Abstract
Background:
Women with node-positive vulvar cancer have a high risk for disease recurrence. Indication criteria for adjuvant radiotherapy are controversial. This study was designed to further understand the role of adjuvant therapy in node-positive disease.
Methods:
Patients with primary squamous-cell vulvar cancer treated at 29 gynecologic cancer centers in Germany from 1998 through 2008 were included in this retrospective exploratory multicenter cohort study. Of 1618 documented patients, 1249 had surgical groin staging and known lymph node status and were further analyzed. All statistical tests were two-sided.
Results:
Four hundred forty-seven of 1249 patients (35.8%) had lymph node metastases (N+). The majority of N+ patients had one (172 [38.5%]) or two (102 [22.8%]) positive nodes. The three-year progression-free survival (PFS) rate of N+ patients was 35.2%, and the overall survival (OS) rate 56.2% compared with 75.2% and 90.2% in node-negative patients (N-). Two hundred forty-four (54.6%) N+ patients had adjuvant therapy, of which 183 (40.9%) had radiotherapy directed at the groins (+/-other fields). Three-year PFS and OS rates in these patients were better compared with N+ patients without adjuvant treatment (PFS: 39.6% vs 25.9%, hazard ratio [HR] = 0.67, 95% confidence interval [CI[= 0.51 to 0.88, P = .004; OS: 57.7% vs 51.4%, HR = 0.79, 95% CI = 0.56 to 1.11, P = .17). This effect was statistically significant in multivariable analysis adjusted for age, Eastern Cooperative Oncology Group, Union internationale contre le cancer stage, grade, invasion depth, and number of positive nodes (PFS: HR = 0.58, 95% CI = 0.43 to 0.78, P < .001; OS: HR = 0.63, 95% CI = 0.43 to 0.91, P = .01).
Conclusion:
This large multicenter study in vulvar cancer observed that adjuvant radiotherapy was associated with improved prognosis in node-positive patients and will hopefully help to overcome concerns regarding adjuvant treatment. However, outcome after adjuvant radiotherapy remains poor compared with node-negative patients. Adjuvant chemoradiation could be a possible strategy to improve therapy because it is superior to radiotherapy alone in other squamous cell carcinomas.
Even large gynecologic cancer centers around the world treat only a few patients with vulvar cancer, a rare disease with two to four women diagnosed per 100 000 per year (1). In contrast to most other malignancies, the incidence of vulvar cancer has recently been rising, leading to increased clinical and scientific interest to improve therapeutic options (2,3).
Patients` prognosis is mainly determined by lymph node status: Five-year disease-specific survival ranges between 70% and 95% in patients with negative inguino-femoral lymph nodes and decreases to 25% to 41% if groin nodes are affected (1,4–6). Adjuvant radiotherapy after surgical excision of the primary tumor and inguino-femoral lymphadenectomy was shown to improve prognosis in patients with nodal involvement (7). The prognostic impact of the number of affected lymph nodes and subsequent benefit of irradiation, however, are controversial (8,9).
The importance of a single intranodal lymph node metastasis is particularly unclear. A potential benefit of adjuvant radiotherapy to groins and pelvis was demonstrated for patients with two or more affected nodes by Homesley et al., but was not observed for women with only one metastasis (7,10). More recent analyses provide evidence that already one intracapsular macrometastasis (>2mm) can lead to impaired prognosis compared with node-negative disease (11), and patients might benefit from adjuvant radiotherapy (6,8). A major discrepancy also prevails between international guideline recommendations: While most international guidelines advise irradiation from two or more affected lymph nodes, German guidelines recommend adjuvant radiotherapy to the groins and pelvis only in patients with three or more positive nodes, one metastasis bigger than 10mm or extracapsular spread (12,13).
Conduction of a well-designed prospective study in a disease as rare as vulvar cancer is extremely difficult. To further understand the role of adjuvant therapy and investigate current treatment practice, we conducted this large exploratory multicenter cohort study prior to planning a possible prospective trial.
Methods
Patients
Patients with primary or recurrent squamous cell vulvar cancer stage IB-IV (Union internationale contre le cancer-tumor, node, metastasis [UICC-TNM]-classification and stage-groupings version 6) treated at 29 Arbeitsgemeinschaft Gynäkologische Onkologie (AGO) cancer centers between 1998 and 2008 were eligible for the Chemo and Radiotherapy in Epithelial Vulvar Cancer (CaRE-1) study (14). Participating institutions could include all patients with the diagnosis of invasive vulvar cancer greater than stage pT1a independent of the mode and initial place of treatment. Patients who were initially treated elsewhere and for disease recurrence in a study center could also be included. Case selection was in the responsibility of the centers and based on their individual documentation systems. Patients with benign or precursor lesions, nonsquamous neoplasia of the vulva, verrucous vulvar cancer, or those with secondary cancers interfering with the treatment of vulvar cancer were not eligible. Patients had to be age 18 years or older.
Data collection was performed retrospectively between February and December 2011. Documentation and analysis was done through a newly designed centralized database by the AGO study group. Surgery reports and histological diagnoses blinded to patient identifiers were sent to the study office on request with in-house monitoring. The study protocol was approved by local ethics committees at each center (leading vote: Hamburg [reference number PV3658]) and registered with clinicaltrials.gov (NCT01304667). Patients provided written informed consent to access their medical records for scientific analysis at first contact with the respective study center. Therefore, no individual consent for the current retrospective analysis was needed.
In the database, tumor characteristics as well as aspects of surgical and nonsurgical treatment were collected including: TNM stage, tumor size, depth of invasion, grade, number and localization of lymph nodes involved, size of nodal metastasis, surgical therapy of vulva and nodes, pathological resection margin, total dose and fields of irradiation, and, if applicable, agent and dosage of chemotherapy as well as date and treatment of recurrent disease and/or date of last contact or death. Furthermore, patient characteristics as Eastern Cooperative Oncology Group (ECOG) performance status and clinically significant comorbidities were documented. To account for possible bias from informative missing values, we introduced the category “unknown” for each variable to keep all patients in the analysis. Supplementary Table 1 (available online) lists the number of missing values per patient, Supplementary Table 2 (available online) opposes patients with three or fewer and more than three missing values.
Statistical Analysis
All analyses were conducted using SAS software version 9.2 (SAS Institute Inc., Cary, NC); tests were two-sided, P values less than or equal to .05 were considered statistically significant. The chi-square test was used to compare categorical data, the independent samples t test for comparing continuous data between subgroups.
Time-to-event data were analyzed using the Kaplan-Meier method, the log-rank test was used to compare survival distributions between groups. Trends in survival related to the number of affected lymph nodes were investigated by means of the log-rank test for trend. For survival analysis only N+ patients with radiotherapy directed at the groins+/-pelvis+/-other fields were included and compared with N+ patients without adjuvant radiotherapy. Patients older than age 90 years (n = 4) were excluded from survival analysis to reduce a potential outcome bias. Overall survival was defined as the time from primary diagnosis to death from any cause. Survivors were censored on the last date they were known to be alive. Progression-free survival was defined as the time from primary diagnosis to disease progression or death from any cause. Survivors without progression were censored on the last date they were known to be alive. Cox proportional hazards models, including known clinicopathologic prognostic factors and adjuvant treatment, were used to identify prognostic relevance for survival (OS, PFS) by estimating hazard ratios with 95% confidence intervals. Proportional hazards assumptions were verified by global and covariable-specific tests based on scaled Schoenfeld residuals and by graphic residual analysis.
