Abstract
Behavioral inhibition (BI) is a temperament identified early in life that is associated with increased risk for anxiety disorders. Amygdala hyperresponsivity, found both in behaviorally inhibited and anxious individuals, suggests that amygdala dysfunction may represent a marker of anxiety risk. However, broader amygdala networks have not been examined in individuals with a history of childhood BI. This study uses resting state fMRI to assess amygdala intrinsic functional connectivity (iFC) in 38 healthy young adults (19 with a history of BI, 19 with no history of BI) selected from a longitudinal study. Centromedial, basolateral, and superficial amygdala iFCs were compared between groups and examined in relation to self-report measures of anxiety. Group differences were observed in amygdala iFC with prefrontal cortex, striatum, anterior insula, and cerebellum. Adults characterized with BI in childhood endorsed greater state anxiety prior to entering the scanner, which was associated with several of the group differences. Findings support enduring effects of BI on amygdala circuitry, even in the absence of current psychopathology.
Keywords: behavioral inhibition, functional connectivity, amygdala, temperament, anxiety, fMRI
Behavioral inhibition (BI) is a temperament identified in the first years of life characterized by hyper-vigilance, heightened reactivity to novelty, and social reticence (Fox, Henderson, Marshall, Nichols, & Ghera, 2005; Kagan, Reznick, & Snidman, 1987). This temperament is shown to be stable with time and to increase risk for anxiety disorders (Rosenbaum et al., 1993). The link to anxiety raises questions regarding the degree to which neural correlates are shared across these two phenotypes, subjects with a history of BI and those with clinical anxiety (Lahat, Hong, & Fox, 2011; Perez-Edgar & Fox, 2005). The identification of a shared neural correlate in BI and anxiety may provide a trait marker that ultimately would inform the neurobiology of risk for anxiety. Evidence implicates altered amygdala function as such a marker, demonstrating that amygdala hyperreactivity persists in adolescents and adults with a history of childhood BI even when they do not exhibit clear behavioral manifestations of anxiety (Perez-Edgar et al., 2007; Schwartz, Wright, Shin, Kagan, & Rauch, 2003). To date, the functional integrity of broader amygdala-based networks has not been systematically examined in relation to BI, despite evidence of disruptions in individuals with anxiety disorders (Etkin, Prater, Schatzberg, Menon, & Greicius, 2009; Roy et al., 2013). The present study uses resting-state fMRI to examine the intrinsic functional connectivity of the amygdala in individuals with and without a history of childhood BI in order to identify putative network-level neural markers of anxiety risk.
Recent studies have identified potential biomarkers of clinical anxiety using resting-state fMRI. These studies examined the intrinsic functional connectivity (iFC) of amygdala subdivisions, including the basolateral (BLA), centromedial (CMA) and superficial (SFA) (Etkin, et al., 2009; Roy, et al., 2013). Compared to healthy peers, adolescents with generalized anxiety disorder (GAD) showed reduced iFC between the CMA and rostral anterior cingulate cortex (rACC), enhanced iFC between the CMA and the insula and enhanced iFC between the SFA and dorsomedial prefrontal cortex (dmPFC) (Roy, et al., 2013). The present work extends these findings by taking a similar approach to assess neural markers of temperamental risk for anxiety in young adults with a documented early history of BI.
Despite the absence of amygdala iFC studies of BI, recent work in related areas can inform study hypotheses. This includes studies in humans on the associations between early-life stress and whole amygdala iFC in adolescence (Burghy et al., 2012), as well as data on amygdala iFC in non-human primate models of BI (Fox et al., 2012; Shackman et al., 2013). Data on task-based amygdala connectivity in BI are particularly relevant. Specifically, in a subset of subjects from the current study, Hardee and colleagues linked early-life BI to perturbed amygdala functional connectivity with anterior insula and dorsolateral prefrontal cortex (dlPFC; Hardee et al., 2013). This study also found that amygdala-insula functional connectivity predicted current internalizing symptoms in the BI group, findings that resembled those in recent report of abnormal CMA-insula iFC in anxious adolescents (Roy, et al., 2013). Further, findings of perturbed amygdala-dlPFC connectivity in this study parallel findings from research on monkeys with anxious temperament and children with anxiety disorders (Birn et al., 2014).
The aim of the present study is to examine enduring BI-related alterations in amygdala iFC in a sample of young adults who were initially assessed as infants. Based on work in animal models of BI (Birn et al., 2014; Fox, et al., 2012; Shackman, et al., 2013) and human studies (Hardee, et al., 2013), we predict that adults with a childhood history of BI will show alterations in amygdala iFC with insula and dlPFC. To examine the unique impact of BI on amygdala circuitry, independent of anxious pathology, we only included participants without current or lifetime anxiety diagnoses in our analyses. We propose that BI-related alterations in amygdala iFC similar to those observed in clinical anxiety represent persistent biomarkers of risk for anxiety, while those not observed in anxiety may represent compensatory mechanisms or markers of resilience. Self-reported anxiety levels are examined to further disentangle iFC markers of early childhood BI from those of current anxiety.
