Grant MB. Inflammation in the pathogenesis of microvascular complications in diabetes. Frontiers in endocrinology. 2012;3:170. doi: 10.3389/fendo.2012.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR, American Diabetes A. European Association for the Study of D: Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qaseem A, Humphrey LL, Sweet DE, Starkey M, Shekelle P. Clinical Guidelines Committee of the American College of P: Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Annals of internal medicine. 2012;156:218–231. doi: 10.7326/0003-4819-156-3-201202070-00011. [DOI] [PubMed] [Google Scholar]
- 13.Hundal RS, Inzucchi SE. Metformin: new understandings, new uses. Drugs. 2003;63:1879–1894. doi: 10.2165/00003495-200363180-00001. [DOI] [PubMed] [Google Scholar]
- 14.Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, Shulman GI. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saenz A, Fernandez-Esteban I, Mataix A, Ausejo M, Roque M, Moher D. Metformin monotherapy for type 2 diabetes mellitus. The Cochrane database of systematic reviews. 2005 doi: 10.1002/14651858.CD002966.pub3. CD002966. [DOI] [PubMed] [Google Scholar]
- 16.Balani J, Hyer SL, Rodin DA, Shehata H. Pregnancy outcomes in women with gestational diabetes treated with metformin or insulin: a case-control study. Diabetic medicine : a journal of the British Diabetic Association. 2009;26:798–802. doi: 10.1111/j.1464-5491.2009.02780.x. [DOI] [PubMed] [Google Scholar]
- 17.Rowan JA, Hague WM, Gao W, Battin MR, Moore MP, Mi GTI. Metformin versus insulin for the treatment of gestational diabetes. The New England journal of medicine. 2008;358:2003–2015. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- 18.Tertti K, Ekblad U, Vahlberg T, Ronnemaa T. Comparison of metformin and insulin in the treatment of gestational diabetes: a retrospective, case-control study. The review of diabetic studies : RDS. 2008;5:95–101. doi: 10.1900/RDS.2008.5.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling S, Birnbaum Y, Nanhwan MK, Thomas B, Bajaj M, Ye Y. MicroRNA-dependent cross-talk between VEGF and HIF1alpha in the diabetic retina. Cellular signalling. 2013;25:2840–2847. doi: 10.1016/j.cellsig.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 20.Straszewski-Chavez SL, Abrahams VM, Alvero AB, Aldo PB, Ma Y, Guller S, Romero R, Mor G. The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta. 2009;30:939–948. doi: 10.1016/j.placenta.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulla MJ, Yu AG, Cardenas I, Guller S, Panda B, Abrahams VM. Regulation of Nod1 and Nod2 in first trimester trophoblast cells. American journal of reproductive immunology. 2009;61:294–302. doi: 10.1111/j.1600-0897.2009.00694.x. [DOI] [PubMed] [Google Scholar]
- 22.Mulla MJ, Salmon JE, Chamley LW, Brosens JJ, Boeras CM, Kavathas PB, Abrahams VM. A role for uric acid and the Nalp3 inflammasome in antiphospholipid antibody- induced IL-1beta production by human first trimester trophoblast. PloS one. 2013;8:e65237. doi: 10.1371/journal.pone.0065237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulla MJ, Myrtolli K, Potter J, Boeras C, Kavathas PB, Sfakianaki AK, Tadesse S, Norwitz ER, Guller S, Abrahams VM. Uric acid induces trophoblast IL-1beta production via the inflammasome: implications for the pathogenesis of preeclampsia. Am J Reprod Immunol. 2011;65:542–548. doi: 10.1111/j.1600-0897.2010.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kavathas PB, Boeras CM, Mulla MJ, Abrahams VM. Nod1, but not the ASC inflammasome, contributes to induction of IL-1beta secretion in human trophoblasts after sensing of Chlamydia trachomatis. Mucosal Immunol. 2013;6:235–243. doi: 10.1038/mi.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kefas BA, Cai Y, Kerckhofs K, Ling Z, Martens G, Heimberg H, Pipeleers D, Van de Casteele M. Metformin-induced stimulation of AMP-activated protein kinase in beta-cells impairs their glucose responsiveness and can lead to apoptosis. Biochemical pharmacology. 2004;68:409–416. doi: 10.1016/j.bcp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Fulop M, Tannenbaum H, Dreyer N. Ketotic hyperosmolar coma. Lancet. 1973;2:635–639. doi: 10.1016/s0140-6736(73)92478-1. [DOI] [PubMed] [Google Scholar]
- 27.Arieff AI, Carroll HJ. Nonketotic hyperosmolar coma with hyperglycemia: clinical features, pathophysiology, renal function, acid-base balance, plasma-cerebrospinal fluid equilibria and the effects of therapy in 37 cases. Medicine. 1972;51:73–94. doi: 10.1097/00005792-197203000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Daugirdas JT, Kronfol NO, Tzamaloukas AH, Ing TS. Hyperosmolar coma: cellular dehydration and the serum sodium concentration. Annals of internal medicine. 1989;110:855–857. doi: 10.7326/0003-4819-110-11-855. [DOI] [PubMed] [Google Scholar]