Competing risk analyses (15,16) and propensity score models with inverse probability of treatment weighting (IPTW) to account for multiple confounding were applied. Both methods are elucidated in the Supplemental Material (available online) (17–19).
Results
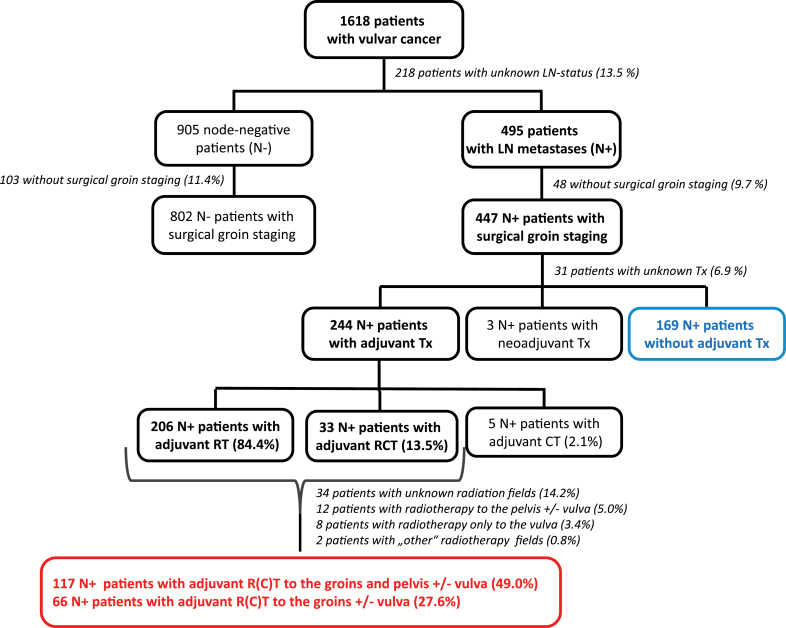
In total, 1618 patients were documented in the database. In 1400 patients, the lymph node status was known. Patients with only clinical, radiological, or bioptic lymph node staging (n = 151) were excluded and further analysis limited to those with surgical groin staging and known lymph node status (n = 1249): 447 (35.8%) node-positive (N+) patients with inguino-femoral lymphadenectomy (LAE) and 802 (64.2%) node-negative (N-) patients with inguino-femoral LAE or sentinel procedure (SLN) only (Figure 1). LAE included resection of lymph nodes above and below the cribriform fascia. If a tumor with a diameter smaller than or equal to 20mm was located strictly lateral with a distance of 1cm or more to the midline, unilateral LAE or SLN was considered safe in cases with clinically negative contralateral groin. In all other cases, a bilateral LAE or SLN was indicated. The majority of N+ patients had one (172 [38.5%]) or two (102 [22.8%]) positive nodes. Detailed characteristics are displayed in Table 1. Patient characteristics of the 151 excluded patients are listed in Supplementary Table 3 (available online).
Figure 1.
Patient characteristics and treatment diagram. CT = chemotherapy; LN = lymph node; Tx = therapy; RT = radiotherapy; RCT = radiochemotherapy.
Table 1.
Characteristics of patients with surgical groin staging
| Characteristic | Total (n = 1249) | N- (n = 802) | N+ (n = 447) | P* (N- vs N+) | N+ without adjuvant treatment (n = 169)† | N+ with adjuvant treatment (n = 244)† | P* (N+ with vs without adjuvant treatment) | N+ with neoadjuvant treatment (n = 3)† |
|---|---|---|---|---|---|---|---|---|
| Median age, y (range) | 67 (20 – 94) | 66 (21–94) | 69 (20 – 94) | <.001 | 72 (20 – 94) | 67 (30 – 87) | .002 | 68 (66 – 80) |
| Tumor stage | <.001 | .38 | ||||||
| pT1b | 474 (38.0%) | 399 (49.8%) | 75 (16.8%) | 30 (17.8%) | 40 (16.4%) | 0 | ||
| pT2 | 650 (52.0%) | 365 (45.5%) | 285 (63.8%) | 111 (65.7%) | 154 (63.1%) | 2 (66.6%) | ||
| pT3 | 115 (9.2%) | 36 (4.5%) | 79 (16.7%) | 24 (14.2%) | 46 (18.9%) | 1 (33.3%) | ||
| pT4 | 7 (0.6%) | 1 (0.1%) | 6 (1.3%) | 4 (2.4%) | 2 (0.8%) | 0 | ||
| Unknown | 3 (0.2%) | 1 (0.1%) | 2 (0.5%) | 0 | 2 (0.8%) | 0 | ||
| Median tumor diameter, mm (range) | 25 (1 – 345) | 20 (1–345) | 35 (2 – 240) | <.001 | 35 (2 – 240) | 35 (2.8 – 200) | .51 | 82.5 (25 – 140) |
| Median depth of invasion, mm (range) | 5 (0.25 – 110) | 4 (0.75–60) | 8 (0.25 - 110) | <.001 | 7 (1 – 70) | 8 (0.25 – 110) | .83 | 27 (4 – 50) |
| Median resection margin, mm (range) | 5 (0.2 – 33) | 5 (0.2–33) | 4 (0.25 – 25) | .03 | 3 (1 – 16) | 4 (0.25 – 25) | .18 | 2 (2 – 2) |
| Resection status of vulvar primary | <.001 | .18 | ||||||
| R0 | 1022 (81.8%) | 703 (87.7%) | 319 (71.4%) | 126 (74.6%) | 173 (70.9%) | 2 (66.7%) | ||
| R1 | 123 (9.9%) | 46 (5.7%) | 77 (17.2%) | 21 (12.4%) | 46 (18.9%) | 1 (33.3%) | ||
| Unknown | 104 (8.3%) | 53 (6.6%) | 51 (11.4%) | 22 (13.0%) | 25 (10.3%) | 0 | ||
| Grading | <.001 | .83 | ||||||
| G1 | 139 (11.1%) | 120 (15.0%) | 19 (4.3%) | 8 (4.7%) | 11 (4.5%) | 0 | ||
| G2 | 768 (61.5%) | 502 (62.6%) | 266 (59.5%) | 100 (59.2%) | 144 (59.0%) | 2 (66.7%) | ||
| G3 | 309 (24.7%) | 158 (19.7%) | 151 (33.8%) | 58 (34.3%) | 81 (33.2%) | 1 (33.3%) | ||
| Unknown | 33 (2.6%) | 22 (2.7%) | 11 (2.5%) | 3 (1.8%) | 8 (3.3%) | 0 | ||
| Positive LN | n/a | .002 | ||||||
| 1 | n/a | n/a | 172 (38.5%) | 86 (50.9%) | 77 (31.6%) | 1 (33.3%) | ||
| 2 | n/a | n/a | 102 (22.8%) | 34 (20.1%) | 57 (23.4%) | 1 (33.3%) | ||
| 3 | n/a | n/a | 62 (13.9%) | 18 (10.7%) | 38 (15.6%) | 1 (33.3%) | ||
| >3 | n/a | n/a | 87 (19.5%) | 25 (14.8%) | 57 (23.4%) | 0 | ||
| Unknown | n/a | n/a | 24 (5.4%) | 6 (3.6%) | (6.2%) | 0 | ||
| ECOG | <.001 | .001 | ||||||
| 0 | 443 (35.5%) | 332 (41.4%) | 111 (24.8%) | 33 (19.5%) | 74 (30.3%) | 0 | ||
| 1 | 199 (15.9%) | 114 (14.2%) | 85 (19.0%) | 24 (14.2%) | 56 (23.0%) | 0 | ||
| 2 | 148 (11.9%) | 86 (10.7%) | 62 (13.9%) | 22 (13.0%) | 33 (13.5%) | 1 (33.3%) | ||
| 3 | 47 (3.8%) | 25 (3.1%) | 22 (4.9%) | 14 (8.3%) | 6 (2.5%) | 0 | ||
| 4 | 5 (0.4%) | 2 (0.3%) | 3 (0.7%) | 1 (0.6%) | 0 | 0 | ||
| Unknown | 407 (32.6%) | 243 (30.3%) | 164 (36.7%) | 75 (44.4%) | 75 (30.7%) | 2 (66.7%) | ||
| Vulva surgery | <.001 | .04 | ||||||
| Wide excision | 128 (10.3%) | 97 (12.1%) | 31 (6.9%) | 20 (11.8%) | 11 (4.5%) | 0 | ||