Method
The current sample was selected from a larger longitudinal cohort that has been followed since 4 months of age. At that time, 433 participants were assessed for motor and emotional reactivity to novel stimuli. A subset of these infants (n = 153), who exhibited either minimal or heightened reactivity, was enrolled in the longitudinal study. Inhibited behavior to novel stimuli was coded at 14 and 24 months and social reticence during standardized social situations with unfamiliar peers was coded at 48 months and 7 years (Fox, Henderson, Rubin, Calkins, & Schmidt, 2001). Parents also completed questionnaires assessing their child’s temperament at each of these time points. Behavioral and questionnaire scores were standardized at each time point and used to create a single composite score of behavioral inhibition (Perez-Edgar, et al., 2007). Participants with scores in the upper half of the distribution were classified as having behaviorally inhibited temperament (BI) and those with scores in the lower half of the distribution were classified as non-behaviorally inhibited (non-BI).
A total of 60 members of this longitudinal cohort completed the current study when they were between the ages of 18 and 21 years. Six (1 BI, 5 non-BI) were excluded from analyses because they were receiving pharmacological treatment at the time of the scan and six (2 BI, 4 non-BI) were excluded due to a lifetime history of anxiety disorders. An additional ten participants (4 BI, 6 non-BI) were excluded due to excessive movement during the scan. A final sample of 38 subjects was used for the current analyses (19 in BI group, 19 in non-BI group; mean age = 19.5 years). All participants were Caucasian, right-handed, and free from current use of psychotropic medication at the time of the scan. The study was approved by the institutional review boards of the University of Maryland, College Park and the National Institute of Mental Health. Informed consent was obtained from participants prior to participation. The presence of current or lifetime psychiatric disorder was assessed by the Structured Clinical Interview for DSM IV (SCID; First, Spitzer, Gibbon, & Williams, 2002). Anxiety levels were assessed in two ways: (1) the Beck Anxiety Inventory (BAI; Beck, Epstein, Brown, & Steer, 1988) was used as a measure of trait anxiety, and (2) the State subscale of the State Trait Anxiety Inventory (STAI-S; Spielberger, Vagg, Barker, Donham, & Westberry, 1980) was used as a measure of state anxiety at the time of the scan.
Data acquisition and image processing
Imaging data were acquired on three NIH 3Tesla scanners with the same acquisition parameters. To control for scanner effects on study results, scanner was entered as a covariate in group analyses as described below. During the resting state (RS-fMRI) scan, participants were instructed to remain still with eyes open while a black screen with a central white crosshair was placed in their field of view. The RS-fMRI sequence consisted of a 6-minute acquisition of 180 EPI functional volumes (TR = 2000 ms; TE = 30 ms; flip angle = 90; FOV = 240x192 mm; acquisition voxel size 3x3x4 mm). T1-weighted anatomical images were obtained for purposes of spatial normalization and localization.
All data were preprocessed at NYU using AFNI (http://afni.nimh.nih.gov/afni/) and FSL (http://www.fmrib.ox.ac.uk/fsl). Preprocessing procedures were identical to those used in previous studies (Di Martino et al., 2009; Roy, et al., 2013) and consisted of slice time correction (interleaved acquisition), motion correction, despiking, spatial smoothing (FWHM=6mm), mean-based intensity normalization of all volumes by the same factor, temporal band-pass filtering (0.009-0.1Hz) and linear and quadratic detrending. Visual inspection of images was conducted at each preprocessing step for quality control. RS-fMRI scans with a maximum displacement greater than 3.1 mm were excluded from further analyses. Linear registration of high resolution structural images to the Montreal Neurological Institute MNI152 template with 2x2x2mm resolution was carried out using the FSL tool FLIRT, and refined using FNIRT nonlinear registration. The preprocessed data were regressed on nine nuisance covariates, removing variance associated with signals derived from white matter and cerebrospinal fluid, six motion parameters, and the global signal, which is included to control for physiological artifacts. The resultant 4-D residual time series was registered to MNI152 space using linear (FLIRT) and nonlinear (FNIRT) procedures for use in subsequent analyses.
