Abstract
Background
Chronic obstructive pulmonary disease (COPD) patients include those who have never smoked. However, risk factors other than smoking in never-smokers have not been elucidated sufficiently. This study investigated the risk factors for COPD among never-smokers in Korea using population-based data.
Methods
The data were retrieved from the Korean National Health and Nutrition Survey IV conducted from 2007 to 2009. Among subjects aged 40 years or older who underwent appropriate pulmonary function tests, never-smokers not diagnosed with asthma and not showing a restrictive pattern on pulmonary function tests were enrolled. Risk factors of COPD in never-smokers were analyzed using logistic regression models.
Results
Among 24,871 participants in the representative Korean cohort, 3,473 never-smokers were enrolled. COPD patients accounted for 7.6% of the never-smokers. In the logistic regression analysis, low education status (odds ratio [OR]: 2.0; 95% confidence interval [CI]: 1.2–3.2), occupational exposure (OR: 2.6; 95% CI: 1.3–5.3), a history of tuberculosis (OR: 4.5; 95% CI: 2.3–8.7), bronchiectasis (OR: 6.0; 95% CI: 1.4–25.4), male sex (OR: 4.2; 95% CI: 2.6–6.7), advanced age (60–69 years vs 40–49 years; OR: 3.8; 95% CI: 2.0–7.0), and being underweight (body mass index <18.5 vs 18.0–24.9 kg/m2; OR: 3.1; 95% CI: 1.0–9.4) were associated with the development of COPD.
Conclusion
Low education status, manual labor, a history of tuberculosis and bronchiectasis, as well as male sex, advanced age and being underweight were risk factors for COPD in Korean never-smokers.
Keywords: socioeconomic status, chronic obstructive pulmonary disease, never-smoker
Introduction
Chronic obstructive pulmonary disease (COPD) is currently a major cause of mortality and imposes a huge socioeconomic burden.1,2 COPD patients are hospitalized or need medical resources including emergency department visits, outpatient visits, or home health care twice as frequently as non-COPD patients.2 In addition, mortality has constantly increased.3 During the natural history of chronic airflow obstruction, smoking reduces the value of the maximal forced expiratory volume in 1 second (FEV1) and increases the rate of FEV1 decline4 and is the strongest risk factor for COPD. However, although the smoking rate has recently decreased, the burden of COPD has increased. The risk factors for COPD, other than smoking, especially in never-smokers, and the prevention for this disease have become targets of interest. Although the development of airflow obstruction is less common than in continuous smokers, 5%–7% of never-smokers develop airflow obstruction.4 Nevertheless, never-smoker COPD has been neglected, and most of the large studies enrolled only ever-smokers, excluding never-smoker subjects. Furthermore, the proportion of never-smokers with COPD is higher in Asia than in other areas.5–7 The authors of our study considered a specific environment of late industrialization in Asian countries and concentrated on variables such as socioeconomic status-associated factors. Risk factors for never-smokers include age, sex, asthma, respiratory illness during childhood, body mass index (BMI), and socioeconomic status in previous studies.8–11 In terms of socioeconomic factors, occupation, marital status, income, and educational status were evaluated.12–14 However, few Asian studies have revealed specific characteristics that may lead to a high incidence of COPD in never-smokers. The present study was designed to identify risk factors for COPD in the never-smoker population from the fourth Korean National Health and Nutrition Examination Survey (NHANES IV), which was conducted from 2007 to 2009.
Methods
Population
The data were retrieved from the Korean NHANES IV, conducted from 2007 to 2009. This was a nationwide cross-sectional survey that used a rolling sampling survey consisting of three independent samples surveyed for 3 years. Stratified sampling was applied to select the participants on the basis of the results of the 2005 census. The aim of this survey was to provide data for the development and evaluation of policies and programs as well as to assess the health and nutritional status of the Korean population. A health interview, health examination, and a nutrition survey were conducted on a nationally representative noninstitutionalized civilian population in Korea.15 From the 24,871 participants, individuals aged 40 years or older and who underwent appropriate pulmonary function tests (PFTs) were included in the current study. PFTs (Vmax Model 2130; SensorMedics, Yorba Linda, CA, USA) were performed and assessed on the basis of the criteria of the American Thoracic Society/European Respiratory Society 2005. Cases were included when the test met acceptability and reproducibility twice or more during a maximum of eight repeated examinations. Among these subjects, never-smokers who had never smoked or smoked less than five packs during their lifetime were enrolled, and patients who had been diagnosed with asthma by a physician and those with a restrictive type of pulmonary function were excluded (Figure 1). COPD was defined by the GOLD criterion based on spirometry tests as a FEV1/forced vital capacity (FVC) ratio less than 70%.16
Figure 1.
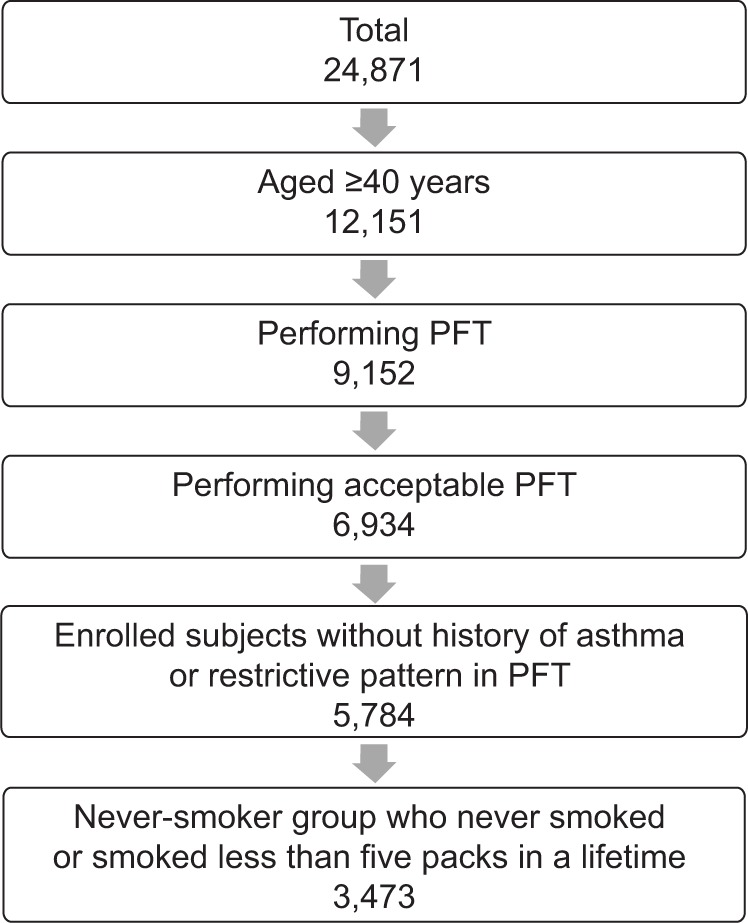
Flow diagram of the process of enrollment of 3,473 never-smoker subjects in the fourth Korean National Health and Nutrition Examination Survey (KNHANES).
