Abstract
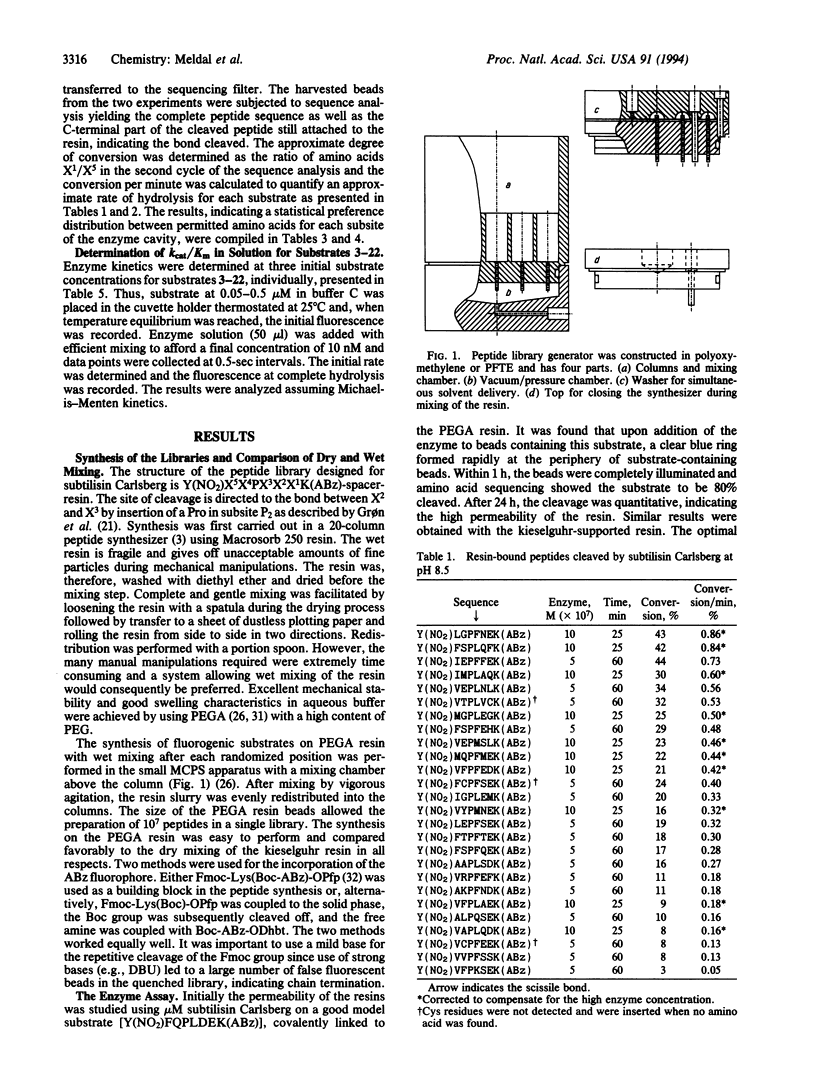
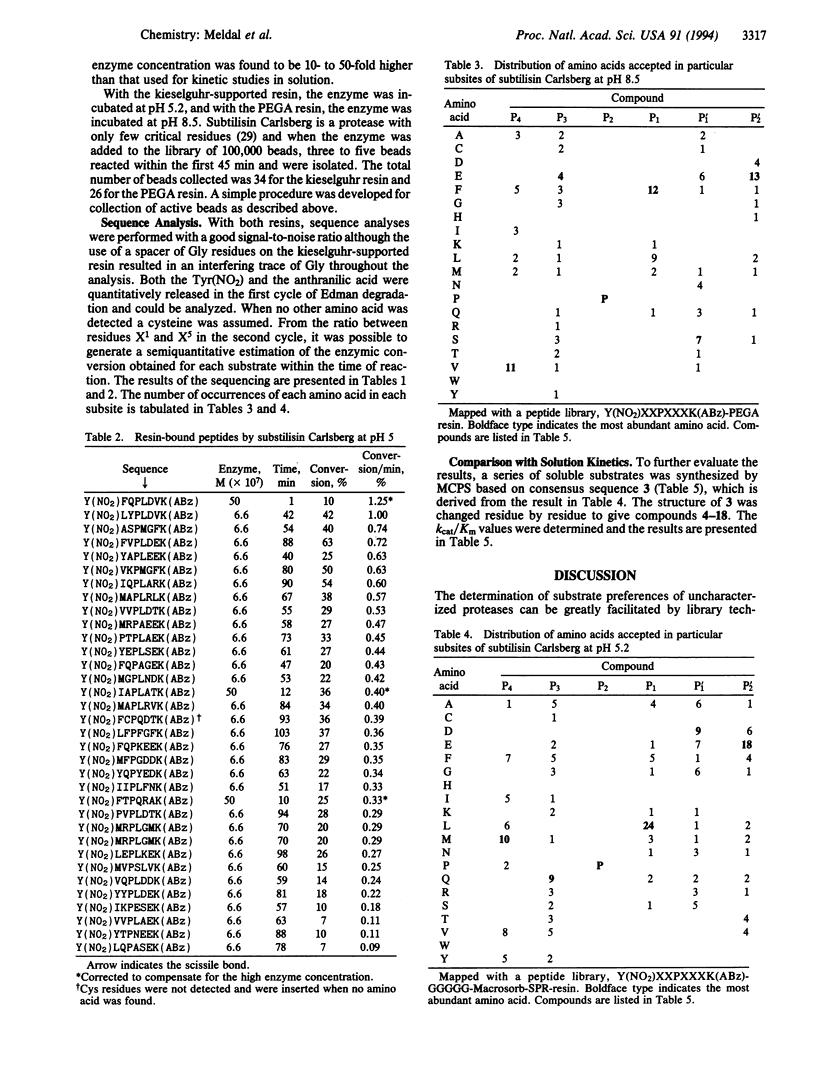
A solid-phase assay for the complete subsite mapping of the active site of endoproteases has been developed. A library of resin-bound protease substrates was synthesized both on kieselguhr-supported polyamide resin and on a polyethylene glycol-poly-(N,N-dimethylacrylamide) copolymer type of resin that allows proteases to diffuse into the interior and perform their catalytic activity. Anthranilic acid and 3-nitrotyrosine were used as an efficient donor-acceptor pair for the resonance energy transfer. The synthesis was performed in a manual library generator that allows simple wet mixing of the beads and parallel washing procedures. After treatment with subtilisin Carlsberg, fluorescing beads were collected and subjected to peptide sequencing, affording the preferred sequences, their cleavage bond, and a semiquantitative estimation of the turnover. A statistical distribution of preferred amino acids was obtained for each subsite. The result was compared with data from kinetic studies in solution.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bech L. M., Sørensen S. B., Breddam K. Mutational replacements in subtilisin 309. Val104 has a modulating effect on the P4 substrate preference. Eur J Biochem. 1992 Nov 1;209(3):869–874. doi: 10.1111/j.1432-1033.1992.tb17359.x. [DOI] [PubMed] [Google Scholar]
- Bech L. M., Sørensen S. B., Breddam K. Significance of hydrophobic S4-P4 interactions in subtilisin 309 from Bacillus lentus. Biochemistry. 1993 Mar 23;32(11):2845–2852. doi: 10.1021/bi00062a016. [DOI] [PubMed] [Google Scholar]
- Chagas J. R., Juliano L., Prado E. S. Intramolecularly quenched fluorogenic tetrapeptide substrates for tissue and plasma kallikreins. Anal Biochem. 1991 Feb 1;192(2):419–425. doi: 10.1016/0003-2697(91)90558-b. [DOI] [PubMed] [Google Scholar]
- Chait B. T., Wang R., Beavis R. C., Kent S. B. Protein ladder sequencing. Science. 1993 Oct 1;262(5130):89–92. doi: 10.1126/science.8211132. [DOI] [PubMed] [Google Scholar]
