Abstract
Background:
Studies have shown a relationship between increased iron content and clinical progression, cognitive impairment, and brain atrophy in patients with multiple sclerosis. Altered phase, as determined by susceptibility-weighted imaging (SWI), can potentially capture iron content changes.
Objective:
The objective of this study was to investigate phase changes in white matter (WM) lesions and subcortical deep-gray matter (SDGM) of patients with relapsing–remitting (RR) MS treated with interferon beta-1a administered subcutaneously versus untreated healthy controls (HCs).
Methods:
We conducted a 24-week, nonrandomized, open-label pilot study of 23 patients with RRMS receiving interferon beta-1a administered subcutaneously and 15 HCs. Patients were imaged on a 3T scanner at baseline, 12, and 24 weeks; changes in phase behavior in WM lesions and regional SDGM [mean phase of low-phase voxels (MP-LPV)], and in SDGM volumes, were measured. Between- and within-group changes were tested using nonparametric statistics adjusted for multiple comparisons.
Results:
The number (p = 0.003) and volume (p < 0.001) of phase WM lesions both significantly decreased among RRMS patients over 24 weeks. At baseline, MP-LPV was lower (suggestive of greater iron content) in total SDGM among RRMS patients versus HCs (p = 0.002). Week 24 MP-LPV changes from baseline were not significantly different between groups in total SDGM or any region except the putamen (−0.0025 radians in RRMS patients versus 0.0035 radians in HCs; p = 0.041).
Conclusions:
Over 24 weeks, phase lesions were reduced significantly in the RRMS group. These preliminary results suggest that SWI-filtered phase may become a useful tool for monitoring RRMS disease activity.
Keywords: iron, MRI, multiple sclerosis, phase assessment
Introduction
Iron is essential for normal brain function and is a cofactor for synthesis of neurotransmitters and many key brain tissues, including myelin [Ortiz et al. 2004]. An imbalance of iron metabolism can influence the pathogenesis of a number of neurological disorders [Hallgren and Sourander, 1958]. The mechanism by which this occurs is unclear, but may involve the generation of reactive oxygen species that cause neurotoxicity, as brain tissue is particularly sensitive to oxidative stress [Sullivan, 2004; Zivadinov et al. 2012].
Brain iron accumulation is associated with ageing in healthy individuals, and has been implicated as a risk factor for neurodegeneration [Bartzokis et al. 2007; Hagemeier et al. 2012a]. An increase in brain iron level has been suggested to be associated with the development, progression, and clinical impact of multiple sclerosis (MS) [Al-Radaideh et al. 2013; Ceccarelli et al. 2011; Hagemeier et al. 2012c, 2013b, 2013c; Jomova and Valko, 2011; Khalil et al. 2011]. The association between gray matter (GM) damage and increased MS disability is increasingly reported, with iron accumulation being observed in structures where volume loss has also occurred [Hagemeier et al. 2013b].
Several magnetic resonance imaging (MRI) techniques enable in vivo imaging of paramagnetic substances, such as iron, in the brain. One such approach, susceptibility-weighted imaging (SWI), may be useful for investigating white matter (WM) lesions and phase changes in subcortical deep GM (SDGM) structures [Haacke et al. 2004, Hagemeier et al. 2012c; 2013b]. SWI takes advantage of magnetic field changes caused by constituents such as iron through their influence on the phase of proton spin [Haacke et al. 2004]. Both post mortem [Bagnato et al. 2011; Liem et al. 2012; Yao et al. 2012] and in vivo [Liem et al. 2012; Yao et al. 2012] studies have confirmed associations between local SWI-filtered phase shift and underlying magnetic susceptibility, which may be affected by increased tissue iron content.
The objective of this study was to investigate changes of WM lesions and SDGM SWI-filtered phase behavior, and of SDGM volumes in patients with relapsing–remitting (RR) MS over 24 weeks of treatment with interferon beta-1a administered subcutaneously (Rebif®, EMD Serono Inc., a subsidiary of Merck KGaA, Darmstadt, Germany) with reference to healthy controls (HCs) not receiving treatment.
Methods
Study design and patients
This was a 24-week, nonrandomized, open-label, two-arm pilot study [ClinicalTrials.gov identifier: NCT01085318] in patients with RRMS receiving interferon beta-1a administered subcutaneously compared with a HC reference group that was conducted at a single center in the United States [Zivadinov et al. 2014].
Patients with RRMS and HCs were eligible for inclusion if they were aged 18–65 years; eligible patients had a diagnosis of RRMS according to the McDonald 2010 criteria [Polman et al. 2010] and disease duration <20 years, and were treatment-naïve or using any of the US Food and Drug Administration (FDA)-approved disease-modifying drugs (except natalizumab, mitoxantrone, or interferon beta-1a administered subcutaneously).
Baseline assessments included physical and neurological exams, MRI scans, and laboratory tests. Neuroimaging assessments were performed at baseline, week 12, and at the week 24/exit visit. Neurological examinations were conducted at screening and the week 24/exit visit. Neurological exams were not blinded. A safety evaluation telephone call was scheduled in week 28 (4 weeks after study exit). Patients with RRMS received interferon beta-1a administered subcutaneously titrated over 4 weeks to a final dose of 44 µg for injection three times weekly for 24 weeks.
Patients and controls gave written informed consent before participation, and the protocol was approved by the University at Buffalo Health Sciences Institutional Review Board. Screening occurred within 2 weeks prior to entering the study.
Study endpoints
Changes in SWI-filtered phase behavior in WM lesion number and volume and in regional SDGM, and changes in regional volumes in the SDGM, were measured at baseline and at 12 and 24 weeks in patients with RRMS treated with interferon beta-1a administered subcutaneously and in untreated HCs. These assessments were secondary endpoints in the study. The primary endpoints were changes in voxel-wise magnetization transfer ratio in normal appearing brain tissue and lesions in RRMS patients taking interferon beta-1a administered subcutaneously compared with that in HCs, as reported previously [Zivadinov et al. 2014]. Safety endpoints included the number of study-emergent adverse events over 24 weeks.