Abbreviation: PFT, pulmonary function test.
Data collection
Information regarding education, occupation, the presence of a partner, income, comorbidities, and secondhand smoke status was obtained through the questionnaires. BMI was calculated from the measured height and weight. Comorbid diseases were defined when diagnosed by a physician. Family income levels were classified into two groups; the upper half was regarded as having a high income and the lower half a low income. A BMI of less than 18.5 was defined as underweight, and a level above 25.0 was defined as overweight. Information regarding secondhand smoke was obtained through a questionnaire about exposure to smoking in the indoor workplace or home. Occupation was classified into six groups: managers or professionals, office work, service or sales, agriculture or fishery work, skilled labor or machine operators, and manual laborers. In the analysis, managerial or professional work, office work, and service or sales were reclassified to a lack of occupational exposure.
Statistical analysis
All statistical analyses were performed using the appropriate sample weights and complex design. The sample weights provided from the Korean NHANES IV were constructed for the sample participants to represent the Korean population by accounting for the complex survey design, survey nonresponse, and poststratification.15 Baseline characteristics were described with crude numbers and weighted frequency, or weighted means and standard errors (SE) for all variables in the total, COPD, and non-COPD groups. Comparison between the two groups was performed using chi-square for categorical variables and t-test for continuous variables. To calculate the odds ratio (OR) for each variable, simple logistic regression analysis was performed. The risk factors for COPD in never-smokers were analyzed by multiple logistic regression models including sex, age, socioeconomic status, and comorbidities. In addition, logistic regression analysis in ever-smokers and total subjects including never-and ever-smokers was performed independently of the never-smokers’ data. Statistical analyses were performed using the SPSS software (version 21.0, IBM Corporation, Armonk, NY, USA), and a P-value less than 0.05 was regarded as indicative of statistical significance.
Results
The total number of participants in the KNHANES IV was 24,871. Among 6,934 participants aged older than 40 years and who underwent PFTs, 1,150 subjects diagnosed with asthma or showing a restrictive type of pulmonary function were excluded. In the remaining 5,784 subjects, 3,473 were never-smokers and were included in the present study (Figure 1). The baseline characteristics, including the comorbidities and socioeconomic factors, of never-smoker subjects are described in Table 1. In the present study, never-smokers accounted for 57.7% of the 5,784 subjects, and COPD patients accounted for 7.6% (n=258) of the 3,473 never-smokers. Never-smokers accounted for 31.7% of all COPD patients. Never-smokers accounted for 12.5% and 81.6% of male and female COPD patients, respectively. The demography and distribution of COPD severity according to the smoking status are presented in Table S1. There were more females in the never-smoker COPD group than in the smoker COPD group (71.6% vs 7.5%, P<0.0001), and patients were older (65.7 vs 62.8, P=0.008). A large proportion of the mild-to-moderate COPD patients in the never-smoker and smoker groups were comparable (P=0.205).
Table 1.
Baseline characteristics of the never-smoker subjects
| Parameters | Total
|
COPD
|
Non-COPD
|
P-value | |||
|---|---|---|---|---|---|---|---|
| N, crude | Weighted, % (SE) | N, crude | Weighted, % (SE) | N, crude | Weighted, % (SE) | ||
| Sex | |||||||
| Male | 420 | 13.9 (0.7) | 71 | 28.4 (3.7) | 349 | 12.7 (0.7) | <0.0001 |
| Female | 3,053 | 86.1 (0.7) | 187 | 71.6 (3.7) | 2,866 | 87.3 (0.7) | |
| Age in years (mean ± SE) | 66±0.9 | 54±0.3 | |||||
| 40–49 | 1,191 | 40.0 (1.2) | 23 | 11.1 (2.6) | 1,168 | 42.4 (1.2) | <0.0001 |
| 50–59 | 1,021 | 28.9 (1.1) | 50 | 20.4 (3.4) | 971 | 29.6 (1.1) | |