Time series extraction
Procedures for amygdala region-of-interest (ROI) definition and time series extraction were identical to those used previously (Roy, et al., 2013; Roy et al., 2009). Standardized amygdala ROIs were defined based on the Juelich histological atlas implemented in FSL, which defines centromedial (CMA), basolateral (BLA), and superficial (SFA) subdivisions based on stereotaxic, probabilistic maps of cytoarchitectonic boundaries (Amunts et al., 2005). Each ROI included only those voxels that exhibited at least a 50% probability of belonging to their given subdivision and overlapping voxels were delegated to the region with the higher probability. Additionally, due to variation in coverage of the medial temporal lobe across participants, a study-specific mask was derived and used to further refine the amygdala ROIs to insure full coverage across all participants. As a result, the basolateral and total amygdala ROIs were reduced by 464mm2 on the left and by 640mm2 on the right. This resulted in final volumes for the left and right BLA ROIs of 1376 mm2 and 1280 mm2, respectively, and 2496 mm2 and 2184 mm2 for the left and right total amygdala, respectively. The sizes of the other amygdala ROIs were the same as used previously (Roy et al., 2009): left and right CMA masks were 176 mm2 and 224 mm2, respectively, and the left and right SFA masks were 952 mm2 and 760 mm2, respectively. Once ROIs were established, the average timeseries across all voxels of each ROI were extracted for each participant for use in subsequent functional connectivity analyses. Statistical Analyses
Voxel-wise Examination of Group Differences in Amygdala iFC
For each participant, first-level multiple regression analyses were conducted using FMRIB Improved Linear Model (FILM) in FSL. For each hemisphere a multiple regression GLM was created that included the time series for each of the three subdivisions resulting in individual subject-level maps of all voxels exhibiting positive and negative iFC with each amygdala subdivision, controlling for the others.
Group-level analyses were carried out using a random-effects ordinary least squares model that included 2 group mean predictors of interest (one for each group), as well as demeaned framewise displacement (an estimate of motion) and scanner as covariates of no interest. Group comparisons were conducted using cluster-level Gaussian Random Field theory for multiple comparison correction, with additional correction for the inclusion of all three amygdala subdivisions (Z > 2.3; p < 0.05/3, corrected). Further examination of amygdala iFC in the regions exhibiting significant group differences was conducted using SPSS 19.0. Average partial regression coefficients (i.e., iFC) were extracted for the significant cluster(s) for each participant. One-sample t-tests were used to determine whether measures of iFC differed significantly from zero. Correlational analyses were conducted to examine the relation of these iFC measures with BI scores and self-reported anxiety symptoms (BAI and STAI-S) across both groups and within groups. Additionally, a multivariate analysis of covariance (MANCOVA) was conducted to examine whether group differences in self-reported anxiety affected group differences in iFC.
Results
As shown in Table 1, no group differences (BI vs. non-BI) were observed for sex, age, IQ, or movement during the resting state scan (mean framewise displacement). No participants had current psychopathology and no group difference emerged in BAI scores. The BI group did endorse significantly higher state anxiety immediately before the fMRI scan than the non-BI group (t[35] = 3.09; p = .005).
Table 1.
Demographic characteristics
| Variable | BI (n = 19) | Non-BI (n = 19) |
|---|---|---|
|
| ||
| Mean Age (SD) | 19.6 (1.0) | 19.5 (.84) |
|
| ||
| # Females (%) | 9 (47.4%) | 11 (57.9%) |
|
| ||
| Mean Estimated IQ (SD) 1 | 116.2 (7.9) | 114.9 (8.9) |
|
| ||
| Scanner Used | ||
| Scanner #1 | 8 (42.1%) | 8 (42.1%) |
| Scanner #2 | 9 (47.4%) | 8 (42.1%) |
| Scanner #3 | 2 (10.5%) | 3 (15.8%) |
|
| ||
| Mean Framewise Displacement (SD) | .10 (.04) | .10 (.04) |
|
| ||
| Beck Anxiety Inventory2 | 4.69 (3.3) | 5.79 (6.8) |
|
| ||
| State-Trait Anxiety Inventory3 | 31.1 (8.6) | 23.9 (5.0) |
IQ data missing for 1 non-BI participant;
Data missing for 3 BI participants and 5 non-BI participants;
Data missing for 1 BI participant, Group difference p < .01.
Voxelwise Group Differences in iFC
Total Amygdala
No significant group differences were observed for the right or left total amygdala.
Centromedial amygdala (CMA)
Compared to non-behaviorally inhibited adults, those with a history of BI showed significantly reduced iFC between the left CMA and a cluster encompassing rostral and subgenual ACC, extending to the caudate and nucleus accumbens. Specifically, BI adults showed significantly negative iFC in this cluster, while non-behaviorally inhibited adults showed non-significant iFC (Table 2 and Figure 1). No group differences were found for the right CMA.
Table 2.