| Partial vulvectomy | 443 (35.5%) | 323 (40.3%) | 120 (26.9%) | 42 (24.9%) | 70 (28.7%) | 0 | ||
| Complete vulvectomy | 662 (53.0%) | 373 (46.5%) | 289 (64.7%) | 105 (62.1%) | 160 (65.6%) | 3 (100%) | ||
| Exenteration | 14 (1.1%) | 7 (0.9%) | 7 (1.6%) | 2 (1.2%) | 3 (1.2%) | 0 | ||
| Surgery type unknown | 2 (0.2%) | 2 (0.3%) | 0 | 0 | 0 | 0 | ||
| No surgery | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Surgery status unknown | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Groin surgery | ||||||||
| Groin dissection | ||||||||
| Unilateral | 245 (19.6%) | 147 (18.3%) | 98 (21.9%) | .56 | 49 (29.0%) | 39 (16.0%) | .002 | 2 (66.7%) |
| Bilateral | 919 (73.6%) | 570 (71.1%) | 349 (78.1%) | 120 (71.0%) | 205 (84.0%) | 1 (33.3%) | ||
| After initial sentinel node dissection | 280 (22.4%) | 178 (22.2%) | 102 (22.8%) | .01 | 52 (30.8%) | 46 (18.9%) | .01 | 0 |
| Primary complete groin dissection | 825 (66.1%) | 514 (64.1%) | 311 (69.6%) | 107 (63.3%) | 175 (71.7%) | 3 (100.0%) | ||
| Unknown if primary or secondary | 59 (4.7%) | 25 (3.1%) | 34 (7.6%) | 10 (5.9%) | 23 (9.4%) | 0 | ||
| Sentinel procedure only | 85 (6.8%) | 85 (10.6%) | 0 | <.001‡ | 0 | 0 | 0 | |
| Pelvic node dissection | 70 (5.6%) | 16 (2.0%) | 54 (12.1%) | <.001 | 16 (9.5%) | 30 (12.3%) | .37 | 1 (33.3%) |
| Median number of dissected groin LNs per patient (range) [IQR] | 15 (1 – 81) [10 – 19] | 14 (1 – 49) [10 – 18] | 15 (1 – 81) [10 – 21] | .01 | 15 (1 – 62) [11 – 20] | 15 (1 – 81) [10 – 21] | .88 | 3 (2 – 7) [2 – 7] |
| Median LN metastasis diameter, mm (range) | n/a | n/a | 20 (0.3 – 100) | n/a | 15 (0.3 – 50) | 20 (1 – 100) | .01 | 15 (15 – 15) |
* All percentages refer to columns. P values were calculated using the Student’s t test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. The latter was applied if any table cell had expected counts less than 5. All tests were two-sided. N+ = node-positive patients, N- = node-negative patients, LN = lymph node.
† 31 missing up to 447 are N+ patients with no information on adjuvant treatment.
‡ P value for groin dissection vs sentinel procedure only.
Most of the patients in the study cohort (1124/1249, 90.0%) had localized tumors (pT1b/pT2) and complete tumor resection (1022/1249, 81.8%). The majority received bilateral groin dissection (919/1249, 73.6%). Median number of excised groin nodes per patient was 15 (interquartile range [IQR] = 10–19), including patients with unilateral procedures and primary and secondary surgeries. Eighty-five of 802 (10.6%) node-negative patients had an SLN procedure only. Four hundred forty-seven of 1249 (35.8%) patients had lymph node metastases (N+). Table 2 displays the treatment characteristics of the node-positive patients with regard to the number of nodes affected. Altogether, 54.6% (244/447) N+ patients received adjuvant therapy; the majority (206/244, 84.4%) was treated with radiotherapy, while 13.5% (33/244) received concomitant chemoradiation. The most frequently applied cytostatic agent was cisplatin (in 72.7% [24/33] as single agent, in 6.1% [2/33] as combination therapy). Radiation therapy was applied heterogeneously particularly in terms of treatment volume and included the inguinal nodes in 183 of the 239 patients (40.9% of all 447 node-positive patients) with adjuvant (chemo)radiation and both the inguinal and pelvic nodes (standard treatment as per Homesley [7]) in 117 of 239 cases, the latter being a subset of the 183 patients. Sixty-six of 239 patients received adjuvant therapy to the inguinal nodes without a pelvic field. Target-specific doses were not collected in the database. The median total dose applied in all N+ patients with adjuvant radiotherapy (n = 239) regardless of the fields irradiated was 50.4 Gy (IQR = 50.4–58.4, 34 missing). The N+ patients with adjuvant radiotherapy directed to the groins (+/-vulva) or the groins and pelvis (+/-vulva) (n = 183) also received a median total dose of 50.4 Gy (IQR = 50.4–56.4, 14 missing). Supplementary Tables 4–7 (available online) summarize the characteristics of patients with and without adjuvant therapy for each nodal subgroup. Variables differing statistically significantly between treatment groups across all nodal subgroups could not be identified, most likely because of small subgroup sizes lacking power for statistical significance. However, a consistent difference in age and performance status between treatment groups could be observed across subgroups revealing that patients of younger age and better constitution were more likely to receive adjuvant treatment than not.
Table 2.