| 60–69 | 790 | 16.9 (0.7) | 80 | 24.1 (3.1) | 710 | 16.3 (0.7) | |
| 70–79 | 429 | 12.5 (0.8) | 93 | 37.5 (4.1) | 336 | 10.4 (0.8) | |
| ≥80 | 42 | 1.7 (0.3) | 12 | 6.9 (2.3) | 30 | 1.3 (0.3) | |
| BMI in kg/m2 | |||||||
| <18.5 | 47 | 1.3 (0.2) | 10 | 1.3 (0.2) | 37 | 1.1 (0.2) | 0.005 |
| 18.5–24.9 | 2,187 | 63.4 (1.1) | 168 | 63.4 (1.1) | 2,019 | 63.1 (1.2) | |
| ≥25.0 | 1,237 | 35.3 (1.2) | 78 | 35.3 (1.2) | 1,159 | 35.8 (1.2) | |
| Secondhand smoke | |||||||
| No | 2,437 | 68.7 (1.1) | 196 | 76.8 (3.5) | 2,241 | 68.0 (1.1) | 0.026 |
| Yes | 1,036 | 31.3 (1.1) | 62 | 23.2 (3.5) | 974 | 32.0 (1.1) | |
| Education level | |||||||
| Elementary school or less | 1,449 | 37.4 (1.2) | 162 | 60.1 (4.1) | 1,287 | 35.5 (1.2) | <0.0001 |
| Middle school | 549 | 16.0 (0.8) | 40 | 17.8 (3.0) | 509 | 15.8 (0.8) | |
| High school | 953 | 29.8 (1.1) | 30 | 10.7 (2.5) | 923 | 31.3 (1.1) | |
| College or more | 518 | 16.9 (1.1) | 25 | 5.1 (1.4) | 493 | 17.3 (1.1) | |
| Occupation | |||||||
| Managers/professional | 232 | 7.5 (0.6) | 10 | 3.1 (1.3) | 222 | 7.8 (0.6) | <0.0001 |
| Office worker | 121 | 3.6 (0.4) | 6 | 2.5 (1.2) | 115 | 3.7 (0.4) | |
| Service/sales worker | 522 | 16.9 (0.9) | 14 | 5.2 (1.9) | 508 | 17.9 (1.0) | |
| Agriculture/fishery worker | 423 | 7.7 (0.9) | 50 | 14.4 (2.9) | 373 | 7.2 (0.8) | |
| Skilled labor/machine operator | 164 | 5.5 (0.5) | 12 | 5.8 (2.3) | 152 | 5.4 (0.7) | |
| Manual laborer (construction, mining) | 412 | 11.8 (0.7) | 39 | 15.5 (3.1) | 376 | 11.4 (0.7) | |
| No labor | 1,576 | 47.0 (1.2) | 124 | 53.5 (4.1) | 1,452 | 46.5 (1.3) | |
| Living with partner | |||||||
| Yes | 2,729 | 80.0 (0.9) | 184 | 68.1 (3.9) | 2,545 | 81.0 (0.9) | <0.0001 |
| No | 735 | 20.0 (0.9) | 73 | 31.9 (3.9) | 662 | 19.0 (0.9) | |
| Family income | |||||||
| High | 1,755 | 54.1 (1.4) | 85 | 37.3 (4.3) | 1,670 | 55.4 (1.4) | <0.0001 |
| Low | 1,647 | 45.9 (1.4) | 159 | 62.7 (4.3) | 1,488 | 44.6 (1.4) | |
| Medical condition | |||||||
| Diabetes mellitus | 277 | 7.7 (0.6) | 29 | 11.9 (2.6) | 248 | 7.3 (0.6) | 0.041 |
| Hypertension | 888 | 24.6 (1.0) | 103 | 38.0 (4.0) | 785 | 23.5 (1.0) | <0.0001 |
| Cancer | 139 | 4.1 (0.4) | 12 | 4.1 (1.4) | 127 | 4.1 (0.4) | 0.948 |
| Lung cancer | 2 | 0 | 2 | 0.4 (0.3) | 0 | 0 | <0.0001 |
| History of pulmonary TB | 180 | 5.4 (0.5) | 32 | 16.8 (3.4) | 139 | 4.3 (0.4) | <0.0001 |
| Bronchiectasis | 19 | 0.6 (0.2) | 8 | 2.7 (1.2) | 11 | 0.4 (0.1) | <0.0001 |
| Chronic liver disease | 82 | 2.3 (0.3) | 1 | 0.2 (0.2) | 81 | 2.4 (0.3) | 0.003 |
| Anemia (Hb <12.0 g/dL) | 443 | 13.4 (0.8) | 26 | 8.0 (1.9) | 417 | 13.3 (0.8) | 0.725 |
Abbreviations: SE, standard error; COPD, chronic obstructive pulmonary disease; BMI, body mass index; TB, tuberculosis; Hb, hemoglobin.
To calculate the OR for each variable and select significant variables for COPD, simple logistic regression analyses were performed. On the basis of the results of univariate analyses, age, sex, secondhand smoke, BMI, education, occupation, partner living, familial income, diabetes mellitus, hypertension, prior tuberculosis history, and bronchiectasis were included in the multiple logistic regression analysis (Table 2). Male sex (OR: 4.2; 95% CI: 2.6–6.7), underweight (BMI <18.5 vs 18.5–24.9 kg/m2; OR: 3.1; 95% CI: 1.0–9.4), and advanced age (60–69 years vs 40–49 years; OR: 3.8; 95% CI: 2.0–7.0) showed a significantly higher risk of COPD. With a 10-year age increase, the OR of COPD was doubled. However, secondhand smoke did not show a relationship with COPD. In terms of socioeconomic status, a low educational level (OR: 2.0; 95% CI: 1.2–3.2) and occupational exposure were COPD risk factors. Participants who worked as manual laborers showed an OR of 2.6 (95% CI: 1.3–5.3) compared with those with a lack of occupational exposure: managers or professionals, office work, service or sales (Table 2). Among the various comorbidities, a history of tuberculosis (OR: 4.5; 95% CI: 2.3–8.7) and bronchiectasis diagnosed by a physician (OR: 6.0; 95% CI: 1.4–25.4) were risk factors for COPD in never-smokers.
Table 2.