MRI assessments
All scans were carried out on a 3T General Electric Signa Excite HD 12.0 (General Electric, Milwaukee, WI, USA) right-handed system using a multichannel head and neck coil. SWI was acquired using a 3D flow-compensated gradient echo sequence with 64 slices, 2-mm slice thickness, field of view (FOV) = 25.6 cm × 19.2 cm, in-plane resolution of 0.5 mm × 1 mm (flip angle = 12° echo time [TE]/repetition time [TR] = 22/40 ms; acquisition time = 8 min 46 s, bandwidth = 13.89 kHz) [Zivadinov et al. 2012, 2014]. Additional sequences included dual fast spin-echo proton density (PD) and T2 weighted image (WI), fluid-attenuated inversion recovery (FLAIR), spin-echo T1WI and a 3D high-resolution T1WI fast spoiled gradient echo sequence with a magnetization-prepared inversion recovery pulse, as reported previously [Zivadinov et al. 2014]. Although perfect blinding between groups is impossible when viewing images, raters were unaware a priori of subjects’ group assignment.
Phase assessment in WM lesions
An overview of the SWI processing, lesion analysis method, and reproducibility results used in the present study is discussed elsewhere [Hagemeier et al. 2012b, 2014]. Each subject’s images and WM lesion maps were coregistered with functional MRI of the brain’s linear image registration tool (FLIRT) using 6 degrees of freedom (rigid body) [Jenkinson and Smith, 2011]. Images were resampled using trilinear interpolation, and WM lesion maps were resampled with nearest-neighbor interpolation using the registration matrix of their associated images. Identification of WM lesions was done using a semi-automated edge-detection contouring/thresholding technique [Zivadinov et al. 2001, 2012].
Classification of phase WM lesions was performed using a manual region-of-interest approach that was previously shown to be reproducible [Hagemeier et al. 2012b, 2014]. WM lesions were identified separately on T2/PD/FLAIR, T1WI, and SWI-filtered phase maps in a blinded manner without a priori knowledge of where the WM lesions were located with respect to the other modality. Only round/oval WM lesions ⩾3 mm in size were included in the study. In addition, a subset of WM lesions was visible on both SWI-filtered phase and T2/PD/FLAIR, and a subset was visible on both SWI-filtered phase and T1WI; these were identified automatically a posteriori as overlapping when one or more voxels overlapped after unblinding was performed.
Low mean phase identification in the SDGM
Segmentation of SDGM structures for volumetric and SWI analysis was done using FLIRT on the 3D T1WI [Patenaude et al. 2011]. Additional structures not identifiable this way (red nucleus, pulvinar nucleus, and substantia nigra) were manually delineated on the most representative SWI-filtered phase slice by a single operator using JIM5 (Xinapse Systems Ltd., Northamptonshire, UK), as reported previously [Zivadinov et al. 2012]. An overview of the analysis method and reproducibility results used in the present study is discussed elsewhere [Zivadinov et al. 2012]. In this study, the mean phase of low-phase voxels (MP-LPV) of each structure was determined by thresholding the phase images to retain only those voxels with phase values lower than two standard deviations below the reference group means, as explained elsewhere [Zivadinov et al. 2012; Hagemeier et al. 2013a]. As a measure of the degree of phase abnormality, the mean phase values of the resulting thresholded voxels were calculated, yielding MP-LPV. Structure-specific maps of voxels with low phase are presented in radians, with lower MP-LPV values suggesting increased iron content.
Statistical methods
Sample size was based on clinical rather than statistical considerations. It was estimated that screening 55 subjects would achieve an intent-to-treat population of 25 patients with RRMS and 15 HCs [Zivadinov et al. 2014]. For testing differences in SWI endpoints between the patients with RRMS group and the HCs, the Wilcoxon rank-sum test was used. For testing differences over time within the RRMS group, the Wilcoxon signed-rank test was used. All statistical tests were two-tailed and were considered significant at an α = 0.05 level of significance. Owing to the exploratory nature of this pilot study and the number of nonindependent endpoints, corrections for multiple comparisons were made for post-baseline comparisons using the Holm–Bonferroni method. Unadjusted p values are reported with those remaining significant after adjustment noted.
Results
In total, 21 of the 23 enrolled patients with RRMS and all 15 HCs completed the study. There were two discontinuations: one due to investigator decision, and one lost to follow up [Zivadinov et al. 2014]. Baseline characteristics were comparable between the study groups, and patients in the RRMS group were representative of a RRMS patient population (Table 1).
Table 1.
Baseline characteristics and drug exposure/compliance of patients with RRMS and healthy controls.
| Patients with RRMS |
HCs |
||
|---|---|---|---|
| n = 23 | n = 15 | ||
| Age, years, mean (SD)a | 39.9 (10.17) | 36.7 (10.31) | |
| Female, n (%)a | 14 (61) | 8 (53) | |
| Race, n (%)a | |||
| White | 20 (87) | 14b (93) | |
| Black | 3 (13) | 0 | |
| Other: Indian | 0 | 1 (7) | |
| Mean (SD) weight, kga | 79.9 (22.25) | 87.0 (18.33) | |
| Mean (SD) height, cma | 171.0 (8.48) | 168.5 (6.99) | |
| Mean (SD) BMI, kg/m2a | 27.2 (6.90) | 30.5 (5.37) | |
| Mean (SD) years since MS diagnosis, range | 6.6 (5.65), 0–20 | — | |
| Mean (SD) years since most recent relapse, range | 1.0 (1.14), 0.1–5.0 | — | |
| Mean (SD) number of relapses in past 12 monthsc | 1.26 (1.18) | — | |
| 0, n (%) | 7 (30) | ||
| 1, n (%) | 7 (30) | ||
| 2, n (%) | 7 (30) | ||
| 4, n (%) | 2 (9) | ||
| Most recent disease-modifying drug use, n (%) | — | ||
| Intramuscular IFN beta 1-a | 8 (35) | ||
| Subcutaneous IFN beta 1-a | 5 (22) | ||
| Glatiramer acetate | 4 (17) | ||
| Natalizumab | 2 (9) | ||
| Intravenous immunoglobulin | 1 (4) | ||
| None | 3 (13) | ||
| Median (range) Expanded Disability Status Scale score | 2.5 (1–5.5) | — | |
| Mean (SD) ambulation distance, meters | 475 (94.2) | — | |
|
| |||
| Study drug exposure and complianced | Interferon beta-1a administered subcutaneously three times per week |
||
| n = 23 | |||
| 8.8 µg | 22 µg | 44 µg | |
|
| |||
| Total dose received, µg, mean (SD) | 52.5 (7.60) | 133.9 (9.17) | 2,174.9 (584.47) |
| Doses missed, mean (SD) | 0 (0.2) | 0 (0) | 5 (7.6) |
| Compliance, %, mean (SD) | 99 (3.5) | 100 (0) | 92 (12.2) |
No significant differences were seen between groups by the Student’s t-test (for age, weight, height, and body mass index) or Fisher’s exact test (for race and gender).