Regions of Significant Group Differences in Amygdala Intrinsic Functional Connectivity
| Seed Region | Target | Cluster Size | X | Y | Z | Max Z | One-Sample t-Test Results |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Left Amygdala | |||||||
|
| |||||||
| Centromedial BI < non-BI |
Subgenual ACC | 824 | 4 | 20 | -6 | 3.77 | BI: t = −6.32, p < .001 BN: t = −.148, p = ns |
|
| |||||||
| Basolateral BI < non-BI |
Middle frontal gyrus (L) | 1124 | −30 | 6 | 56 | 3.83 | BI: t = −2.78, p = .012 BN: t = 5.39, p < .001 |
| Frontal operculum (R) | 843 | 38 | 14 | 12 | 4.07 | BI: t = −5.82, p < .001 BN: t = 1.09, p = ns |
|
|
| |||||||
| Superficial BI < non-BI |
Posterior Cingulate/ Lingual Gyrus | 2103 | −14 | −44 | −2 | 3.53 | BI: t = −2.79, p = .012 BN: t = 4.12, p= .001 |
| Occipital Pole | 885 | 16 | −92 | −10 | 3.73 | BI: t = −2.55, p = .02 BN: t = 3.90, p = .001 |
|
|
| |||||||
| Right Amygdala | |||||||
|
| |||||||
| Basolateral BI > non-BI |
Cerebellum | 1121 | 20 | −62 | −20 | 3.67 | BI: t = 3.99, p = .001 BN: t = −2.43, p < .03 |
|
| |||||||
| Superficial BI < non-BI |
Insula/ Temporal Pole | 770 | 36 | 0 | −20 | 4.81 | BI: t = −2.10, p = .05 BN: t = 4.58, p < .001 |
Figure 1.
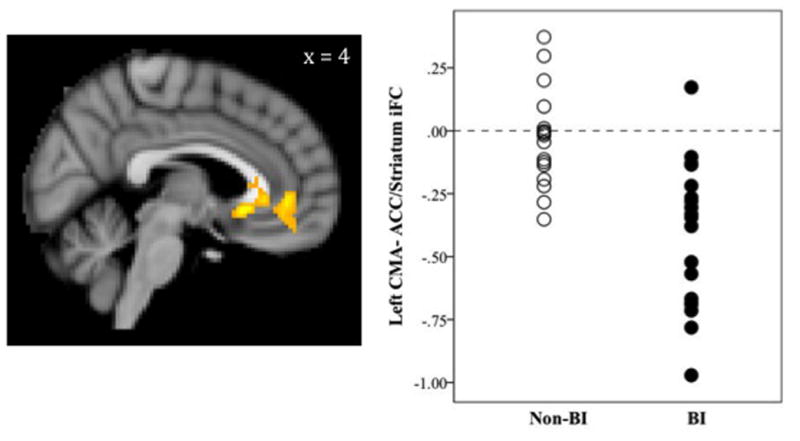
BI-Related Differences in Centromedial Amygdala (CMA) Intrinsic Functional Connectivity
Image in radiological convention (right = left, left = right); iFC = intrinsic functional connectivity; ACC = anterior cingulate cortex; BI = Behaviorally inhibited group; Non-BI = Non-behaviorally inhibited group
Basolateral amygdala (BLA)
As shown in Table 2 and Figure 2, significant group differences in left BLA iFC were observed in two clusters. First, adults characterized in childhood with behavioral inhibition exhibited significantly negative iFC between the left BLA and a large cluster extending from left dlPFC to frontal operculum. Non-behaviorally inhibited adults showed significantly positive iFC in this cluster resulting in significant group differences. Second, the BI group exhibited significantly negative iFC between left BLA and a cluster of voxels extending from right frontal operculum to striatal regions (caudate and globus pallidus). The non-BI group showed no significant iFC in this cluster. For the right BLA, adults characterized in childhood with BI showed significantly positive iFC with the cerebellum, while the non-behaviorally inhibited adults showed negative iFC between right BLA and cerebellum (see Figure 2).
Figure 2.