Treatment characteristics of node-positive patients (n = 447)
| Characteristic | N+ (n = 447) | 1 pos LN (n = 172) | 2 pos LN (n = 102) | 3 pos LN (n = 62) | >3 pos LN (n = 87) | Unknown number of positive LNs (n = 24) |
|---|---|---|---|---|---|---|
| No Tx | ||||||
| Number of pts | 169 (37.8%) | 86 (50.0%) | 34 (33.3%) | 18 (29.0%) | 25 (28.7%) | 6 (25.0%) |
| Neoadjuvant Tx | ||||||
| Number of pts | 3 (0.7%) | 1 (0.6%) | 1 (1.0%) | 1 (1.6%) | 0 | 0 |
| Unknown Tx | ||||||
| Number of pts | 31 (6.9%) | 8 (4.7%) | 10 (9.8%) | 5 (8.1%) | 5 (5.8%) | 3 (12.5%) |
| Adjuvant Tx | ||||||
| Number of pts | 244 (54.6%) | 77 (44.8%) | 57 (55.9%) | 38 (61.3%) | 57 (65.5%) | 15 (62.5%) |
| Radio/chemotherapy* | ||||||
| Radiotherapy only | 206 (84.4%) | 74 (96.1%) | 48 (84.2%) | 32 (84.2%) | 40 (70.2%) | 12 (80.0%) |
| Chemotherapy only | 5 (2.1%) | 1 (1.3%) | 1 (1.8%) | 0 | 3 (5.3%) | 0 |
| Radio- chemotherapy | 33 (13.5%) | 2 (2.6%) | 8 (14.0%) | 6 (15.8%) | 14 (24.6%) | 3 (20.0%) |
| Radiation field† | ||||||
| Groins +/- vulva | 66 (27.6%) | 26 (34.2%) | 16 (28.6%) | 8 (21.1%) | 14 (25.9%) | 2 (13.3%) |
| Groins and pelvis +/- vulva | 117 (49.0%) | 34 (44.7%) | 31 (55.4%) | 19 (50.0%) | 26 (48.2%) | 7 (46.7%) |
| Pelvis +/- vulva | 12 (5.0%) | 5 (6.6%) | 2 (3.6%) | 2 (5.3%) | 3 (5.6%) | 0 |
| Vulva only | 8 (3.4%) | 5 (6.6%) | 1 (1.8%) | 1 (2.6%) | 0 | 1 (6.7%) |
| Other (vagina) | 2 (0.8%) | 0 | 1 (1.8%) | 1 (2.6%) | 0 | 0 |
| Unknown | 34 (14.2%) | 6 (7.9%) | 5 (8.9%) | 7 (18.4%) | 11 (20.4%) | 5 (33.3%) |
| Median LN metastasis diameter, mm (range) | 20 (1 – 100) | 8 (1 – 70) | 20 (2 – 80) | 30.5 (8 – 100) | 35 (12 – 70) | 29 (29 – 29)‡ |
* All percentages refer to columns. Percentages refer to patients who received adjuvant treatment. LN = lymph node; N+ = node-positive patients; Tx = therapy.
† Percentages refer to patients who received adjuvant (chemo)radiation.
‡ Only one known LN metastasis diameter.
Median follow-up in the study cohort (n = 1249) was 39.4 months (95% confidence interval [CI] = 36.8 to 43.2, IQR = 11.8–71.4). Disease recurred in 360 of 1249 patients after a median of 12.6 months. Table 3 describes localization of disease recurrence with regard to nodal involvement. While 89 N+ patients had vulvar recurrence only, 25 (28.1%) of these had previously received adjuvant radiotherapy to the vulva (+/-other fields).
Table 3.
Localization of disease recurrence with regard to nodal involvement
| Localization of disease recurrence | Total (n = 1249) 360 recurrences | N- (n = 802) 169 recurrences | N+ (n =447) 191 recurrences |
|---|---|---|---|
| Vulva (+/- other localizations) | 266 | 132 | 134 |
| thereof vulva only | 200 | 111 | 89 |
| Groins (+/- other localizations) | 103 | 39 | 64 |
| thereof groins only | 43 | 24 | 19 |
| Pelvis (+/- other localizations) | 31 | 10 | 21 |
| thereof pelvis only | 5 | 2 | 3 |
| Distant (+/- other localizations) | 68 | 16 | 52 |
| thereof distant only | 17 | 1 | 16 |
| Unknown | 8 | 5 | 3 |
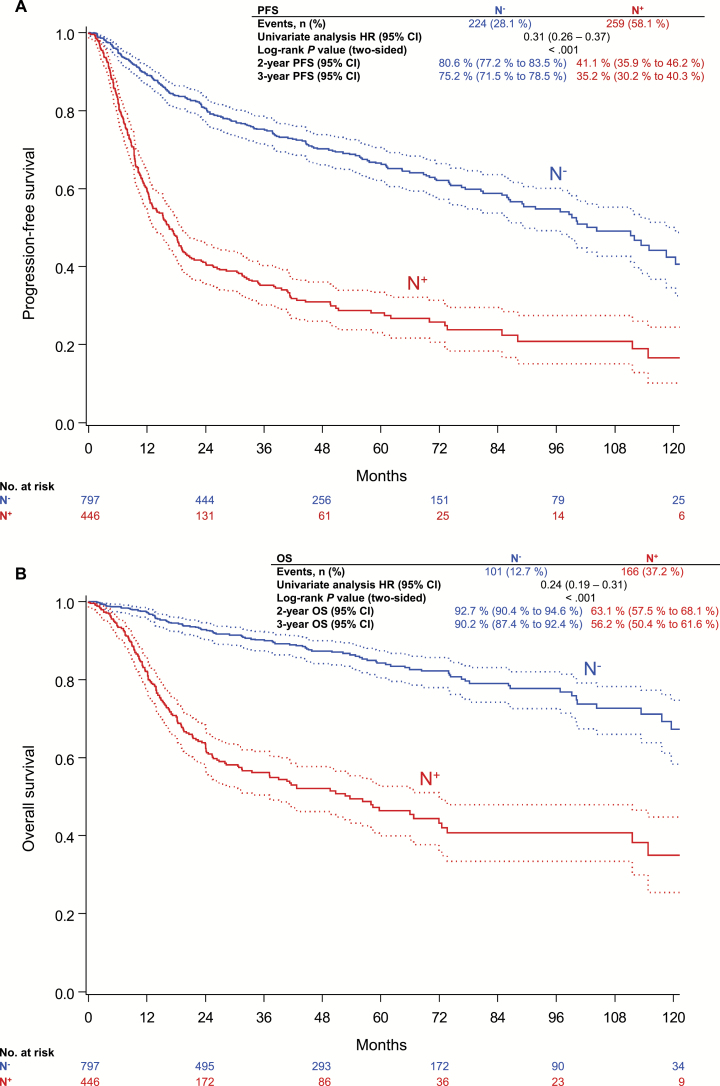
Three-year progression-free survival (PFS) rate of N+ patients was 35.2%, compared with 75.2% in N- patients (P < .001) (Figure 2A); three-year overall survival (OS) rates were 56.2% and 90.2%, respectively (P < .001) (Figure 2B). Altogether 267 of 1249 patients died, 136 (50.9%) clearly disease related; 87 of 267 (32.6%) deaths were indicated as “death from unknown cause,” and 44 of 267 (16.5%) as “death from other causes.” Of these 131 not clearly disease-related deaths, 72 occurred in the N+ and 59 in the N- cohort.
Figure 2.
Lymph node status and outcome. A) Progression-free survival. B) overall survival. P values were calculated using the two-sided log-rank test. CI = confidence interval; HR = hazard ratio; N- = node-negative; N+ = node-positive; OS = overall survival; PFS = progression-free survival.