Includes one patient of Hispanic ethnicity (all other subjects were not Hispanic).
Patients reported the same number of relapses for the past 24 months.
Compliance was calculated at study visits using subject diaries and returned drug. Exposure was calculated from compliance. BMI, body mass index; HC, healthy control; IFN, interferon; RRMS, relapsing–remitting multiple sclerosis; SD, standard deviation.
Analysis of phase lesion number and volume
Both the number and volume of SWI-filtered phase WM lesions significantly decreased in the RRMS interferon beta-1a administered subcutaneously group from baseline to 24 weeks (Figure 1 and Table 2). This was consistent for both the SWI-filtered phase WM lesions alone (Figure 2) and for the overlapping SWI-filtered phase + T2/PD/FLAIR, and to some extent was also true for overlapping SWI-filtered phase + T1WI (for number of lesions but not volume). The decreases from baseline for the SWI-filtered phase WM lesions alone remained significant after multiple comparison adjustment. Trends towards reduced SWI-filtered phase WM lesion number and volume were observed at the 12-week MRI.
Figure 1.
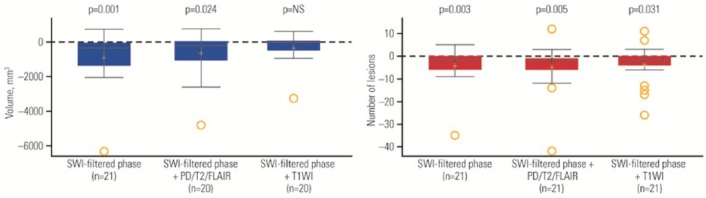
Change from baseline to week 24 in volume (left panel) and number (right panel) of susceptibility-weighted imaging (SWI)-filtered phase white matter (WM) lesions in patients with relapsing–remitting multiple sclerosis (RRMS).
In the box plots, the yellow + represents the mean; the solid line represents the median; the dashed line represents no change; the colored boxes represent the middle 50% of data; the top and bottom of the box represent the first and third quartiles; the open circles are outliers. The ‘whisker lines’ above and below the boxes represent the largest and smallest values that are not considered to be outliers. p values shown are for the difference between RRMS and healthy controls (HCs) from the Wilcoxon rank-sum test. FLAIR, fluid-attenuated inversion recovery; NS, not significant; PD, proton density; T1WI, T1 weighted image.
Table 2.
Volume and number of SWI-filtered phase WM lesions at baseline and change from baseline to week 12 and week 24.
| Baseline |
Week 12 change from baseline |
Week 24 change from baseline |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients with RRMS | HCs | p valuea | Patients with RRMS | HCs | p valuea | Patients with RRMS | HCs | p valuea | |
| Volume on SWI-filtered phase in mm3, mean (SD) | 2,620 (2,289.6) | 175 (160.5) | <0.001 | −271 (895.1) | −32 (130.1) | NS | −849 (1,479.4)b | −63 (154.5) | 0.011c |
| n (missing) | 22 (1) | 14 (1) | 22 (1) | 14 (1) | 21 (2) | 12 (3) | |||
| Number of lesions on SWI-filtered phase, mean (SD) | 17.4 (14.47) | 2.6 (1.45) | <0.001 | −0.6 (3.78) | −0.2 (1.52) | NS | −4 (8.1)b | −1 (1.4) | NS |
| n (missing) | 22 (1) | 15 (0) | 22 (1) | 15 (0) | 21 (2) | 13 (2) | |||
| Volume on SWI + PD/T2/FLAIR in mm3, mean (SD) | 1,342 (1,555.9) | – | – | −185 (539.0) | – | −610 (1,243.0)b,c | |||
| n (missing) | 22 (1) | 0 (15) | 22 (1) | 0 (15) | 20 (3) | 0 (15) | |||
| Number of lesions on SWI + PD/T2/FLAIR, mean (SD) | 16.6 (15.03) | 0.0 (0.00) | <0.001 | –0.9 (4.29) | 0.0 (0.00) | NS | −4.9 (10.21)b,c | 0.0 (0.00) | 0.001 |
| n (missing) | 22 (1) | 15 (0) | 22 (1) | 15 (0) | 21 (2) | 13 (2) | |||
| Volume on SWI + T1WI in mm3, mean (SD) | 788 (1,047.3) | – | – | −91 (422.1) | – | −305 (785.1) | – | ||
| n (missing) | 20 (3) | 0 (15) | 20 (3) | 0 (15) | 20 (3) | 0 (15) | |||
| Number of lesions on SWI + T1WI, mean (SD) | 10.8 (11.20) | 0.0 (0.00) | <0.001 | 0.2 (3.54) | 0.0 (0.00) | NS | −3.6 (8.22)b,c | 0.0 (0.00) | 0.003 |
| n (missing) | 22 (1) | 15 (0) | 22 (1) | 15 (0) | 21 (2) | 13 (2) | |||
For the difference between patients and HCs from the Wilcoxon rank-sum test.
Significant difference from zero within group from the Wilcoxon signed-rank test.
Not significant after adjustment for multiple comparisons with the Holm–Bonferroni correction. Comparisons at baseline were not adjusted for multiple comparisons.
FLAIR, fluid-attenuated inversion recovery; HC, healthy control; NS, not significant; PD, proton density; RRMS, relapsing–remitting multiple sclerosis; SD, standard deviation; SWI, susceptibility-weighted imaging; T1WI, T1 weighted image; WM, white matter.