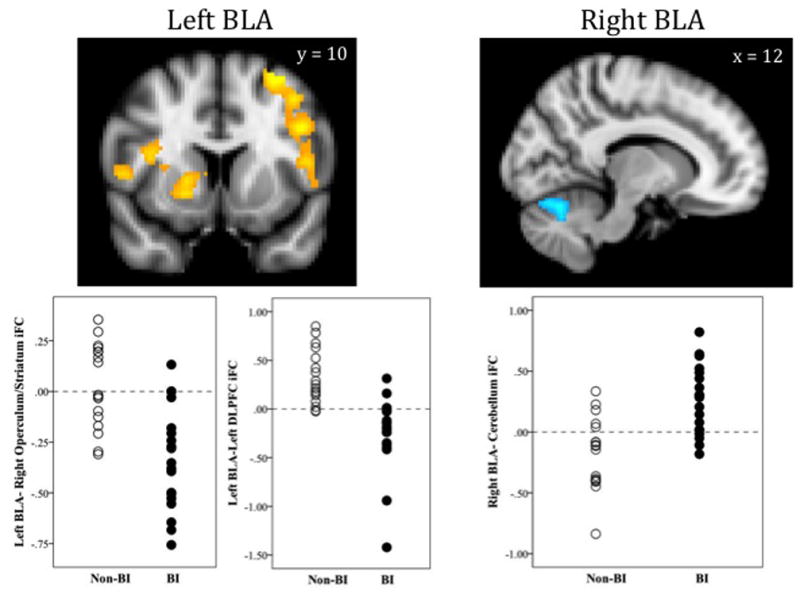
BI-Related Differences in Basolateral Amygdala (BLA) Intrinsic Functional Connectivity
Images in radiological convention (right = left, left = right); iFC = intrinsic functional connectivity; DLPFC = dorsolateral prefrontal cortex; BI = Behaviorally inhibited group; Non-BI = Non-behaviorally inhibited group
Superficial amygdala (SFA)
As shown in Figure 3, significant group differences were observed in the iFC between the left SFA and two posterior clusters. One cluster encompasses the posterior cingulate cortex and lingual gyrus, while the other resides in the occipital pole. Group differences were also observed in the iFC between right SFA and a cluster including the ventral anterior insula and temporal pole (Figure 3). As shown in Table 2, adults characterized in childhood with BI showed significantly negative iFC, while the non-behaviorally inhibited adults showed significantly positive iFC in all three of these clusters.
Figure 3.
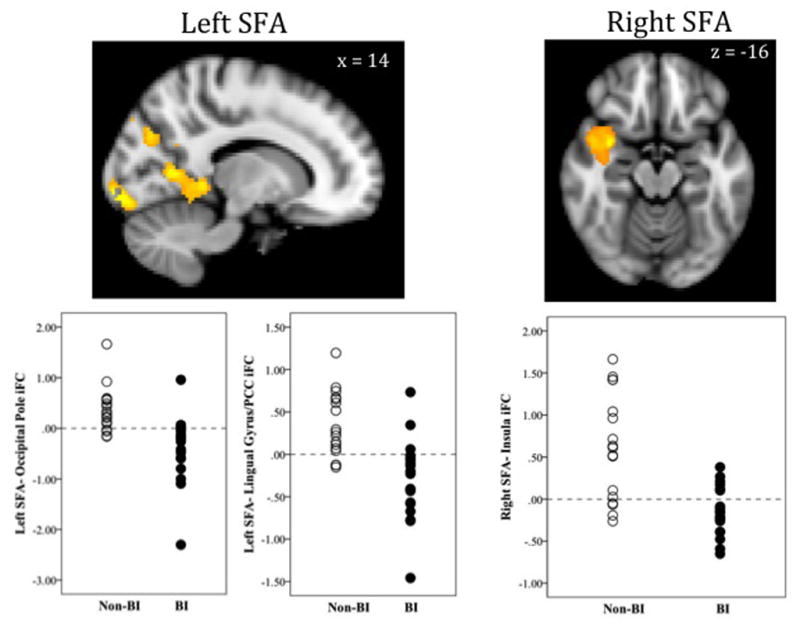
BI-Related Differences in Superficial Amygdala (SFA) Intrinsic Functional Connectivity
Images in radiological convention (right = left, left = right); iFC = intrinsic functional connectivity; PCC = posterior cingulate cortex; BI = Behaviorally inhibited group; Non-BI = Non-behaviorally inhibited group
Relationship between Self-Reported Anxiety and Amygdala iFC
Correlational analyses were conducted between anxiety scores (STAI-S, BAI) and iFC measures for the total sample and for either group separately. No significant associations emerged for the BAI. Across the whole sample, STAI-S scores were moderately correlated with iFC of the left CMA (r = −.40, p = .015), right BLA (r = .38, p = .02), and right SFA r = −.37, p = .02). However, these correlations were no longer statistically significant after controlling for multiple comparisons (p = .05/7 = .007). No significant correlations were observed within the BI group alone, while a moderate correlation was observed in the BN group between STAI-S scores and iFC between right SFA and the insula (r = −.53, p = .02). To assess whether these STAI-S scores were contributing to any of the results discussed above, a multivariate ANCOVA was conducted to assess group differences while controlling for STAI-S scores in all seven significant iFC clusters. The overall effect of group was significant (F[7, 28] = 10.5, p < .001) with no effect of STAI-S scores (F[7, 28] = .42 , p = ns). Group differences also remained significant for all seven iFC clusters (p < .003 for all).