- 29.Mulla MJ, Myrtolli K, Brosens JJ, Chamley LW, Kwak-Kim JY, Paidas MJ, Abrahams VM. Antiphospholipid antibodies limit trophoblast migration by reducing IL-6 production and STAT3 activity. American journal of reproductive immunology. 2010;63:339–348. doi: 10.1111/j.1600-0897.2009.00805.x. [DOI] [PubMed] [Google Scholar]
- 30.Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA. IL-1beta processing in host defense: beyond the inflammasomes. PLoS Pathog. 2010;6:e1000661. doi: 10.1371/journal.ppat.1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000;21(Suppl A):S25–30. doi: 10.1053/plac.1999.0522. [DOI] [PubMed] [Google Scholar]
- 32.Inadera H, Tachibana S, Takasaki I, Tatematsu M, Shimomura A. Hyperglycemia perturbs biochemical networks in human trophoblast BeWo cells. Endocrine journal. 2010;57:567–577. doi: 10.1507/endocrj.k10e-045. [DOI] [PubMed] [Google Scholar]
- 33.R Cawyer C, Horvat D, Leonard D, Allen SR, Jones RO, Zawieja DC, Kuehl TJ, Uddin MN. Hyperglycemia impairs cytotrophoblast function via stress signaling. American Journal of Obstetrics and Gynecology. 2014 doi: 10.1016/j.ajog.2014.04.033. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Chen K, Zhang J, Zhang W, Zhang J, Yang J, Li K, He Y. ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. The international journal of biochemistry & cell biology. 2013;45:932–943. doi: 10.1016/j.biocel.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Meng XF, Wang XL, Tian XJ, Yang ZH, Chu GP, Zhang J, Li M, Shi J, Zhang C. Nod-like receptor protein 1 inflammasome mediates neuron injury under high glucose. Molecular neurobiology. 2014;49:673–684. doi: 10.1007/s12035-013-8551-2. [DOI] [PubMed] [Google Scholar]
- 36.Rusterholz C, Hahn S, Holzgreve W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Seminars in immunopathology. 2007;29:151–162. doi: 10.1007/s00281-007-0071-6. [DOI] [PubMed] [Google Scholar]
- 37.Matsubara K, Ochi H, Kitagawa H, Yamanaka K, Kusanagi Y, Ito M. Concentrations of serum granulocyte-colony-stimulating factor in normal pregnancy and preeclampsia. Hypertension in pregnancy. 1999;18:95–106. doi: 10.3109/10641959909009614. [DOI] [PubMed] [Google Scholar]
- 38.Mellembakken JR, Solum NO, Ueland T, Videm V, Aukrust P. Increased concentrations of soluble CD40 ligand, RANTES and GRO-alpha in preeclampsia--possible role of platelet activation. Thromb Haemost. 2001;86:1272–1276. [PubMed] [Google Scholar]
- 39.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. The New England journal of medicine. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh HL, Lin CC, Hsiao LD, Yang CM. High glucose induces reactive oxygen species- dependent matrix metalloproteinase-9 expression and cell migration in brain astrocytes. Molecular neurobiology. 2013;48:601–614. doi: 10.1007/s12035-013-8442-6. [DOI] [PubMed] [Google Scholar]
- 41.Xuan YH, Huang BB, Tian HS, Chi LS, Duan YM, Wang X, Zhu ZX, Cai WH, Zhu YT, Wei TM, Ye HB, Cong WT, Jin LT. High-Glucose Inhibits Human Fibroblast Cell Migration in Wound Healing via Repression of bFGF-Regulating JNK Phosphorylation. PLoS One. 2014;9:e108182. doi: 10.1371/journal.pone.0108182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulla MJ, Myrtolli K, Brosens JJ, Chamley LW, Kwak-Kim JY, Paidas MJ, Abrahams VM. Antiphospholipid Antibodies Limit Trophoblast Migration by Reducing IL-6 Production and STAT3 Activity. Am J Reprod Immunol. 2010;63:339–348. doi: 10.1111/j.1600-0897.2009.00805.x. [DOI] [PubMed] [Google Scholar]
- 43.Gabbe SG, Graves CR. Management of diabetes mellitus complicating pregnancy. Obstetrics and gynecology. 2003;102:857–868. doi: 10.1016/j.obstetgynecol.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Singh H, Murphy HR, Hendrieckx C, Ritterband L, Speight J. The challenges and future considerations regarding pregnancy-related outcomes in women with pre-existing diabetes. Current diabetes reports. 2013;13:869–876. doi: 10.1007/s11892-013-0417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilcock C, Bailey CJ. Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica; the fate of foreign compounds in biological systems. 1994;24:49–57. doi: 10.3109/00498259409043220. [DOI] [PubMed] [Google Scholar]
- 46.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. The Journal of clinical investigation. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glund S, Treebak JT, Long YC, Barres R, Viollet B, Wojtaszewski JF, Zierath JR. Role of adenosine 5'-monophosphate-activated protein kinase in interleukin-6 release from isolated mouse skeletal muscle. Endocrinology. 2009;150:600–606. doi: 10.1210/en.2008-1204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Effect of metformin on glucose-mediated modulation of trophoblast cytokine/chemokine secretion. Sw.71 cells were with glucose at 5mM (black bars) or 50mM (gray bars) in the presence of media or metformin (0.5mM) (n=3). Supernatants were analyzed for cytokines/chemokines by multiplex assays. Barcharts show changes in IL-17, IFNγ, TNFα and MIP-1β (*p<0.05; ns=not significant).