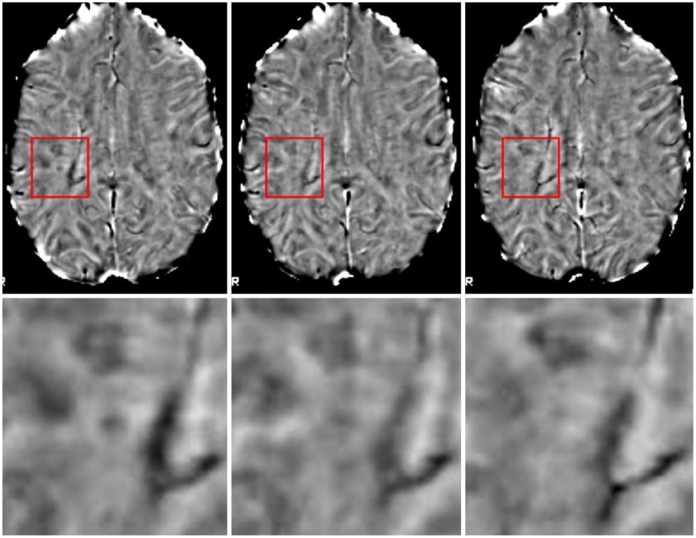
Figure 2.
Phase-visible lesion resolution over time. Images were linearly coregistered to Montreal Neurologic Institute (MNI) space to allow unbiased visualization. The top row shows the full slices for reference, while the lower row provides enlargements of the boxes centered around the lesion. From left to right, images are of baseline, 3-month follow up, and 6-month follow up. The lesion in the center is clearly visible at baseline, partially resolved at 3 months, and almost completely resolved at 6 months.
Analysis of MP-LPV changes in the SDGM over the 24-week follow up
MP-LPV was significantly lower at baseline, suggestive of increased iron content in patients with RRMS compared with HCs in the total SDGM (p = 0.002), as well as in the caudate (p < 0.001), putamen (p = 0.007), globus pallidus (p = 0.023), thalamus (p = 0.017), and pulvinar nucleus (p = 0.013) regions (Table 3).
Table 3.
MP-LPV values at baseline and absolute change from baseline at week 24.
| Baseline |
Week 24 change from baseline |
|||||
|---|---|---|---|---|---|---|
| Patients with RRMS |
HCs |
Patients with RRMS |
HCs |
|||
| Mean (SD) MP-LPV, radians | Mean (SD) MP-LPV, radians | p valuea | Mean (SD) MP-LPV, radians | Mean (SD) MP-LPV, radians | p valuea | |
| n (missing) | 23 (0) | 15 (0) | 21 (2) | 13 (2) | ||
| Total SDGM | −0.170 (0.030) | −0.140 (0.019) | 0.002 | 0.0009 (0.0217) | 0.0051 (0.0070)b,c | NS |
| Caudate | −0.190 (0.025) | −0.169 (0.019) | <0.001 | 0.0017 (0.0123) | 0.0040 (0.0112) | NS |
| Putamen | −0.205 (0.050) | −0.169 (0.023) | 0.007 | −0.0025 (0.0448) | 0.0035 (0.0098) | 0.041c |
| Globus pallidus | −0.207 (0.054) | −0.175 (0.019) | 0.023 | 0.0020 (0.0277) | 0.0045 (0.0123) | NS |
| Thalamus | −0.106 (0.020) | −0.091 (0.013) | 0.017 | 0.0003 (0.0137) | −0.0004 (0.0079) | NS |
| Pulvinar nucleus | −0.178 (0.044) | −0.145 (0.022) | 0.013 | −0.0082 (0.0276) | −0.0099 (0.0285) | NS |
| Hippocampus | −0.176 (0.052) | −0.154 (0.037) | NS | 0.0081 (0.0266) | 0.0107 (0.0193)b,c | NS |
| Red nucleus | −0.246 (0.037) | −0.242 (0.032) | NS | −0.0027 (0.0306) | 0.0077 (0.0300) | NS |
| Substantia nigra | −0.359 (0.069) | −0.495 (0.705) | NS | 0.0061 (0.0420) | 0.0228 (0.0330) | NS |
For the difference between patients and HCs from the Wilcoxon rank-sum test.
Significant difference from zero within group from the Wilcoxon signed-rank test.
Not significant after adjustment for multiple comparisons with the Holm–Bonferroni correction. Comparisons at baseline were not adjusted for multiple comparisons.
Lower MP-LPV values are believed to be indicative of higher iron content.
HC, healthy control; MP-LPV, mean phase of low-phase voxels; NS, not significant; RRMS, relapsing–remitting multiple sclerosis; SD, standard deviation; SDGM, subcortical deep-gray matter.
Change from baseline to week 24 in MP-LPV was not significantly different between groups in the total SDGM or in any of the regions, except for the putamen (−0.0025 radians versus 0.0035 radians in RRMS patients and HCs, respectively; p = 0.041); however, this difference was not significant after adjustment for multiple comparisons (Table 3 and Supplement Figure). Within the RRMS group only, the caudate showed a significant difference in MP-LPV during the first 12 weeks of the study (an increase in radians, suggestive of a decrease in iron content) compared with the second 12 weeks (a decrease in radians, suggestive of an increase in iron content, p = 0.001), which was still significant after adjustment for multiple comparisons (p = 0.0121; data not shown).
Analysis of normalized SDGM volume changes over the 24-week follow up
Patients with RRMS had significantly lower volumes compared with HCs for the total SDGM, putamen, globus pallidus, and thalamus (p < 0.01) at baseline, but no significant differences were measured for the caudate or hippocampus (Table 4).
Table 4.