Discussion
This is the first study to examine amygdala intrinsic functional connectivity (iFC) in young adults with a documented history of the early childhood temperament of behavioral inhibition (BI). Group differences in iFC were not observed for the total amygdala but were found across all three amygdala subdivisions suggesting that childhood BI is associated with connectivity of the neural circuits that are key to emotion processing. These results extend previous task-based findings of BI-related alterations in amygdala function (Perez-Edgar, et al., 2007; Schwartz, et al., 2003).
Overall, findings support predictions based on previous work and suggest that early childhood BI is associated with disruptions in functional circuits similar to those observed in anxiety disorders, even when such psychopathology has never developed. For example, group differences observed in amygdala circuits implicated in fear learning and regulation were similar to those observed between individuals with and without anxiety disorders. These circuits consist of the amygdala-subgenual ACC, particularly associated with fear extinction and expression (Etkin, Egner, & Kalisch, 2011; Milad et al., 2007; Quirk, Likhtik, Pelletier, & Pare, 2003) and the amygdala-cerebellum, particularly associated with fear learning and memory (Sacchetti, Sacco, & Strata, 2007; Sacchetti, Scelfo, & Strata, 2005; Supple, Leaton, & Fanselow, 1987). This is further supported by a recent study suggesting that behaviorally inhibited adults exhibit facilitated fear learning even in the absence of an anxiety disorder (Myers, VanMeenen, McAuley, Pang, & Servatius, 2012). Further work directly comparing behavioral measures of fear learning and iFC in the same sample is needed to explore these putative relationships.
As predicted, group differences emerged in amygdala iFC with the anterior insula, a network implicated in attention orienting as well as integration of environmental inputs and interoceptive state, processes that are disrupted in anxiety disorders (Hoehn-Saric, Schlund, & Wong, 2004; Paulus & Stein, 2010) and BI (Perez-Edgar et al., 2010; Perez-Edgar et al., 2011). The iFC of right SFA with right anterior insula was found to be negative in the BI group while it was positive in the non-BI group. Previous work shows similar negative amygdala-insula functional connectivity in adults characterized in childhood with BI during threat processing (Hardee, et al., 2013). In addition, associations between insula volume and similar temperamental traits such as shyness have been reported (Yang et al., 2013). Although not initially hypothesized, group differences in amygdala- striatal iFC are consistent with previous task-based studies showing altered striatal function in behaviorally inhibited adolescents (Bar-Haim et al., 2009; Guyer et al., 2006; Helfinstein et al., 2011) and adolescents with an anxiety disorder (Guyer et al., 2012). In the present work, adults characterized with BI in childhood exhibited negative iFC of the striatum with both the left BLA and CMA, while the non-BI group showed no significant iFC in these circuits, a finding consistent with previous work with anxious adolescents (Roy et al., 2013). Amygdalastriatal interactions have been implicated in avoidance learning, a mechanism involved in active coping with fear (Delgado, Jou, LeDoux, & Phelps, 2009; LeDoux & Gorman, 2001). However, the specific functions affected by this BI-related disruption in amygdala-striatum iFC, such as impaired avoidance learning, remain to be examined.
Finally, in line with our predictions, we observed group differences in BLA iFC with the dlPFC, a region implicated in strategic emotion regulation processes, particularly in relation to the amygdala (Goldin, McRae, Ramel, & Gross, 2008.) These group differences in BLA-dlPFC iFC are similar to previous task-based data showing greater negative functional connectivity between amygdala and dlPFC in young adults with a history of childhood BI when viewing angry faces (Hardee, et al., 2013) and in adults with social anxiety disorder when viewing fearful faces (Prater, Hosanagar, Klumpp, Angstadt, & Phan, 2013). However, they are in contrast to resting state findings that show positive iFC between the BLA and dlPFC in adults with generalized anxiety disorder and no significant iFC (Etkin, et al., 2009) or negative iFC (Roy et al., 2009) in non-anxious comparisons. In the present study, the non-BI group showed positive iFC between these regions. Thus, these results are not easily interpreted in the context of previous work. The functional connectivity between these two regions may depend upon whether cognitive strategies are being deployed. For example, under stress, such as when observing negative stimuli, these strategies may be engaged leading to negative iFC. Similarly, in the present study, the adults characterized with BI in childhood exhibited greater state anxiety during the scan, relative to those without childhood BI, which might be driving a similar pattern. These ideas remain speculative as measures of thought processes or cognitive regulation during the scan were not obtained during the current study.