Clear trends of decreasing PFS and OS in patients with increasing numbers of lymph node metastases were observed (P < .001, by two-sided log-rank tests for trend). Three-year PFS rates were 47.6% (95% CI = 38.9% to 55.8%) in patients with one, 27.6% (95% CI = 18.0% to 38.1%) in patients with two, 33.1% (95% CI = 20.4%to 46.3%) in patients with three, and 21.2% (95% CI = 12.0% to 32.2%) in patients with more than three positive lymph nodes; three-year OS rates were 72.8% (95% CI = 64.0% to 79.8%), 50.1% (95% CI = 37.1% to 61.8%), 44.8% (95% CI = 30.2% to 58.5%), and 33.0% (95% CI = 20.0% to 46.5%), respectively.
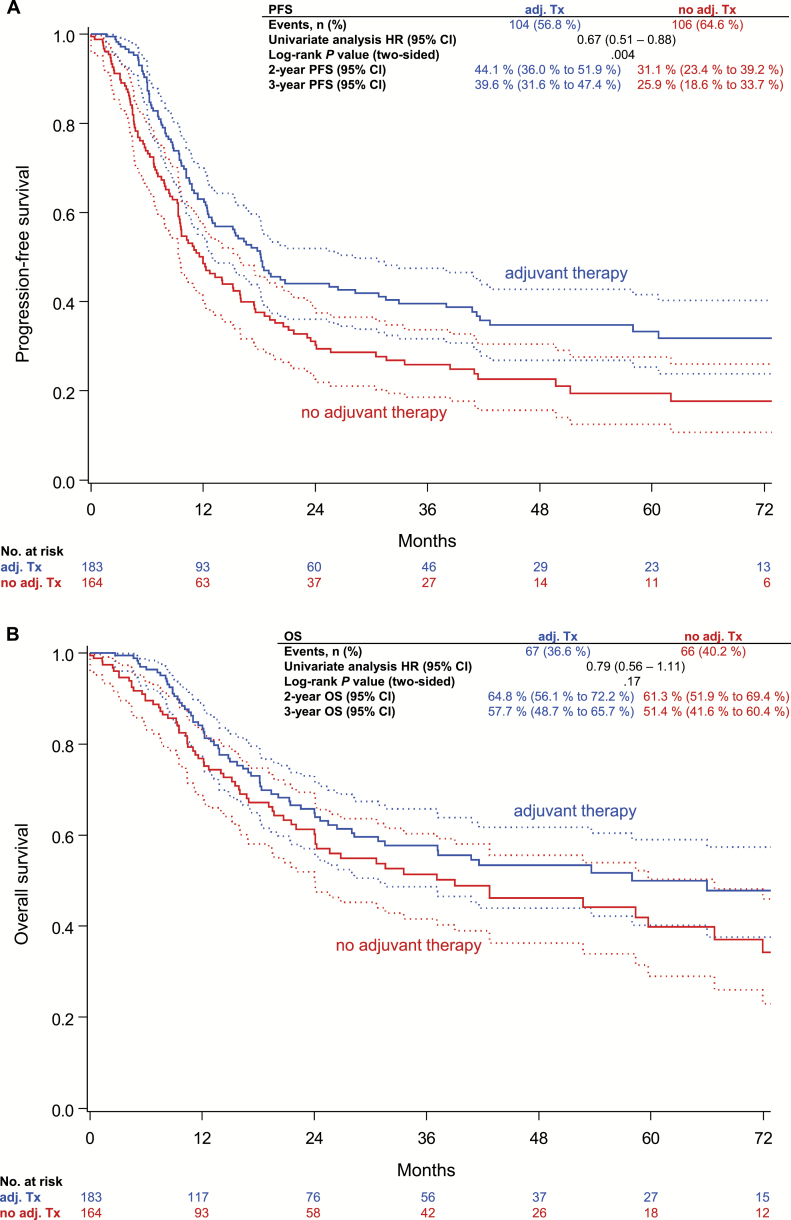
For the analysis of adjuvant radiotherapy and disease recurrence, only patients younger than age 90 years with radiotherapy directed at the groins+/-pelvis+/-vulva (n = 183) or without adjuvant radiotherapy (n = 165) were included. Three-year PFS rate in N+ patients receiving adjuvant therapy was statistically significantly better compared with N+ patients without adjuvant treatment (39.6% vs 25.9%, HR = 0.67, 95% CI = 0.51 to 0.88, P = .004), whereas the difference in three-year OS rate was statistically not significant (57.7% vs 51.4%, HR = 0.79, 95% CI = 0.56 to 1.11, P = .17) (Figure 3). Similar results were obtained after control for multiple confounding by inverse probability of treatment weighting (IPTW) based on the propensity score (PFS: 39.7% vs 24.5%, HRIPTW = 0.65, 95% CI = 0.49 to 0.86, P IPTW = .003; OS: 57.6% vs 51.9%, HRIPTW = 0.80, 95% CI = 0.55 to 1.15, P IPTW = .22).
Figure 3.
Progression-free survival (A) and overall survival of (B) node-positive patients with regard to adjuvant radiotherapy to the groins +/-pelvis +/-vulva. P values were calculated using the two-sided log-rank test. CI = confidence interval; HR = hazard ratio; OS = overall survival; PFS = progression-free survival; Tx = therapy.
Supplementary Figures 1–4 (available online) show the corresponding competing risk graphics with similar results regarding cancer-related outcomes compared with the overall analyses.
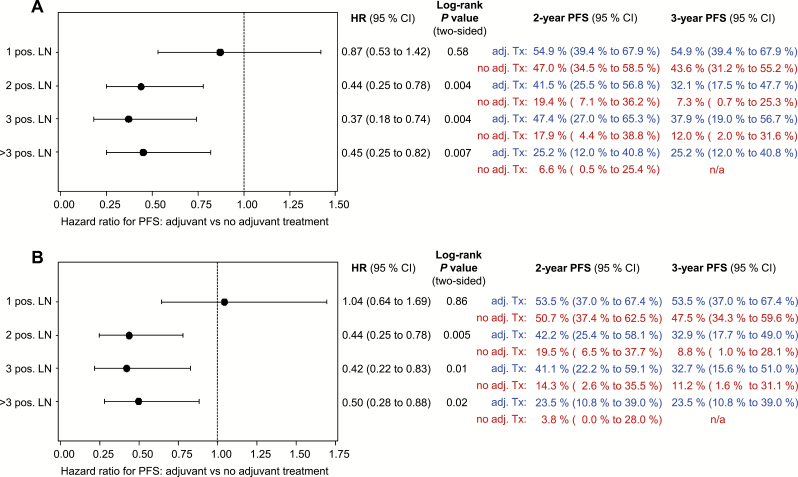
Looking at adjuvant therapy by univariate subgroup analysis with regard to the number of affected nodes, PFS rate was statistically significantly higher for patients with adjuvant radiotherapy in case of two or more affected nodes. The hazard ratio was 0.44 in patients with two positive nodes (95% CI = 0.25 to 0.78, P = .004) (Figure 4A); the hazard ratio was 0.37 in patients with three (95% CI = 0.18 to 0.74, P = .004), and it was 0.45 in patients with more than three positive nodes (95% CI = 0.25 to 0.82, P = .007). With control for multiple confounding, HRsIPTW were 0.44 (95% CI = 0.25 to 0.78, P = .005) in patients with two, 0.42 (95% CI = 0.22 to 0.83, P = .01) in patients with three, and 0.50 (P = .02, 95% CI = 0.28 to 0.88) in patients with more than three three positive nodes (Figure 4B). Regarding the size of metastasis and subsequent better outcome with adjuvant treatment, a cutoff value in the subgroup of patients with only one positive node could not be identified. In multivariable analysis of the different nodal subgroups (adjustment for age, ECOG, UICC [Union internationale contre le cancer] stage, grade, and invasion depth) adjuvant radiotherapy was associated with better PFS (HR = 0.88, P = .67 [95% CI = 0.50 to 1.56]/HRIPTW = 0.93, P = .79 [95% CI = 0.51 to 1.67] in patients with one, HR = 0.31, P = .005 [95% CI = 0.14 to 0.71]/HRIPTW = 0.24, P < .001 [95% CI = 0.11 to 0.56] in patients with two, HR = 0.40, P = .05 [95% CI = 0.16 to 0.98]/HRIPTW = 0.32, P = .009 [95% CI = 0.13 to 0.79] in patients with three, and 0.52, P = .09 [95% CI = 0.24 to 1.10]/HRIPTW = 0.44, P = .10 [95% CI = 0.17 to 1.17] in patients with more than three positive nodes) reaching statistical significance only in patients with two or three positive nodes, for both the naïve and confounder-adjusted estimates.