Regional normalized SDGM volumes at baseline and percentage change from baseline at week 24.
| Baseline volume (mm3) |
% change from baseline at week 24 |
||||||
|---|---|---|---|---|---|---|---|
| Patients with RRMS |
HCs |
Patients with RRMS |
HCs |
||||
| Mean (SD) | Mean (SD) | p valuea | Mean (SD) | p valueb | Mean (SD) | p valuea | |
| n (missing) | 23 (0) | 15 (0) | 21 (2) | 13 (2) | |||
| SDGM | 42,620 (4,475) | 46,934 (2,748) | 0.002 | −2.09 (2.81) | <0.001 | 0.20 (1.82) | 0.011c |
| Caudate | 6,526 (987) | 7,002 (622) | NS | −1.53 (5.09) | 0.039c | −2.12 (5.28) | NS |
| Putamen | 9,076 (1,111) | 10,091 (750) | 0.004 | −4.55 (10.93) | 0.012c | 0.58 (3.298) | NS |
| Globus pallidus | 3,250 (386) | 3,575 (182) | 0.009 | −0.79 (5.72) | NS | −0.54 (4.36) | NS |
| Thalamus | 13,908 (1,743) | 15,551 (1,294) | 0.004 | −1.44 (1.76) | <0.001 | 0.06 (1.96) | NS |
| Hippocampus | 6,931 (658) | 7,315 (563) | NS | −1.71 (4.78) | NS | 2.07 (5.04) | 0.041c |
For the difference between patients with RRMS and HCs from the Wilcoxon rank-sum test.
For the difference from zero within patient group from the Wilcoxon signed-rank test.
Not significant after adjustment for multiple comparisons with the Holm–Bonferroni correction. Comparisons at baseline were not adjusted for multiple comparisons.
HC, healthy control; NS, not significant; RRMS, relapsing–remitting multiple sclerosis; SD, standard deviation; SDGM, subcortical deep-gray matter.
As a group, patients with RRMS had significant decreases in volume from baseline to 24 weeks in some of the brain regions (Table 4). Only the differences in the total SDGM and thalamus remained significant after adjustment for multiple comparisons. There were no significant changes from baseline to 24 weeks within the HC group.
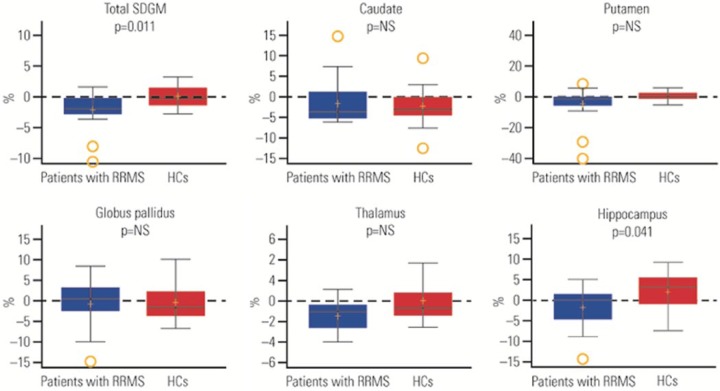
From baseline to 24 weeks, changes in normalized volume were significantly different between patients with RRMS and HCs in the total SDGM (–2.09% and 0.20% in RRMS and HC groups, respectively; p = 0.011) and hippocampus (–1.71% and 2.07% in RRMS and HC groups, respectively; p = 0.041). The differences between groups did not remain significant after adjustment for multiple comparisons (Figure 3 and Table 4).
Figure 3.
Percentage change from baseline to week 24 in normalized volume of brain subcortical deep gray matter (SDGM) structural regions.
In the box plots, the yellow + represents the mean; the solid line represents the median; the dashed line represents no change; the colored boxes represent the middle 50% of data; the top and bottom of the box represent the first and third quartiles; the open circles are outliers. The ‘whisker lines’ above and below the boxes represent the largest and smallest values that are not considered to be outliers. p values shown are for the difference between relapsing–remitting multiple sclerosis (RRMS) and healthy controls (HCs) from Wilcoxon rank-sum test. NS, not significant.
Safety and tolerability
Almost all patients with RRMS (22/23) and 8/15 HCs had ⩾1 study-emergent adverse effect; 16 of the patients had adverse effects considered to be related to study treatment (Supplement Table).
Discussion
In this pilot longitudinal study, one of the first to investigate the SWI-filtered phase changes in WM lesions in RRMS patients over 24 weeks, both the number and volume of SWI-filtered phase WM lesions were significantly decreased from baseline in RRMS patients receiving treatment with interferon beta-1a administered subcutaneously. This effect has to be interpreted with caution, but could potentially be related to the effect of interferon beta-1a administered subcutaneously on the iron and myelin content in the WM lesions [Yao et al. 2012; Hagemeier et al. 2014; Bian et al. 2013; Eissa et al. 2009; Haacke et al. 2009; Mehta et al. 2013; Yablonskiy et al. 2012]. However, without the use of randomized, controlled study design, it is difficult to attribute the observed effect directly to the treatment with interferon beta-1a. The accumulation of iron in some, but not all, WM lesions suggests a specific, potentially disease-relevant process; however, its pathophysiological significance remains unknown [Bian et al. 2013; Mehta et al. 2013]. It is also unknown whether the increased iron deposition in WM lesions of patients with MS is a result of debris from myelin/oligodendrocyte breakdown, concentrated iron in macrophages (that phagocytize the myelin/oligodendrocytes), or the product of hemorrhages from damaged brain vessels [Haacke et al. 2004, 2009], or whether it represents a direct contribution to brain pathology. A recent cross-validation study of phase imaging with immunohistochemical examination of autoptic MS tissue [Mehta et al. 2013] reported that iron is present in nonphagocytosing, M1-polarized microglia/macrophages at the rim of chronic active WM demyelinating lesions. This suggests that phase imaging has potential to visualize specific, chronic proinflammatory activity in established MS lesions. In fact, phase hypointense lesions are more prevalent in patients with active relapsing than with secondary progressive MS [Hagemeier et al. 2012b; Mehta et al. 2013]. The RRMS patients enrolled in this 24-week study had active disease, with 16 of 23 patients presenting with a relapse in the 12 months prior to baseline. Therefore, a significant decrease in number and volume of existing SWI-filtered phase WM lesions over 24 weeks may represent the decrease of chronic pro-inflammatory activity in established MS lesions. However, little is known at this time about phase lesion changes over the short- or long-term follow up in MS patients, so the findings from this study have to be interpreted with caution [Bian et al. 2013].