Risk factors for COPD in the never-smoker subjects
| Factor | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% confidence interval | P-value | Odds ratio | 95% confidence interval | P-value | |
| Sex | ||||||
| Male | 2.7 | 1.9–4.0 | <0.0001 | 4.2 | 2.6–6.7 | <0.0001 |
| Female | Reference | Reference | ||||
| Age in years | ||||||
| 40–49 | Reference | Reference | ||||
| 50–59 | 2.6 | 1.4–5.0 | 0.003 | 1.7 | 0.9–3.3 | 0.135 |
| 60–69 | 5.68 | 3.3–9.8 | <0.0001 | 3.8 | 2.0–7.0 | <0.0001 |
| 70–79 | 13.8 | 7.6–25.2 | <0.0001 | 9.2 | 4.6–18.7 | <0.0001 |
| ≥80 | 20.5 | 8.1–51.6 | <0.0001 | 17.7 | 6.3–49.4 | <0.0001 |
| BMI (kg/m2) | ||||||
| <18.5 | 3.1 | 1.3–7.1 | <0.0001 | 3.1 | 1.0–9.4 | 0.045 |
| 18.5–24.9 | Reference | Reference | ||||
| ≥25.0 | 0.8 | 0.5–1.1 | 0.11 | 0.7 | 0.5–0.9 | 0.046 |
| Secondhand smoke | ||||||
| No | Reference | Reference | ||||
| Yes | 0.6 | 0.4–0.9 | 0.027 | 1.1 | 0.7–1.7 | 0.711 |
| Education level | ||||||
| High school or more | Reference | Reference | ||||
| Middle school or less | 3.4 | 2.2–5.1 | <0.0001 | 2.0 | 1.2–3.2 | 0.007 |
| Occupation | ||||||
| Manager/professional/office worker/service/sales worker | Reference | Reference | ||||
| Agriculture/fishery worker | 5.4 | 2.9–10.2 | <0.0001 | 1.9 | 0.9–4.1 | 0.563 |
| Skilled labor/machine operator | 2.9 | 1.1–7.5 | 0.027 | 1.3 | 0.5–3.5 | 0.100 |
| Manual laborer (construction, mining) | 3.7 | 1.9–7.2 | <0.0001 | 2.6 | 1.3–5.3 | 0.007 |
| No labor | 3.1 | 1.8–5.3 | <0.0001 | 1.5 | 0.8–3.1 | 0.226 |
| Living with partner | ||||||
| Yes | Reference | Reference | ||||
| No | 2.0 | 1.4–2.9 | <0.0001 | 1.2 | 0.8–1.8 | 0.301 |
| Family income | ||||||
| High | Reference | Reference | ||||
| Low | 2.1 | 1.4–3.0 | <0.0001 | 0.9 | 0.6–1.4 | 0.710 |
| Diabetes mellitus | ||||||
| No | Reference | Reference | ||||
| Yes | 1.7 | 1.0–3.0 | 0.043 | 1.1 | 0.6–1.9 | 0.888 |
| Hypertension | ||||||
| No | Reference | Reference | ||||
| Yes | 2.0 | 1.4–2.8 | <0.0001 | 0.9 | 0.6–1.3 | 0.467 |
| Anemia | ||||||
| No | Reference | – | – | – | ||
| Yes | 1.1 | 0.6–1.9 | 0.725 | |||
| History of pulmonary tuberculosis | ||||||
| No | Reference | Reference | ||||
| Yes | 4.5 | 2.7–7.5 | <0.0001 | 4.5 | 2.3–8.7 | <0.0001 |
| Bronchiectasis | ||||||
| No | Reference | Reference | ||||
| Yes | 7.0 | 2.2–22.2 | 0.007 | 6.0 | 1.4–25.4 | 0.016 |
| Cancer | ||||||
| No | Reference | – | – | – | ||
| Yes | 1.0 | 0.5–2.09 | 0.948 | |||
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease.
For further information, the results of a logistic regression analysis of risk factors for COPD in total subjects and ever-smokers are presented in Table S2. When the risk factors between never-smokers and smokers were compared, bronchiectasis was found to be a significant risk factor among never-smokers. The OR of pulmonary tuberculosis was higher in never-smokers compared with the OR in smokers (Table 2, Table S2).
Discussion
Socioeconomic status and comorbidities were strongly related to COPD in never-smoker subjects. A low level of education, employment as a manual laborer, and a history of tuberculosis or bronchiectasis were associated with an increased prevalence of COPD in never-smokers. In previous studies, the parameters of socioeconomic status such as education, occupation, marital status, and familial income were closely related to health condition and affected the onset and prognosis of COPD, particularly in never-smokers.9,12,14,17,18 There is no doubt that smoking is a major risk factor for COPD. However, never-smokers account for some proportion of COPD. According to data from the National Health and Nutrition Examination Survey (NHANES) III, a population survey that represents the US population, 1.6% of male COPD patients and 12.2% of female COPD patients were never-smokers.19 In Korea, the burden of never-smoker COPD is higher than the 6.1%–24.9% reported previously in Spanish and American studies.8,19,20 According to a study based on the Korean NHANES II in 2001, the proportion of never-smoker COPD patients among all COPD patients was 32.1%.5 These Korean data, and our present results, support previous Chinese data that showed a high proportion of never-smokers (38.6%) as having COPD.6 This high proportion of never-smoker COPD patients suggests that risk factors other than smoking may influence the development of COPD, especially in Asia. Thus, investigations of the major risk factors other than smoking are required.
Old age and being underweight are associated with COPD.13,21 In the natural course of FEV1 change among healthy never-smokers, the annualized rate of decline is 19.6 mL/year and 17.6 mL/year after a peak in males and females, respectively.4 There may also be an association between being underweight and having COPD, although further studies on the causative relationship are still needed.6,13 In a previous study with a mean follow-up of 10 years, being underweight at baseline was a risk factor for subsequently developing COPD.22 Other studies have explained how malnutrition associated with underweight subjects contributes to an increased susceptibility to infection or respiratory muscle wasting and subsequently compromised lung function.23,24
In the present study, the final educational level was associated with COPD. Previous studies have reported a positive relationship between a lower educational level and the prevalence of COPD,13,25,26 although others have reported contradictory results.27,28 A large study of 49,363 participants in The People’s Republic of China showed relationships among low education, low familial income, and COPD, regardless of residing in a rural or urban area.18 However, familial income was not a risk factor for COPD in our study. The difference in risk factors in various countries could be due to differences in lifestyles and multifactorial relationships among risk factors.25 However, the relationship between low socioeconomic status, represented by education status, and COPD was observed again in our study. A link between socioeconomic status and COPD might be exposure to the burning of biomass such as wood. The use of biomass fuel is associated with low socioeconomic status,29 and indoor exposure to smoke from such burning has been reported to be an important risk factor of chronic airway disease.30,31 Traditionally, indoor exposure affects females more than males, especially in Asian countries with late industrialization. Women are usually exposed to smoke inhalation, from wood or charcoal used for cooking and heating, while performing housework.32 This could partly explain the reason for COPD among never-smoker females. However, we did not measure biomass exposure, and a direct relationship between biomass exposure and COPD could not be shown.