Figure 4.
Forest plot of progression-free survival in nodal subgroups with regard to adjuvant radiotherapy to the groins +/-pelvis +/-vulva. Results unadjusted (A) and confounder-adjusted by inverse probability of treatment weighting (B). P values were calculated using the two-sided log-rank test. CI = confidence interval; HR = hazard ratio; LN = lymph node; PFS = progression-free survival.
In multivariable analysis of the node-positive patients with adjuvant radiotherapy directed to the groins+/-pelvis+/-vulva (n = 183) and those without adjuvant radiotherapy (n = 165) adjusted for age, ECOG, UICC stage, grade, invasion depth, and number of positive nodes, the effect of adjuvant therapy on PFS and OS remained consistent (PFS: HR = 0.58 [95% CI = 0.43 to 0.78, P < .001]/HRIPTW = 0.58 [95% CI = 0.42 to 0.79, P = .001], OS: HR = 0.63 [95% CI = 0.43 to 0.91, P = .01]/HRIPTW = 0.67 [95% CI = 0.44 to 1.00, P = .05]) (Table 4; Supplementary Table 8, available online). Besides adjuvant treatment, the number of positive nodes was also an independent prognostic factor for PFS and OS as well as age for OS. Testing for interactions between the effect of adjuvant therapy and number of affected lymph nodes or age did not yield statistically significant results (interaction with number of nodes [PFS: P = .16; OS: P = .29], interaction with age [PFS: P = .94; OS: P = .23]). Multivariable analyses with categorized age (Supplementary Table 9, available online) showed similar results to those including age as a continuous covariable.
Table 4.
Multivariable analysis of progression-free survival and overall survival in node-positive patients (n = 346/348, 2 observations deleted because of missing values)
| Outcome | HR (95% CI) | P* |
|---|---|---|
| Progression-free survival | ||
| Adjuvant therapy to groins +/- pelvis +/- vulva vs none | 0.58 (0.43 to 0.78) | <.001 |
| ECOG1 vs 0 | 2.46 (1.51 to 4.03) | <.001 |
| ECOG2 vs 0 | 1.80 (1.07 to 3.02) | .03 |
| ECOG>2 vs 0 | 2.41 (1.15 to 5.04) | .02 |
| ECOG unknown† vs 0 | 2.31 (1.47 to 3.61) | <.001 |
| T2 vs T1 | 1.36 (0.89 to 2.08) | .16 |
| T3 vs T1 | 1.01 (0.59 to 1.73) | .96 |
| T4 and T unknown† vs T1 | 5.17 (1.75 to 15.27) | .003 |
| Age (per year) | 1.01 (1.00 to 1.02) | .24 |
| Depth of invasion >3–6mm vs 0–3 mm | 0.92 (0.50 to 1.72) | .80 |
| Depth of invasion >6mm vs 0–3 mm | 0.87 (0.50 to 1.72) | .64 |
| Depth of invasion unknown† vs 0–3 mm | 0.92 (0.52 to 1.63) | .77 |
| Grade 2 vs 1 | 1.62 (0.87 to 3.03) | .13 |
| Grade 3 vs 1 | 1.95 (1.02 to 3.69) | .04 |
| Grade unknown† vs 1 | 1.79 (0.61 to 5.21) | .29 |
| 2 positive lymph nodes vs 1 | 2.08 (1.41 to 3.08) | <.001 |
| 3 positive lymph nodes vs 1 | 2.02 (1.28 to 3.19) | .002 |
| >3 positive lymph nodes vs 1 | 3.05 (2.00 to 4.66) | <.001 |
| Unknown† number of pos. nodes vs 1 | 1.88 (0.89 to 3.97) | .10 |
| Overall survival | ||
| Adjuvant therapy to groins +/- pelvis +/- vulva vs none | 0.63 (0.43 to 0.91) | .01 |
| ECOG1 vs 0 | 1.71 (0.92 to 3.18) | .09 |
| ECOG2 vs 0 | 1.66 (0.88 to 3.15) | .12 |
| ECOG>2 vs 0 | 1.51 (0.58 to 3.92) | .39 |
| ECOG unknown† vs 0 | 1.68 (0.96 to 2.93) | .07 |
| T2 vs T1 | 1.48 (0.86 to 2.55) | .15 |
| T3 vs T1 | 1.16 (0.60 to 2.23) | .66 |
| T4 and T unknown† vs T1 | 3.68 (0.81 to 16.77) | .09 |
| Age (per year) | 1.03 (1.02 to 1.05) | <.001 |
| Depth of invasion >3–6mm vs 0–3 mm | 0.94 (0.45 to 1.97) | .88 |
| Depth of invasion >6mm vs 0–3 mm | 0.79 (0.39 to 1.62) | .53 |
| Depth of invasion unknown† vs 0–3 mm | 0.66 (0.33 to 1.34) | .25 |
| Grade 2 vs 1 | 1.22 (0.57 to 2.61) | .61 |
| Grade 3 vs 1 | 1.77 (0.82 to 3.81) | .15 |
| Grade unknown† vs 1 | 2.04 (0.48 to 8.73) | .34 |
| 2 positive lymph nodes vs 1 | 2.52 (1.51 to 4.22) | <.001 |
| 3 positive lymph nodes vs 1 | 2.97 (1.69 to 5.24) | <.001 |
| >3 positive lymph nodes vs 1 | 4.80 (2.78 to 8.28) | <.001 |
| Unknown† number of pos. nodes vs 1 | 1.49 (0.56 to 3.99) | .43 |
* P values were calculated using the Student’s t test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. The latter was applied if any table cell had expected counts less than 5. All tests were two-sided. HR = hazard ratio; CI = confidence interval.
† Number of missing values per covariable assigned to category “unknown”: ECOG 123 (35.3%), tumor stage one (0.3%), age one (0.3%), depth of invasion 157 (45.1%), grade seven (2.0%), number of positive nodes 15 (4.3%).