Although phase lesions may be indicative of iron, they may be even more affected by myelin changes [Yao et al. 2012; Bian et al. 2013; Chen et al. 2014]. In a recent study in patients with clinically isolated syndrome who converted to clinically definite MS within a 3-year follow-up period versus those who did not, converters had significantly (p = 0.008) more phase lesions, but not T2WM or T1WM lesions; although the phase contrast could have been influenced by demyelination or other factors, the predictive value of phase lesions could imply a role in early disease pathology [Hagemeier et al. 2014]. These findings are in line with observations that phase contrast observed in MS WM lesions is dynamic and may be visible at early disease onset [Yablonskiy et al. 2012; Chen et al. 2014]. However, others have reported that ring-like phase WM lesions remained unchanged over several years [Bian et al. 2013]. A better understanding of phase behavior in active and chronic WM lesions in MS patients necessitates further longitudinal studies with larger sample size and heterogeneous disease subgroups. However, the findings from the present study can help guide the design of these future investigations, given that trends towards lower SWI-filtered phase number and volume of WM lesions were observed as early as 2 months after interferon beta-1a administered subcutaneously was titrated to the full dose.
There were significantly decreased MP-LPV and normalized volumes of the various SDGM structures in patients with RRMS compared with HCs at baseline, further validating the biological relevance of these MRI outcomes. However, the majority of these MRI outcomes did not appear to progress in patients with RRMS over 24 weeks.
SWI-filtered phase has been proposed as a method to indirectly measure increased iron content [Haacke et al. 2004; Hagemeier et al. 2013a]. MP-LPV was significantly lower in the RRMS group than in HCs at baseline in the total SDGM and a number of its component regions (caudate, putamen, globus pallidus, thalamus, and pulvinar nucleus), suggesting higher iron content in the SDGM of MS patients. After 24 weeks, patients with RRMS showed no significant changes in MP-LPV compared with HCs, other than a possible effect in the putamen that was not supported after adjustment for multiple comparisons.
Paramagnetic substances, mostly in the form of ferritin and iron, influence the phase of proton spin. The changes in mean phase measures are most likely caused by iron; however, it is possible that phase shifts could also potentially be caused by other factors [Bagnato et al. 2011; Yao et al. 2012], for example, by the diamagnetic properties of myelin [Schweser et al. 2011]. Among this study’s investigations were phase changes in the SDGM structures, an approach believed to minimize the confounding effects of myelin [Langkammer et al. 2012a]. However, it has to be noted that some SDGM structures, most notably the thalamus and hippocampus, have relatively high myelin content, which could potentially have influenced phase measurements.
Baseline volumes of SDGM regional structures were lower in patients with RRMS than in HCs, possibly indicating regional brain atrophy. A recent study with 5 years’ follow up provided evidence that SDGM atrophy is greater in patients with disability progression than in stable patients [Zivadinov et al. 2013]. The specific volume reductions in SDGM regions (putamen, global pallidus, and thalamus) are in line with previous reports, in which greater atrophy of certain SDGM regions in RRMS patients has been described [Zivadinov et al. 2013].
There are a number of limitations to this study. Given that no differences in MP-LPV changes were seen between RRMS patients and HCs over 24 weeks, one could question whether the 24-week follow up is sufficiently long enough to measure detectable SWI-filtered phase changes in the SDGM, and whether longer follow-up studies are needed to elucidate this issue further. While the treatment with interferon beta-1a administered subcutaneously may have also influenced SWI-filtered phase changes in the SDGM of the RRMS patients compared with HCs, it is not possible to establish a ‘cause–effect’ relationship without a control group of MS patients. Therefore, potential benefit of interferon beta-1a administered subcutaneously on changes in the iron content of the SDGM as measured by SWI-filtered phase should be established in a head-to-head comparison with untreated or comparator-treated patients. However, the inclusion of a healthy group in a current study provided a valuable baseline, allowing evaluation of this technique for normal variation between individuals against which changes in SWI-filtered phase could be compared. It should be noted that an ultimate goal of MS therapy would be to normalize patient SWI-filtered phase changes over time towards the age-related changes observed in HCs. There are inherent limitations of the SWI-filtered phase-based imaging method. Although this method is sensitive to iron changes, it can be affected by a number of other factors, in particular myelin, calcium phosphates, and deoxygenated blood [Langkammer et al. 2012b]. In addition, different forms of iron and containment structures (hemosiderin, ferritin) cannot be distinguished although they have different biological impacts [Haacke et al. 2009]. The phase changes, reflecting the tissue magnetic properties, may also be explained by changes of the chemical form of iron, rather than the total iron concentration [Schweser et al. 2011]. SWI-filtered phase depends not only on the tissue magnetic susceptibility, and thus indirectly on the tissue iron concentration, but also on the tissue structure, which can be influenced by amount of atrophy [Schweser et al. 2013]. Therefore, in future studies, more advanced techniques such as quantitative susceptibility mapping and MR relaxometry may help to provide more exact iron concentration changes over time [Schweser et al. 2011]. The results of phase estimation of red nucleus, substantia nigra, and pulvinar have to be interpreted with caution, as the iron concentration of these regions was performed on the most representative slice, rather than over the whole volume of the structure. Also, our segmentation method did not correct for presence of large veins in the SDGM [Zivadinov et al. 2012]. It is therefore possible that larger veins and/or differences in oxygen extraction might have some impact on our results. However, we did not find previously that segmentation and exclusion of the veins affected the reproducibility of our method or the overall iron concentration between MS patients and controls in the SDGM (unpublished data). Furthermore, manual or automated removal of the veins from SDGM may also underestimate true differences between controls and MS patients, since iron commonly deposits around perivascular spaces in MS patients [LeVine, 1997].
In conclusion, RRMS patients showed significant decreases in both number and volume of SWI-filtered phase WM lesions over the 24-week follow up. No significant differences between patients with RRMS and HCs in change from baseline in MP-LPV or normalized volumes were detected after 24 weeks after adjusting for multiple comparisons. While these preliminary results should be interpreted with caution, the study showed that SWI-filtered phase may become a useful tool for monitoring disease activity among RRMS patients.