Being male was a risk factor in our study, in line with other studies.6,10 Although a large number of smoking male subjects were excluded and a small number of males were included in this study to focus on never-smokers, being male was still a dominant risk factor. Contrary to our result, an international population-based study, the Burden of Obstructive Lung Disease (BOLD) study, reported being female as a high risk factor for COPD.9 The male dominance in COPD was variable depending on the severity of airway obstruction, and male was a risk factor for COPD, but only for moderate and severe COPD in previous studies.8,10 The difference in distribution of COPD severity between study populations might explain the difference in sex dominance. Some investigators have emphasized that exposure to indoor pollution in early life influences the development of COPD in never-smoker males as well as in never-smoker females.6,33
The present study showed a high risk of COPD in manual laborers employed in the construction or mining industry. Although the amount of occupational exposure was not assessed using direct methods such as particulate matter (PM), significant exposure to inorganic dust in the workplace was likely. Construction workers exposed to inorganic dust have increased mortality from COPD, particularly among never-smokers.34 US population-based data showed that employment in construction and record-processing is associated with never-smoker COPD.35 Causal associations among various occupational exposures and COPD, and related dose-dependency, have been revealed in previous studies.36 A lower OR in skilled laborers or machine operators compared to a previous study might be due to the simple occupational classification used in the present study.35 According to the results of the Comparative Risk Assessment project of the World Health Organization (WHO), occupational factors were responsible for 13% of COPD development.37 Although the classifications of occupations and outcomes differed among the groups, other studies have reported consistent results concerning the role of occupational exposure in COPD.12,17,37 Considering that more males tend to be exposed to occupational dust than females,38 the male dominance in never-smoker COPD might be partly explained.
Secondhand smoke, a risk factor for decreased lung function, did not show a relationship with COPD in our study. Although many cross-sectional studies have revealed such a relationship,39 several longitudinal epidemiologic studies have not shown a relationship or clinical significance.40–43 There are inconsistent results regarding a possible association between secondhand smoke and lung function, which may be attributable to methodological differences among studies.44 For example, when a questionnaire includes exposure during childhood or total duration of exposure, or when the subjects are limited to workers with high exposure levels, the study results may be different. The survey used in the present study was conducted on the Korean general population, and the exposure level of secondhand smoke might not be high. Furthermore, the total duration of exposure during one’s lifetime was not considered in this questionnaire.
Marital status can be a risk factor for COPD.45,46 The relationship between marital status and COPD can be explained in part by nutritional factors.47 Subjects who lived alone were found to have poorer nutritional status, particularly COPD patients.14 In our study, living alone did not exhibit a strong association with COPD.
Bronchiectasis as a complication of infection can cause airflow obstruction, the severity of which depends on the degree of elastic and muscular destruction of the bronchial wall.48 Other mechanisms – such as bronchospasm, bronchial secretion, or accompanying emphysematous changes – can also contribute to the development of airway obstruction.49 In the present study, bronchiectasis was closely associated with COPD in never-smokers. Unlike in the never-smokers, bronchiectasis was not a significant risk factor for COPD in ever-smokers, presented in Table S2. Bronchiectasis is a contributing factor only in never-smokers or is associated with a greater risk for COPD compared with ever-smokers. Based on the US health care claims system, the estimated prevalence of bronchiectasis is 52.3 cases per 100,000 persons. Although the prevalence of bronchiectasis in persons aged ≥75 years increased substantially to 271.8 per 100,000 persons,50 it was lower than the 0.6% in this study, which was limited to never-smokers. Asian countries, including Korea, have a relatively high prevalence of bronchiectasis,51 and the impact of bronchiectasis on the development of COPD, especially among never-smokers in Asia, should be emphasized.
A history of tuberculosis was associated with COPD independently of bronchiectasis. A previous population-based study reported a similar result. Lamprecht et al9 reported that a history of tuberculosis resulted in an OR of 1.47 for moderate-to-very severe COPD in males and an OR of 1.65 in females. In addition, there is a close relationship between pulmonary function insufficiency and a history of tuberculosis.52,53 Pulmonary function insufficiency in patients previously treated for tuberculosis presents as extensive fibrosis and endobronchial stricture following tuberculosis.54 These previous reports are supported by our results with an OR of 4.5 in never-smokers. This value was greater than the OR of 2.3 in ever-smokers as well as other previously reported values. The relationship between previously treated tuberculosis and subsequent COPD may be associated with a high rate of never-smoker COPD in endemic areas such as Asia, including Korea. Indeed, controlling the tuberculosis epidemic may prevent some cases of COPD.55
The present study had several limitations. The diagnosis of bronchiectasis was based on a questionnaire about history of diagnosis by a physician without radiologic information. For this reason, the real number of patients with bronchiectasis might be greater compared with our data. In addition, the definition of COPD was based on the prebronchodilator FEV1/FVC ratio. Although patients with asthma diagnosed by physicians were excluded in this study, it is possible that undiagnosed asthma patients were mingled with the COPD patients. The questionnaires in this study did not measure biomass smoke or biologic dust exposure. The direct relationship between environmental exposure and COPD could not be analyzed, which was also a limitation. In addition, due to the small numbers of several variable categories such as male individuals and underweight subjects, the contribution of these risk factors toward developing COPD might be less obvious. Nevertheless, this study tried to clarify the impact of several socioeconomic factors and comorbidities on never-smoker COPD and examined their relationship. A low education status, low labor status, and a previous history of tuberculosis and bronchiectasis were found to be important risk factors for COPD in Korean never-smokers.