- Dreyer T. Substrate specificity of proteinase yscA from saccharomyces cerevisiae. Carlsberg Res Commun. 1989;54(3):85–97. doi: 10.1007/BF02908301. [DOI] [PubMed] [Google Scholar]
- Furka A., Sebestyén F., Asgedom M., Dibó G. General method for rapid synthesis of multicomponent peptide mixtures. Int J Pept Protein Res. 1991 Jun;37(6):487–493. doi: 10.1111/j.1399-3011.1991.tb00765.x. [DOI] [PubMed] [Google Scholar]
- Grøn H., Breddam K. Interdependency of the binding subsites in subtilisin. Biochemistry. 1992 Sep 22;31(37):8967–8971. doi: 10.1021/bi00152a037. [DOI] [PubMed] [Google Scholar]
- Grøn H., Meldal M., Breddam K. Extensive comparison of the substrate preferences of two subtilisins as determined with peptide substrates which are based on the principle of intramolecular quenching. Biochemistry. 1992 Jul 7;31(26):6011–6018. doi: 10.1021/bi00141a008. [DOI] [PubMed] [Google Scholar]
- Houghten R. A., Pinilla C., Blondelle S. E., Appel J. R., Dooley C. T., Cuervo J. H. Generation and use of synthetic peptide combinatorial libraries for basic research and drug discovery. Nature. 1991 Nov 7;354(6348):84–86. doi: 10.1038/354084a0. [DOI] [PubMed] [Google Scholar]
- Juliano L., Chagas J. R., Hirata I. Y., Carmona E., Sucupira M., Oliveira E. S., Oliveira E. B., Camargo A. C. A selective assay for endooligopeptidase A based on the cleavage of fluorogenic substrate structurally related to enkephalin. Biochem Biophys Res Commun. 1990 Dec 14;173(2):647–652. doi: 10.1016/s0006-291x(05)80084-1. [DOI] [PubMed] [Google Scholar]
- Lam K. S., Salmon S. E., Hersh E. M., Hruby V. J., Kazmierski W. M., Knapp R. J. A new type of synthetic peptide library for identifying ligand-binding activity. Nature. 1991 Nov 7;354(6348):82–84. doi: 10.1038/354082a0. [DOI] [PubMed] [Google Scholar]
- Maggiora L. L., Smith C. W., Zhang Z. Y. A general method for the preparation of internally quenched fluorogenic protease substrates using solid-phase peptide synthesis. J Med Chem. 1992 Oct 16;35(21):3727–3730. doi: 10.1021/jm00099a001. [DOI] [PubMed] [Google Scholar]