Of note, some variables had a considerable amount of missing values (assigned to the category “unknown” for analysis): ECOG in 123 patients (35.3%), tumor stage in one (0.3%), age in one (0.3%), depth of invasion in 157 (45.1%), grade in seven (2.0%), and number of positive nodes in 15 patients (4.3%). Multivariable analyses excluding ECOG and depth of invasion showed, however, consistent results regarding adjuvant radiotherapy (adjuvant therapy vs none PFS: HR = 0.52, 95% CI = 0.39 to 0.70, P < .001; OS: HR = 0.58, 95% CI = 0.40 to 0.84, P = .004). Further multivariable analyses including cause-specific endpoints are presented in Supplementary Tables 10–12 (available online). Corresponding to the results of the overall analyses, adjuvant therapy was identified as a statistically significant predictor in multivariable analyses for cancer-related PFS and survival (adjuvant therapy vs none PFS: HR = 0.58, 95% CI = 0.42 to 0.81, P = .001; survival: HR = 0.59, 95% CI = 0.36 to 0.97, P = .04) (Supplementary Table 10, available online). All results of the confounder-adjusted models by IPTW supported the unadjusted findings.
Discussion
The present study analyzes current clinical practice and indication criteria of adjuvant treatment in node-positive vulvar cancer. It represents a large multicenter cohort where adjuvant radiotherapy is associated with better outcome in node-positive patients.
Even though lymph node metastases are clearly the most important prognostic factor in vulvar cancer and outcome is already impaired with only one affected node, parameters determining indication of adjuvant radiotherapy after groin dissection are highly controversial and remain subject of discussion (6,9,11). In the 1980s, the Gynecologic Oncology Group published GOG37, investigating the value of pelvic lymphadenectomy compared with irradiation of groins and pelvis after vulvectomy and inguino-femoral lymphadenectomy in a group of 114 patients (7). A survival benefit for patients with two or more positive groin nodes could be observed in the irradiation group. Even after more than 20 years, discussion on the study design and interpretation of these results is ongoing. The main reasons for reservation to generally implement adjuvant inguinal and pelvic radiotherapy are a higher pelvic recurrence rate observed in the radiation group (6.8% vs 1.8% in the surgery group) and the fact that no adjuvant radiotherapy to the groins was applied in the surgery group despite positive groin nodes, with a high rate of groin recurrences (23.6%) in this group and consecutive poor outcome. The positive effect of radiotherapy on survival in GOG37 might therefore be mainly gained by irradiation of the groins and not the pelvis with the latter being a potential overtreatment in patients with low risk of pelvic metastasis. This idea is especially present in patients with two positive groin nodes since risk for pelvic disease appears to increase substantially from three or more affected groin nodes (20). Although not supported by high-level evidence, some people therefore argue for surgical assessment of the pelvic nodes in patients with positive groins and application of pelvic radiation only in case of metastasis in this area (21). These aspects, however, can only partially explain the poor penetrance of GOG37 results we observed, especially with only 61.7% of the patients with three or more affected nodes receiving adjuvant radiotherapy to the groins.
Our study could not identify uniform indication criteria for adjuvant treatment when comparing node-positive patients receiving treatment with those who did not: Despite negative guideline recommendation, almost half of the patients with one positive node underwent adjuvant treatment without having larger nodal metastases. Treatment criteria in the other nodal groups were similarly ambiguous. Heterogeneity might partially be explained by the assumedly high number of elderly vulvar cancer patients with clinically significant comorbidities, as well as the orphan status of the disease. This could result in individualized treatment decisions rather than institutional standard approaches. Younger patients received adjuvant therapy more frequently than older patients, interaction analysis between age and treatment effect, however, did not reveal a significant interaction. To account for selection bias and potential confounding, we performed a propensity score analysis. Results were similar to the overall analysis, confirming the association of adjuvant radiotherapy with improved outcome for node-positive vulvar cancer.
Despite the fact that only half of the patients in the “adjuvant radiotherapy” group received the “Homesley schema” of pelvic field, bilateral inguinal nodes ± vulva, the results of our study suggest that adjuvant radiotherapy is associated with better outcome in patients with two or more positive lymph nodes in univariate and multivariable analysis. Outcome might have been even better if treatment had been more standardized and comprehensive. In line with the findings of GOG37, our results will hopefully help to overcome concerns regarding the reliability of the study and improve guideline adherence.
Unfortunately, the situation of patients with single lymph node metastasis remains unclear. Prognosis was statistically significantly impaired in this subgroup in our study as well as in other published series (22), but we could not observe a statistically significant difference with and without adjuvant radiotherapy. The statistically nonsignificant result of the interaction analysis between number of positive nodes and treatment effect might, however, suggest a potential positive effect of adjuvant radiotherapy in node-positive vulvar cancer irrespective of the number of affected lymph nodes. Even though the present study represents a very large multicenter population, the subgroup of 77 patients with a single positive node receiving adjuvant radiotherapy might be too small to show a statistically significant treatment effect, especially if such an effect is potentially restricted to a fraction of single node-positive patients. A recently published analysis of 75 patients from the Netherlands could likewise not demonstrate a benefit of adjuvant radiotherapy in these patients while a Surveillance Epidemiology and End Results (SEER) analysis observed more favorable five-year survival in patients with one positive lymph node undergoing radiotherapy compared with patients without adjuvant treatment (8,23). In the SEER analysis, however, size and characteristics of the nodal metastases were not known. Some previous analyses have suggested a relation between size of nodal metastasis and therapeutic benefit that could not be reproduced in our cohort (24,25).
Another notable result of our study is that prognosis of patients with node-positive disease remains poor even after adjuvant treatment. Improvement of adjuvant therapy is therefore urgently needed, especially with the increasing incidence of vulvar cancer (26). A possible option to improve prognosis might be an intensified adjuvant treatment in analogy to other squamous cell cancers such as anal or cervical cancer applying chemoradiation instead of radiotherapy alone. There have already been small series of adjuvant chemoradiation in vulvar cancer showing promising activity of the combined treatment (27). This and especially the encouraging response rates from studies on neoadjuvant chemoradiation in advanced vulvar cancer encourage further investigation of this concept (28,29).
Because of the retrospective design of our study, several limitations, especially the potential bias in case selection, missing data, and variations in primary management have to be acknowledged. However, gathering data of more than 1600 patients from 29 centers through this project enabled the analysis of a meaningful patient cohort in a reasonable time frame despite the low incidence of this disease. The reported AGO CaRE-1 study will hopefully build the basis for an upcoming prospective randomized international trial investigating the role of adjuvant chemoradiation in vulvar cancer. Successful operation of such a trial, however, implies substantial logistic challenges and will probably take a long time to recruit even if performed in widespread international collaboration. Until then, large retrospective studies such as the present are essential and provide important information on a disease as rare as node-positive vulvar cancer.
Funding
This work was partially supported by Medac oncology.
Supplementary Material
The funder had no role in design and conduct of the study, the collection, management, analysis, or interpretation of the data, the preparation, review, or approval of the manuscript, nor the decision to submit the manuscript for publication.
The authors declare that there is no conflict of interest regarding the present study
We thank Cordula Petersen for continuous support in all aspects and questions regarding radiation oncology, Christine zu Eulenburg for help regarding the propensity score modelling, the KKS statisticians and staff for thorough data management and the AGO study group staff for invaluable organizational help and the Norddeutsche Gesellschaft für Gynäkologische Onkologie (NOGGO) for support.