Conclusion
The consideration of never-smokers as a proportion of COPD patients has been low, especially in Asia, including Korea. In this population, never-smokers account for more than 30% of COPD patients. This study revealed that low educational status, manual labor, a history of pulmonary tuberculosis, and bronchiectasis as well as being male, old, and underweight are risk factors for COPD in never-smokers. This result supports the association between low socioeconomic status and COPD and emphasizes the importance of chronic lung injury due to infection in never-smoker COPD compared with that in ever-smokers.
Supplementary materials
Table S1.
Demography and severity of never-smoker COPD and smoker COPD
| Factors | Never-smoker COPD N (%) |
Smoker COPD N (%) |
P-value |
|---|---|---|---|
| Subjectsa | 258 | 562 | – |
| Sex, female | 187 (71.6) | 40 (7.5) | <0.0001 |
| Age, mean ± SE | 65.7±0.9 | 62.8±0.6 | 0.008 |
| COPD severity | 0.205 | ||
| Mild | 108 (44.8) | 247 (43.3) | |
| Moderate | 142 (53.5) | 282 (52.0) | |
| Severe | 6 (1.6) | 29 (4.4) | |
| Very severe | 0 | 3 (0.3) | |
Notes:
Patients with COPD were recruited from 5,784 subjects designated in Figure 1.
Abbreviations: COPD, chronic obstructive pulmonary disease; SE, standard error.
Table S2.
Multivariate analysis of risk factors for COPD in total subjects and ever-smokers
| Factors | Total
|
Ever-smoker
|
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Sex | ||||||
| Male | 5.9 | 4.5–7.8 | <0.0001 | 2.6 | 1.5–4.5 | 0.001 |
| Female | Reference | Reference | ||||
| Age (years) | ||||||
| 40–49 | Reference | Reference | ||||
| 50–59 | 2.4 | 1.7–3.5 | <0.0001 | 2.8 | 1.8–4.4 | <0.0001 |
| 60–69 | 5.9 | 4.0–8.7 | <0.0001 | 7.2 | 4.6–11.5 | <0.0001 |
| 70–79 | 13.4 | 8.8–20.5 | <0.0001 | 15.6 | 9.0–27.0 | <0.0001 |
| ≥80 | 16.2 | 7.3–36.3 | <0.0001 | 8.4 | 2.9–24.7 | <0.0001 |
| BMI (kg/m2) | ||||||
| <18.5 | 2.1 | 1.1–4.1 | 0.022 | 1.6 | 0.72–3.3 | 0.245 |
| 18.5–24.9 | Reference | Reference | ||||
| ≥25.0 | 0.7 | 0.5–0.9 | 0.006 | 0.7 | 0.5–1.0 | 0.049 |
| Secondhand smoking | ||||||
| No | Reference | Reference | ||||
| Yes | 1.2 | 0.9–1.5 | 0.266 | 1.1 | 0.8–1.6 | 0.458 |
| Education level | ||||||
| High school or more | Reference | Reference | ||||
| Middle school or less | 1.7 | 1.3–2.2 | <0.0001 | 1.6 | 1.2–2.2 | 0.005 |
| Occupation | ||||||
| Manager/professional/office worker/service/sales worker | Reference | Reference | ||||
| Agriculture/fishery worker | 1.4 | 0.9–2.1 | 0.156 | 1.2 | 0.7–1.9 | 0.535 |
| Skilled labor/machine operator | 1.0 | 0.7–1.6 | 0.909 | 0.9 | 0.6–1.5 | 0.769 |
| Manual laborer (construction, mining) | 2.2 | 1.5–3.1 | <0.0001 | 1.2 | 1.2–3.4 | 0.006 |
| No labor | 1.5 | 1.0–2.1 | 0.044 | 1.4 | 0.9–2.2 | 0.146 |
| Living with partner | ||||||
| Yes | Reference | Reference | ||||
| No | 1.3 | 1.0–1.7 | 0.077 | 1.2 | 0.8–1.4 | 0.441 |
| Family income | ||||||
| High | Reference | Reference | ||||
| Low | 1.0 | 0.8–1.3 | 0.929 | 1.0 | 0.81–1.54 | 0.764 |
| Diabetes mellitus | ||||||
| No | Reference | Reference | ||||
| Yes | 1.2 | 0.8–1.6 | 0.382 | 1.2 | 0.8–1.7 | 0.297 |
| Hypertension | ||||||
| No | Reference | Reference | ||||
| Yes | 0.7 | 0.6–0.9 | 0.003 | 0.6 | 0.5–0.8 | 0.002 |
| Pulmonary tuberculosis | ||||||
| No | Reference | Reference | ||||
| Yes | 2.9 | 1.9–4.3 | <0.0001 | 2.3 | 1.5–3.6 | <0.0001 |
| Bronchiectasis | ||||||
| No | Reference | Reference | ||||
| Yes | 2.7 | 1.0–7.6 | 0.06 | 1.0 | 0.4–2.7 | 0.966 |
Abbreviations: COPD, chronic obstructive pulmonary disease; OR, odds ratio; CI, confidence interval; BMI, body mass index.
Footnotes
Disclosure
The authors report no conflicts of interest. The authors are responsible for the content and writing of the paper.