Supplementary Material
Acknowledgments
The authors thank Rachel Johnatty of Caudex Medical, USA (supported by EMD Serono, Inc., a subsidiary of Merck KGaA, Darmstadt, Germany, and by Pfizer Inc, New York, NY) for editorial assistance in preparing the initial draft of the manuscript, collating the comments of authors and other named contributors, and assembling tables and figures.
Footnotes
Conflict of interest statement: R Zivadinov received personal compensation from Teva Pharmaceuticals, Biogen Idec, EMD Serono, Inc., Novartis, Claret, and Sanofi-Genzyme for speaking and consultant fees. He also received financial support for research activities from Biogen Idec, Teva Pharmaceuticals, Claret, Sanofi-Genzyme, Novartis, and EMD Serono, Inc. M Dwyer received consulting fees from EMD Serono, Inc., and Claret. S Markovic-Plese received personal compensation from Genzyme Inc. and EMD Serono, Inc., for consultant fees. She also received research grants from Biogen Idec, EMD Serono, Inc., Genzyme Inc., and Novartis. B Hayward and F Dangond are employees of EMD Serono, Inc., a subsidiary of Merck KGaA, Darmstadt, Germany. B Weinstock-Guttman has received honoraria as a speaker and a consultant for Biogen Idec, Teva Pharmaceuticals, EMD Serono, Inc., Pfizer, Novartis, Genzyme and Sanofi, and Acorda, and has also received research funds from Biogen Idec, Teva Pharmaceuticals, EMD Serono, Inc., Genzyme and Sanofi, Novartis, and Acorda. N Bergsland, M Heininen-Brown, E Carl, and C Kennedy have nothing to disclose.
Funding: This study was sponsored by EMD Serono, Inc., Rockland, MA, USA (a subsidiary of Merck KGaA, Darmstadt, Germany) and by Pfizer Inc, New York, NY, USA. The sponsor provided logistical support, support for investigator meetings, central statistical support, and data analysis.
Contributor Information
Robert Zivadinov, Buffalo Neuroimaging Analysis Center, Department of Neurology, School of Medicine and Biomedical Sciences, MRI Imaging Clinical Translational Research Center, University at Buffalo, State University of New York, 100 High Street, Buffalo, NY 14203, USA.
Michael Dwyer, Buffalo Neuroimaging Analysis Center, Department of Neurology, School of Medicine and Biomedical Sciences, MRI Imaging Clinical Translational Research Center, University at Buffalo, State University of New York, 100 High Street, Buffalo, NY 14203, USA.
Silva Markovic-Plese, Department of Neurology, Microbiology and Immunology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Brooke Hayward, US Medical Affairs, EMD Serono, Inc., Rockland, MA, USA.
Niels Bergsland, Buffalo Neuroimaging Analysis Center, Department of Neurology, School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, Buffalo, NY, USA.
Mari Heininen-Brown, Buffalo Neuroimaging Analysis Center, Department of Neurology, School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, Buffalo, NY, USA.
Ellen Carl, Buffalo Neuroimaging Analysis Center, Department of Neurology, School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, Buffalo, NY, USA.
Cheryl Kennedy, Buffalo Neuroimaging Analysis Center, Department of Neurology, School of Medicine and Biomedical Sciences, University at Buffalo, State University of New York, Buffalo, NY, USA.
Fernando Dangond, US Medical Affairs, EMD Serono, Inc., Rockland, MA, USA.
Bianca Weinstock-Guttman, Baird MS Center, Department of Neurology, State University of New York at Buffalo, Buffalo, NY, USA.
References
- Al-Radaideh A., Wharton S., Lim S., Tench C., Morgan P., Bowtell R., et al. (2013) Increased iron accumulation occurs in the earliest stages of demyelinating disease: an ultra-high field susceptibility mapping study in Clinically Isolated Syndrome. Mult Scler 19: 896–903. [DOI] [PubMed] [Google Scholar]
- Bagnato F., Hametner S., Yao B., van Gelderen P., Merkle H., Cantor F., et al. (2011) Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain 134: 3602–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G., Tishler T., Lu P., Villablanca P., Altshuler L., Carter M., et al. (2007) Brain ferritin iron May influence age- and gender-related risks of neurodegeneration. Neurobiol Aging 28: 414–423. [DOI] [PubMed] [Google Scholar]
- Bian W., Harter K., Hammond-Rosenbluth K., Lupo J., Xu D., Kelley D., et al. (2013) A serial in vivo 7T magnetic resonance phase imaging study of white matter lesions in multiple sclerosis. Mult Scler 19: 69–75. [DOI] [PubMed] [Google Scholar]
- Ceccarelli A., Rocca M., Perego E., Moiola L., Ghezzi A., Martinelli V., et al. (2011) Deep grey matter T2 hypo-intensity in patients with paediatric multiple sclerosis. Mult Scler 17: 702–707. [DOI] [PubMed] [Google Scholar]
- Chen W., Gauthier S., Gupta A., Comunale J., Liu T., Wang S., et al. (2014) Quantitative susceptibility mapping of multiple sclerosis lesions at various ages. Radiology 271: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissa A., Lebel R., Korzan J., Zavodni A., Warren K., Catz I., et al. (2009) Detecting lesions in multiple sclerosis at 4.7 tesla using phase susceptibility-weighting and T2-weighting. J Magn Reson Imaging 30: 737–742. [DOI] [PubMed] [Google Scholar]
- Haacke E., Xu Y., Cheng Y., Reichenbach J. (2004) Susceptibility weighted imaging (SWI). Magn Reson Med 52: 612–618. [DOI] [PubMed] [Google Scholar]
- Haacke E., Makki M., Ge Y., Maheshwari M., Sehgal V., Hu J., et al. (2009) Characterizing iron deposition in multiple sclerosis lesions using susceptibility weighted imaging. J Magn Reson Imaging 29: 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeier J., Geurts J., Zivadinov R. (2012a) Brain iron accumulation in aging and neurodegenerative disorders. Expert Rev Neurother 12: 1467–1480. [DOI] [PubMed] [Google Scholar]
- Hagemeier J., Heininen-Brown M., Poloni G., Bergsland N., Magnano C., Durfee J., et al. (2012b) Iron deposition in multiple sclerosis lesions measured by susceptibility-weighted imaging filtered phase: a case control study. J Magn Reson Imaging 36: 73–83. [DOI] [PubMed] [Google Scholar]