We also thank the additional investigators and staff at the 29 clinical sites for their work with clinical data and followup: Kreisklinik Altoetting; Charité - University Medicine Berlin Campus Virchow; University Hospital Dresden; Essen KEM; Essen University Hospital; University Hospital Frankfurt; Alb Fils Kliniken Goeppingen; University Hospital Freiburg; University Hospital Goettingen; University Hospital Greifswald; University Offenbach; Oberhavel Kliniken Oranienburg; Elblandkliniken Radebeul; Städtisches Klinikum Solingen; University Hospital Tuebingen; Klinikum Wolfsburg.
This study was presented in part at ASCO Annual Meeting 2012.
References
- 1. Beller U, Quinn MA, Benedet JL, et al. Carcinoma of the vulva. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S7–S27. [DOI] [PubMed] [Google Scholar]
- 2. Hampl M, Deckers-Figiel S, Hampl JA, Rein D, Bender HG. New aspects of vulvar cancer: changes in localization and age of onset. Gynecol Oncol. 2008;109(3):340–345. [DOI] [PubMed] [Google Scholar]
- 3. Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006;107(5):1018–1022. [DOI] [PubMed] [Google Scholar]
- 4. Gadducci A, Cionini L, Romanini A, Fanucchi A, AR G. Old and new perspectives in the management of high-risk, locally advanced or recurrent, and metastatic vulvar cancer. Crit Rev Oncol/Hematol. 2006;60(3):227–241. [DOI] [PubMed] [Google Scholar]
- 5. Woelber L, Mahner S, Voelker K, et al. Clinicopathological prognostic factors and patterns of recurrence in vulvar cancer. Anticancer Res. 2009;29(2):545–552. [PubMed] [Google Scholar]
- 6. Woelber L, Eulenburg C, Choschzick M, et al. Prognostic role of lymph node metastases in vulvar cancer and implications for adjuvant treatment. Int J Gynecol Cancer. 2012;22(3):503–508. [DOI] [PubMed] [Google Scholar]
- 7. Homesley HD, Bundy BN, Sedlis A, Adcock L. Radiation therapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obstet Gynecol. 1986;68(6):733–740. [PubMed] [Google Scholar]
- 8. Parthasarathy A, Cheung MK, Osann K, et al. The benefit of adjuvant radiation therapy in single-node-positive squamous cell vulvar carcinoma. Gynecol Oncol. 2006;103(3):1095–1099. [DOI] [PubMed] [Google Scholar]
- 9. Woelber L, Kock L, Gieseking F, et al. Clinical management of primary vulvar cancer. Eur J Cancer. 2011;47(15):2315–2321. [DOI] [PubMed] [Google Scholar]
- 10. Kunos C, Simpkins F, Gibbons H, Tian C, Homesley H. Radiation therapy compared with pelvic node resection for node-positive vulvar cancer: a randomized controlled trial. Obstet Gynecol. 2009;114(3):537–546. [DOI] [PubMed] [Google Scholar]
- 11. Oonk MH, de Hullu JA, van der Zee AG. Current controversies in the management of patients with early-stage vulvar cancer. Curr Opin Oncol. 2010;22(5):481–486. [DOI] [PubMed] [Google Scholar]
- 12. AGO Kommission Vulva Vagina. Interdisziplinäre S2k Leitlinie für die Diagnostik und Therapie des Vulvakarzinoms und seiner Vorstufen. München: W. Zuckschwerdt Verlag; 2009. [Google Scholar]
- 13. Royal College of Obstetricians and Gynaecologists: Guidelines for the Diagnosis and Management of Vulval Carcinoma, 2014. http://www.rcog.org.uk/womens-health/clinical-guidance/vulval-carcinoma-guidelines-diagnosis-and-management.
- 14. American Joint Committee on Cancer. AJCC Cancer Staging Manual In. 6th ed., 2002. https://cancerstaging.org/references-tools/deskreferences/Pages/default.aspx. [Google Scholar]
- 15. Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18(8):2301–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wolbers M, Koller MT, Stel VS, et al. Competing risks analyses: objectives and approaches. Eur Heart J. 2014;35(42):2936–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crowson CS, Schenck LA, Green AB, Atkinson EJ, Therneau T. The Basics of Propensity Scoring and Marginal Structural Models. Department of Health Sciences Research, Mayo Clinic Rochester, Minnesota;. In: Department of Health Sciences Research, Mayo Clinic Rochester, Minnesota, 2013. [Google Scholar]
- 19. Rosenbaum P, Rubin D. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 20. Hacker NF, Berek JS, Lagasse LD, Leuchter RS, Moore JG. Management of regional lymph nodes and their prognostic influence in vulvar cancer. Obstet Gynecol. 1983;61(4):408–412. [PubMed] [Google Scholar]
- 21. Klemm P, Marnitz S, Kohler C, Braig U, Schneider A. Clinical implication of laparoscopic pelvic lymphadenectomy in patients with vulvar cancer and positive groin nodes. Gynecol Oncol. 2005;99(1):101–105. [DOI] [PubMed] [Google Scholar]
- 22. Oonk MH, van Hemel BM, Hollema H, et al. Size of sentinel-node metastasis and chances of non-sentinel-node involvement and survival in early stage vulvar cancer: results from GROINSS-V, a multicentre observational study. Lancet Oncol. 2010;11(7):646–652. [DOI] [PubMed] [Google Scholar]
- 23. Fons G, Groenen SMA, Oonk MHM, et al. Adjuvant radiotherapy in patients with vulvar cancer and one intra capsular lymph node metastasis is not beneficial. Gynecol Oncol. 2009;114(2):343–345. [DOI] [PubMed] [Google Scholar]
- 24. Origoni M, Sideri M, Garsia S, Carinelli SG, Ferrari AG. Prognostic value of pathological patterns of lymph node positivity in squamous cell carcinoma of the vulva stage III and IVA FIGO. Gynecol Oncol. 1992;45(3):313–316. [DOI] [PubMed] [Google Scholar]
- 25. van der Velden J, van Lindert AC, Lammes FB, et al. Extracapsular growth of lymph node metastases in squamous cell carcinoma of the vulva. The impact on recurrence and survival. Cancer. 1995;75(12):2885–2890. [DOI] [PubMed] [Google Scholar]
- 26. Jones RW, Baranyai J, Stables S. Trends in squamous cell carcinoma of the vulva: the influence of vulvar intraepithelial neoplasia. Obstet Gynecol. 1997;90(3):448–452. [DOI] [PubMed] [Google Scholar]
- 27. Han SC, Kim DH, Higgins SA, Carcangiu ML, BM K. Chemoradiation as primary or adjuvant treatment for locally advanced carcinoma of the vulva. Int J Radiation Oncol Biol Phys. 2000;47(5):1235–1244. [DOI] [PubMed] [Google Scholar]
- 28. Moore DH, Ali S, Koh WJ, et al. A phase II trial of radiation therapy and weekly cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: a gynecologic oncology group study. Gynecol Oncol. 2012;124(3):529–533. [DOI] [PubMed] [Google Scholar]
- 29. Moore DH, Thomas GM, Montana GS, Saxer A, Gallup DG, Olt G. Preoperative chemoradiation for advanced vulvar cancer: a phase II study of the Gynecologic Oncology Group. Int J Radiation Oncol Biol Phys. 1998;42(1):79–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.