References
- 1.Johnson NB, Hayes LD, Brown K, Hoo EC, Ethier KA. CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors–United States, 2005–2013. MMWR Surveill Summ. 2014;63(Suppl 4):3–27. [PubMed] [Google Scholar]
- 2.D’Souza AO, Shah M, Dhamane AD, Dalal AA. Clinical and economic burden of COPD in a medicaid population. COPD. 2014;11(2):212–220. doi: 10.3109/15412555.2013.836168. [DOI] [PubMed] [Google Scholar]
- 3.Brown DW, Pleasants RA. Mortality from chronic obstructive pulmonary disease among adults aged 25 years or older in North Carolina. South Med J. 2011;104(1):20–23. doi: 10.1097/SMJ.0b013e3181fcda00. [DOI] [PubMed] [Google Scholar]
- 4.Kohansal R, Martinez-Camblor P, Agusti A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009;180(1):3–10. doi: 10.1164/rccm.200901-0047OC. [DOI] [PubMed] [Google Scholar]
- 5.Kim DS, Kim YS, Jung KS, et al. Prevalence of chronic obstructive pulmonary disease in Korea: a population-based spirometry survey. Am J Respir Crit Care Med. 2005;172(7):842–847. doi: 10.1164/rccm.200502-259OC. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Wang C, Yao W, et al. COPD in Chinese nonsmokers. Eur Respir J. 2009;33(3):509–518. doi: 10.1183/09031936.00084408. [DOI] [PubMed] [Google Scholar]
- 7.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374(9691):733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 8.Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest. 2005;128(3):1239–1244. doi: 10.1378/chest.128.3.1239. [DOI] [PubMed] [Google Scholar]
- 9.Lamprecht B, McBurnie MA, Vollmer WM, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139(4):752–763. doi: 10.1378/chest.10-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celli BR, Halbert RJ, Nordyke RJ, Schau B. Airway obstruction in never smokers: results from the Third National Health and Nutrition Examination Survey. Am J Med. 2005;118(12):1364–1372. doi: 10.1016/j.amjmed.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 11.Sexton P, Black P, Wu L, et al. Chronic obstructive pulmonary disease in non-smokers: a case-comparison study. COPD. 2014;11(1):2–9. doi: 10.3109/15412555.2013.800853. [DOI] [PubMed] [Google Scholar]
- 12.Kainu A, Rouhos A, Sovijarvi A, Lindqvist A, Sarna S, Lundback B. COPD in Helsinki, Finland: socioeconomic status based on occupation has an important impact on prevalence. Scand J Public Health. 2013;41(6):570–578. doi: 10.1177/1403494813484554. [DOI] [PubMed] [Google Scholar]
- 13.Kanervisto M, Vasankari T, Laitinen T, Heliovaara M, Jousilahti P, Saarelainen S. Low socioeconomic status is associated with chronic obstructive airway diseases. Respir Med. 2011;105(8):1140–1146. doi: 10.1016/j.rmed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Odencrants S, Bjustrom T, Wiklund N, Blomberg K. Nutritional status, gender and marital status in patients with chronic obstructive pulmonary disease. J Clin Nurs. 2013;22(19–20):2822–2829. doi: 10.1111/jocn.12222. [DOI] [PubMed] [Google Scholar]
- 15.Kweon S, Kim Y, Jang MJ, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES) Int J Epidemiol. 2014;43(1):69–77. doi: 10.1093/ije/dyt228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 17.Matheson MC, Benke G, Raven J, et al. Biological dust exposure in the workplace is a risk factor for chronic obstructive pulmonary disease. Thorax. 2005;60(8):645–651. doi: 10.1136/thx.2004.035170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin P, Zhang M, Li Y, Jiang Y, Zhao W. Prevalence of COPD and its association with socioeconomic status in China: findings from China Chronic Disease Risk Factor Surveillance 2007. BMC Public Health. 2011;11:586. doi: 10.1186/1471-2458-11-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coultas DB, Mapel D, Gagnon R, Lydick E. The health impact of undiagnosed airflow obstruction in a national sample of United States adults. Am J Respir Crit Care Med. 2001;164(3):372–377. doi: 10.1164/ajrccm.164.3.2004029. [DOI] [PubMed] [Google Scholar]
- 20.Pena VS, Miravitlles M, Gabriel R, et al. Geographic variations in prevalence and underdiagnosis of COPD: results of the IBERPOC multicentre epidemiological study. Chest. 2000;118(4):981–989. doi: 10.1378/chest.118.4.981. [DOI] [PubMed] [Google Scholar]
- 21.Benedik B, Farkas J, Kosnik M, Kadivec S, Lainscak M. Mini nutritional assessment, body composition, and hospitalisations in patients with chronic obstructive pulmonary disease. Respir Med. 2011;105(Suppl 1):S38–S43. doi: 10.1016/S0954-6111(11)70009-9. [DOI] [PubMed] [Google Scholar]
- 22.Harik-Khan RI, Fleg JL, Wise RA. Body mass index and the risk of COPD. Chest. 2002;121(2):370–376. doi: 10.1378/chest.121.2.370. [DOI] [PubMed] [Google Scholar]
- 23.Chandra RK. Cell-mediated immunity in nutritional imbalance. Fed Proc. 1980;39(13):3088–3092. [PubMed] [Google Scholar]
- 24.Thurlbeck WM. Diaphragm and body weight in emphysema. Thorax. 1978;33(4):483–487. doi: 10.1136/thx.33.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nizankowska-Mogilnicka E, Mejza F, Buist AS, et al. Prevalence of COPD and tobacco smoking in Malopolska region–results from the BOLD study in Poland. Pol Arch Med Wewn. 2007;117(9):402–410. [PubMed] [Google Scholar]
- 26.Menezes AM, Perez-Padilla R, Jardim JR, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366(9500):1875–1881. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 27.Lindberg A, Jonsson AC, Ronmark E, Lundgren R, Larsson LG, Lundback B. Ten-year cumulative incidence of COPD and risk factors for incident disease in a symptomatic cohort. Chest. 2005;127(5):1544–1552. doi: 10.1378/chest.127.5.1544. [DOI] [PubMed] [Google Scholar]