- Hagemeier J., Weinstock-Guttman B., Bergsland N., Heininen-Brown M., Carl E., Kennedy C., et al. (2012c) Iron deposition on SWI-filtered phase in the subcortical deep gray matter of patients with clinically isolated syndrome May precede structure-specific atrophy. AJNR Am J Neuroradiol 33: 1596–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeier J., Dwyer M., Bergsland N., Schweser F., Magnano C., Heininen-Brown M., et al. (2013a) Effect of age on MRI phase behavior in the subcortical deep gray matter of healthy individuals. AJNR Am J Neuroradiol 34: 2144–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeier J., Weinstock-Guttman B., Heininen-Brown M., Poloni G., Bergsland N., Schirda C., et al. (2013b) Gray matter SWI-filtered phase and atrophy are linked to disability in MS. Front Biosci (Elite Ed) 5: 525–532. [DOI] [PubMed] [Google Scholar]
- Hagemeier J., Yeh E., Brown M., Bergsland N., Dwyer M., Carl E., et al. (2013c) Iron content of the pulvinar nucleus of the thalamus is increased in adolescent multiple sclerosis. Mult Scler 19: 567–576. [DOI] [PubMed] [Google Scholar]
- Hagemeier J., Heininen-Brown M., Gabelic T., Guttuso T., Jr., Silvestri N., Lichter D., et al. (2014) Phase white matter signal abnormalities in patients with clinically isolated syndrome and other neurologic disorders. AJNR Am J Neuroradiol 35: 1916-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren B., Sourander P. (1958) The effect of age on the non-haemin iron in the human brain. J Neurochem 3: 41–51. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. (2001) A global optimisation method for robust affine registration of brain images. Med Image Anal 5: 143–156. [DOI] [PubMed] [Google Scholar]
- Jomova K., Valko M. (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283: 65–87. [DOI] [PubMed] [Google Scholar]
- Khalil M., Langkammer C., Ropele S., Petrovic K., Wallner-Blazek M., Loitfelder M., et al. (2011) Determinants of brain iron in multiple sclerosis: a quantitative 3T MRI study. Neurology 77: 1691–1697. [DOI] [PubMed] [Google Scholar]
- Langkammer C., Krebs N., Goessler W., Scheurer E., Yen K., Fazekas F., et al. (2012a) Susceptibility induced gray-white matter MRI contrast in the human brain. Neuroimage 59: 1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langkammer C., Schweser F., Krebs N., Deistung A., Goessler W., Scheurer E., et al. (2012b) Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. Neuroimage 62: 1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine S. (1997) Iron deposits in multiple sclerosis and Alzheimer’s disease brains. Brain Res 760: 298–303. [DOI] [PubMed] [Google Scholar]
- Liem M., Lesnik Oberstein S., Versluis M., Maat-Schieman M., Haan J., Webb A., et al. (2012) 7 T MRI reveals diffuse iron deposition in putamen and caudate nucleus in CADASIL. J Neurol Neurosurg Psychiatry 83: 1180–1185. [DOI] [PubMed] [Google Scholar]
- Mehta V., Pei W., Yang G., Li S., Swamy E., Boster A., et al. (2013) Iron is a sensitive biomarker for inflammation in multiple sclerosis lesions. PLoS One 8: e57573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz E., Pasquini J., Thompson K., Felt B., Butkus G., Beard J., et al. (2004) Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J Neurosci Res 77: 681–689. [DOI] [PubMed] [Google Scholar]
- Patenaude B., Smith S., Kennedy D., Jenkinson M. (2011) A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 56: 907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman C., Reingold S., Banwell B., Clanet M., Cohen J., Filippi M., et al. (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweser F., Deistung A., Lehr B., Reichenbach J., et al. (2011) Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism? Neuroimage 54: 2789–2807. [DOI] [PubMed] [Google Scholar]
- Schweser F., Dwyer M., Deistung A., Reichenbach R., Zivadinov R., et al. (2013) Impact of tissue atrophy on high-pass filtered MRI signal phase-based assessment in large-scale group-comparison studies: a simulation study. Front Phys 1: 1–9. [Google Scholar]
- Sullivan J. (2004) Is stored iron safe? J Lab Clin Med 144: 280–284. [DOI] [PubMed] [Google Scholar]
- Yablonskiy D., Luo J., Sukstanskii A., Iyer A., Cross A., et al. (2012) Biophysical mechanisms of MRI signal frequency contrast in multiple sclerosis. Proc Natl Acad Sci U S A 109: 14212–14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao B., Bagnato F., Matsuura E., Merkle H., van Gelderen P., Cantor F., et al. (2012) Chronic multiple sclerosis lesions: characterization with high-field-strength MR imaging. Radiology 262: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R., Rudick R., De Masi R., Nasuelli D., Ukmar M., Pozzi-Mucelli R., et al. (2001) Effects of IV methylprednisolone on brain atrophy in relapsing-remitting MS. Neurology 57: 1239–1247. [DOI] [PubMed] [Google Scholar]
- Zivadinov R., Heininen-Brown M., Schirda C., Poloni G., Bergsland N., Magnano C., et al. (2012) Abnormal subcortical deep-gray matter susceptibility-weighted imaging filtered phase measurements in patients with multiple sclerosis: a case-control study. Neuroimage 59: 331–339. [DOI] [PubMed] [Google Scholar]
- Zivadinov R., Bergsland N., Dolezal O., Hussein S., Seidl Z., Dwyer M., et al. (2013) Evolution of cortical and thalamus atrophy and disability progression in early relapsing–remitting MS during 5 years. AJNR Am J Neuroradiol 34: 1931–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R., Dwyer M., Markovic-Plese S., Kennedy C., Bergsland N., Ramasamy D., et al. (2014) Effect of treatment with interferon beta-1a on changes in voxel-wise magnetization transfer ratio in normal appearing brain tissue and lesions of patients with relapsing-remitting multiple sclerosis: a 24-week, controlled pilot study. PLoS One 9: e91098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.