- Meldal M., Breddam K. Anthranilamide and nitrotyrosine as a donor-acceptor pair in internally quenched fluorescent substrates for endopeptidases: multicolumn peptide synthesis of enzyme substrates for subtilisin Carlsberg and pepsin. Anal Biochem. 1991 May 15;195(1):141–147. doi: 10.1016/0003-2697(91)90309-h. [DOI] [PubMed] [Google Scholar]
- Meldal M., Holm C. B., Bojesen G., Jakobsen M. H., Holm A. Multiple column peptide synthesis, Part 2 (1, 2). Int J Pept Protein Res. 1993 Mar;41(3):250–260. doi: 10.1111/j.1399-3011.1993.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Paul S., Sun M., Mody R., Tewary H. K., Stemmer P., Massey R. J., Gianferrara T., Mehrotra S., Dreyer T., Meldal M. Peptidolytic monoclonal antibody elicited by a neuropeptide. J Biol Chem. 1992 Jul 5;267(19):13142–13145. [PubMed] [Google Scholar]
- Philipp M., Bender M. L. Kinetics of subtilisin and thiolsubtilisin. Mol Cell Biochem. 1983;51(1):5–32. doi: 10.1007/BF00215583. [DOI] [PubMed] [Google Scholar]
- Riordan J. F., Sokolovsky M., Vallee B. L. Environmentally sensitive tyrosyl residues. Nitration with tetranitromethane. Biochemistry. 1967 Jan;6(1):358–361. doi: 10.1021/bi00853a053. [DOI] [PubMed] [Google Scholar]
- Stöcker W., Ng M., Auld D. S. Fluorescent oligopeptide substrates for kinetic characterization of the specificity of Astacus protease. Biochemistry. 1990 Nov 13;29(45):10418–10425. doi: 10.1021/bi00497a018. [DOI] [PubMed] [Google Scholar]
- Szwajcer-Dey E., Rasmussen J., Meldal M., Breddam K. Proline-specific endopeptidases from microbial sources: isolation of an enzyme from a Xanthomonas sp. J Bacteriol. 1992 Apr;174(8):2454–2459. doi: 10.1128/jb.174.8.2454-2459.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen S. B., Bech L. M., Meldal M., Breddam K. Mutational replacements of the amino acid residues forming the hydrophobic S4 binding pocket of subtilisin 309 from Bacillus lentus. Biochemistry. 1993 Sep 7;32(35):8994–8999. doi: 10.1021/bi00086a003. [DOI] [PubMed] [Google Scholar]
- Yaron A., Carmel A., Katchalski-Katzir E. Intramolecularly quenched fluorogenic substrates for hydrolytic enzymes. Anal Biochem. 1979 May;95(1):228–235. doi: 10.1016/0003-2697(79)90210-0. [DOI] [PubMed] [Google Scholar]