Compared to the non-BI group, adults characterized in childhood with BI endorsed greater state anxiety (STAI-S) immediately prior to entering the scanning room; trait anxiety (BAI scores) did not differ between groups. Of note, STAI-S scores of both groups were relatively low, within the normative range, and not indicative of clinical levels of anxiety. However, this still suggests that temperamental vulnerability may continue to be apparent under stressful or novel circumstances even in psychiatrically healthy individuals. Further, these state anxiety levels correlated with iFC of several amygdala circuits when examined across groups. While this is highly confounded due to the significant group differences in STAI-C scores, it is notable as such correlations were specific to regions of amygdala iFC involved in fear learning and expression, as defined above. Increased reactivity to the stress of the MRI environment in adults with childhood BI may be a reflection of disrupted amygdala circuitry, particularly in these circuits. However, the design of this study does not allow us to dissociate the roles of BI and state anxiety on iFC.
The present findings should be interpreted with the following limitations. First, the sample sizes are relatively small, reducing our ability to detect significant effects. However, this is a unique longitudinal sample, extremely well-characterized since age 4 months. Therefore, the participants are fairly homogeneous within each group, which mitigates, to a certain extent, the limited sample sizes. Second, since these functional imaging data were acquired in young adulthood without neural measures obtained earlier in development, we cannot know whether observed connectivity differences were the cause (already present early on), or result (emerged with age), of early childhood behavioral inhibition. Third, we selected a sample of adults characterized with BI that was devoid of individuals with an anxiety disorder in order to examine perturbations in neural circuitry associated with BI without the confounds of anxious pathology. While an advantage for sample homogeneity, this prevented us from examining the potential modulation by clinical anxiety of the BI findings and more fully test models of risk and resiliency. To do this, a substantially larger longitudinal study would need to be conducted so that by young adulthood, there would be a sufficient number of participants in each of four groups (BI-anxious, BI-nonanxious, non-BI-anxious, non-BI-nonanxious) that a full factorial analysis could be conducted. Finally, data were collected across three scanners which might have contributed to variability, further reducing power. However, we estimate such variability to be small because the three scanners were of the same brand (3 Tesla GE) and the acquisition sequence was identical across scanners. In addition, we used scanner as a nuisance covariate in all analyses.
In summary, the present findings extend previous task-based evidence of alterations in neural function amongst adults characterized in childhood with BI. Critically, these adults carried no current or lifetime anxiety diagnoses, providing us with a sample of healthy resilient individuals who started their life with a fearful temperament. Thus, among our findings, those that are similar to reports of previous anxiety studies suggest that these circuits may reflect early long-lasting markers of temperamental risk for anxiety. Conversely, BI-related alterations in amygdala-dlPFC circuits that have not previously been observed in anxiety may represent compensatory or protective mechanisms that support resilience. This resilience at the trait level (i.e., BAI) may not carry over to the state level (STAI-S), as evidenced by greater state anxiety at the time of the scan in the adults characterized in childhood with BI. State anxiety may have its own impact on amygdala circuits, as evidenced by the moderate associations observed between STAI-S scores and several amygdala iFC measures. Large, longitudinal studies combining task-based and resting state methods, and including individuals who differ on BI as well as anxiety pathology would be needed to further investigate these different interpretations.
Highlights.
We assess amygdala functional connectivity associated with childhood behavioral inhibition
Resting state fMRI was used to examine amygdala intrinsic functional connectivity
Adults with a history of childhood BI show altered amygdala functional connectivity
Acknowledgments
This research was supported by the National Institute of Mental Health (5U01MH093349-02; 3U01MH093349-02S1 [PI: N.A. Fox]; K01-MH073569 [PI: K. Perez-Edgar]).
Footnotes
Financial Disclosures
The authors have no financial relationships to disclose.
References
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, et al. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychological Science. 2009;20:1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Shackman AJ, Oler JA, Williams LE, McFarlin DR, Rogers GM, et al. Evolutionarily conserved prefrontal-amygdalar dysfunction in early-life anxiety. Molecular Psychiatry. 2014 doi: 10.038/mp.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience. 2012;15:1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Jou RL, LeDoux JE, Phelps EA. Avoiding negative outcoes: Tracking the mechanisms of avoidance learning in humans during fer conditioning. Frontiers in Behavioral Neuroscience. 2009;3:1–9. doi: 10.3389/neuro.08.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Shehzad Z, Kelly C, Roy AK, Gee DG, Uddin LQ, et al. Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiatry. 2009;166:891–899. doi: 10.1176/appi.ajp.2009.08121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of General Psychiatry. 2009;66:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. SCID-I/NP (for DSM-IV) Non-patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fox AS, Oler JA, Shelton SE, Nanda SA, Davidson RJ, Roseboom PH, et al. Central amygdala nucleus (Ce) gene expression linked to increased trait-like Ce metabolism and anxious temperament in young primates. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18108–18113. doi: 10.1073/pnas.1206723109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Dev. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural basis of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577– 586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez-Edgar K, et al. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. The American journal of psychiatry. 2012;169:205–212. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardee JE, Benson BE, Bar-Haim Y, Mogg K, Bradley BP, Chen G, et al. Patterns of Neural Connectivity During an Attention Bias Task Moderates Associations Between Early Childhood Temperament and Internalizing Symptoms in Young Adulthood. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein SM, Benson B, Perez-Edgar K, Bar-Haim Y, Detloff A, Pine DS, et al. Striatal responses to negative monetary outcomes differ between temperamentally inhibited and non-inhibited adolescents. Neuropsychologia. 2011;49:479–485. doi: 10.1016/j.neuropsychologia.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn-Saric R, Schlund MW, Wong SH. Effects of citalopram on worry and brain activation in patients with generalized anxiety disorder. Psychiatry Res. 2004;131:11–21. doi: 10.1016/j.pscychresns.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Jarcho JM, Fox NA, Pine DS, Etkin A, Leibenluft E, Shechner T, et al. The neural correlates of emotion-based cognitive control in adults with early childhood behavioral inhibition. Biological Psychology. 2013;92:306–314. doi: 10.1016/j.biopsycho.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Dev. 1987;58:1459–1473. [PubMed] [Google Scholar]
- Lahat A, Hong M, Fox NA. Behavioural inhibition: is it a risk factor for anxiety? International Review of Psychiatry. 2011;23:248–257. doi: 10.3109/09540261.2011.590468. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Gorman JM. A call to action: Overcoming anxiety through active coping. American Journal of Psychiatry. 2001;158:1953– 1955. doi: 10.1176/appi.ajp.158.12.1953. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Myers CE, VanMeenen KM, McAuley JD, Beck KD, Pang KCH, Servatius RJ. Behaviorally-inhibited temperament is associated with severity of PTSD symptoms and faster eyeblink conditioning in veterans. Stress. 2012;15:31– 44. doi: 10.3109/10253890.2011.578184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain structure & function. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, Bar-Haim Y, McDermott JM, Chronis-Tuscano A, Pine DS, Fox NA. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010;10:349–357. doi: 10.1037/a0018486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, Fox NA. Temperament and anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2005;14:681–706. viii. doi: 10.1016/j.chc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Perez-Edgar K, Reeb-Sutherland BC, McDermott JM, White LK, Henderson HA, Degnan KA, et al. Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. Journal of Abnormal Child Psychology. 2011;39:885–895. doi: 10.1007/s10802-011-9495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35:1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL. Aberrant amygdala- frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depression and Anxiety. 2013;30:234– 241. doi: 10.1002/da.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum JF, Biederman J, Bolduc-Murphy EA, Faraone SV, Chaloff J, Hirshfeld DR, et al. Behavioral inhibition in childhood: a risk factor for anxiety disorders. Harv.Rev. Psychiatry. 1993;1:2–16. doi: 10.3109/10673229309017052. [DOI] [PubMed] [Google Scholar]
- Roy AK, Fudge JL, Kelly C, Perry JS, Daniele T, Carlisi C, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:290– 299. e292. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Sacco T, Strata P. Reversible inactivation of amygdala and cerebellum but not perirhinal cortex impairs reactivated fear memories. The European Journal of Neuroscience. 2007;25:2875–2884. doi: 10.1111/j.1460-9568.2007.05508.x. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Scelfo B, Strata P. The cerebellum: synaptic changes and fear conditioning. The Neuroscientist. 2005;11:217–227. doi: 10.1177/1073858405276428. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants "grown up": adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, Oler JA, Shelton SE, Davidson RJ, Kalin NH. Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6145–6150. doi: 10.1073/pnas.1214364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Vagg PR, Barker LR, Donham GW, Westberry LG. The factor structure of the state-trait anxiety inventory. In: Sarason IG, Spielberger CD, editors. Stress and Anxiety. Washington, DC: Hemisphere Publishing Corporation; 1980. pp. 95–109. [Google Scholar]
- Supple WF, Jr, Leaton RN, Fanselow MS. Effects of cerebellar vermal lesions on species-specific fear responses, neophobia, and taste-aversion learning in rats. Physiology & Behavior. 1987;39:579–586. doi: 10.1016/0031-9384(87)90156-9. [DOI] [PubMed] [Google Scholar]
- Yang X, Kendrick KM, Wu Q, Chen T, Lama S, Cheng B, et al. Structural and functional connectivity changes in the brain associated with shyness but not with social anxiety. PloS ONE. 2013;8:e63151. doi: 10.1371/journal.pone.0063151. [DOI] [PMC free article] [PubMed] [Google Scholar]