- 28.Lokke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: a 25 year follow up study of the general population. Thorax. 2006;61(11):935–939. doi: 10.1136/thx.2006.062802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fullerton DG, Semple S, Kalambo F, et al. Biomass fuel use and indoor air pollution in homes in Malawi. Occup Environ Med. 2009;66(11):777–783. doi: 10.1136/oem.2008.045013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekici A, Ekici M, Kurtipek E, et al. Obstructive airway diseases in women exposed to biomass smoke. Environ Res. 2005;99(1):93–98. doi: 10.1016/j.envres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Zhou Y, Wang X, et al. Biomass fuels are the probable risk factor for chronic obstructive pulmonary disease in rural South China. Thorax. 2007;62(10):889–897. doi: 10.1136/thx.2006.061457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon SB, Bruce NG, Grigg J, et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med. 2014;2(10):823–860. doi: 10.1016/S2213-2600(14)70168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orozco-Levi M, Garcia-Aymerich J, Villar J, Ramirez-Sarmiento A, Anto JM, Gea J. Wood smoke exposure and risk of chronic obstructive pulmonary disease. Eur Respir J. 2006;27(3):542–546. doi: 10.1183/09031936.06.00052705. [DOI] [PubMed] [Google Scholar]
- 34.Bergdahl IA, Toren K, Eriksson K, et al. Increased mortality in COPD among construction workers exposed to inorganic dust. Eur Respir J. 2004;23(3):402–406. doi: 10.1183/09031936.04.00034304. [DOI] [PubMed] [Google Scholar]
- 35.Hnizdo E. Association between chronic obstructive pulmonary disease and employment by industry and occupation in the US population: a study of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2002;156(8):738–746. doi: 10.1093/aje/kwf105. [DOI] [PubMed] [Google Scholar]
- 36.Omland O, Wurtz ET, Aasen TB, et al. Occupational chronic obstructive pulmonary disease: a systematic literature review. Scand J Work Environ Health. 2014;40(1):19–35. doi: 10.5271/sjweh.3400. [DOI] [PubMed] [Google Scholar]
- 37.Fingerhut M, Nelson DI, Driscoll T, et al. The contribution of occupational risks to the global burden of disease: summary and next steps. Med Lav. 2006;97(2):313–321. [PubMed] [Google Scholar]
- 38.Marchetti N, Garshick E, Kinney GL, et al. Association between occupational exposure and lung function, respiratory symptoms, and high-resolution computed tomography imaging in COPDGene. Am J Respir Crit Care Med. 2014;190(7):756–762. doi: 10.1164/rccm.201403-0493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu CF, Feng NH, Chong IW, et al. Second-hand smoke and chronic bronchitis in Taiwanese women: a health-care based study. BMC Public Health. 2010;10:44. doi: 10.1186/1471-2458-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lebowitz MD. Influence of passive smoking on pulmonary function: a survey. Prev Med. 1984;13(6):645–655. doi: 10.1016/s0091-7435(84)80014-6. [DOI] [PubMed] [Google Scholar]
- 41.Brunekreef B, Fischer P, Remijn B, van der Lende R, Schouten J, Quanjer P. Indoor air pollution and its effect on pulmonary function of adult non-smoking women: III. Passive smoking and pulmonary function. Int J Epidemiol. 1985;14(2):227–230. doi: 10.1093/ije/14.2.227. [DOI] [PubMed] [Google Scholar]
- 42.Jaakkola MS, Jaakkola JJ, Becklake MR, Ernst P. Passive smoking and evolution of lung function in young adults. An 8-year longitudinal study. J Clin Epidemiol. 1995;48(3):317–327. doi: 10.1016/0895-4356(94)00157-l. [DOI] [PubMed] [Google Scholar]
- 43.Carey IM, Cook DG, Strachan DP. The effects of environmental tobacco smoke exposure on lung function in a longitudinal study of British adults. Epidemiology. 1999;10(3):319–326. [PubMed] [Google Scholar]
- 44.Jaakkola MS, Jaakkola JJ. Effects of environmental tobacco smoke on the respiratory health of adults. Scand J Work Environ Health. 2002;28(Suppl 2):52–70. [PubMed] [Google Scholar]
- 45.Noda T, Ojima T, Hayasaka S, Hagihara A, Takayanagi R, Nobutomo K. The health impact of remarriage behavior on chronic obstructive pulmonary disease: findings from the US longitudinal survey. BMC Public Health. 2009;9:412. doi: 10.1186/1471-2458-9-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kosarev VV, Babanov SA. Age and gender aspect of epidemiology of chronic obstructive pulmonary disease. Adv Gerontol. 2010;23(4):630–635. [PubMed] [Google Scholar]
- 47.Hanson C, Rutten EP, Wouters EF, Rennard S. Diet and vitamin D as risk factors for lung impairment and COPD. Transl Res. 2013;162(4):219–236. doi: 10.1016/j.trsl.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Jordan TS, Spencer EM, Davies P. Tuberculosis, bronchiectasis and chronic airflow obstruction. Respirology. 2010;15(4):623–628. doi: 10.1111/j.1440-1843.2010.01749.x. [DOI] [PubMed] [Google Scholar]
- 49.Pande JN, Jain BP, Gupta RG, Guleria JS. Pulmonary ventilation and gas exchange in bronchiectasis. Thorax. 1971;26(6):727–733. doi: 10.1136/thx.26.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clin Pulm Med. 2005;12(4):205–209. [Google Scholar]
- 51.Tsang KW, Tipoe GL. Bronchiectasis: not an orphan disease in the East. Int J Tuberc Lung Dis. 2004;8(6):691–702. [PubMed] [Google Scholar]
- 52.Chung KP, Chen JY, Lee CH, et al. Trends and predictors of changes in pulmonary function after treatment for pulmonary tuberculosis. Clinics (Sao Paulo) 2011;66(4):549–556. doi: 10.1590/S1807-59322011000400005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasipanodya JG, Miller TL, Vecino M, et al. Pulmonary impairment after tuberculosis. Chest. 2007;131(6):1817–1824. doi: 10.1378/chest.06-2949. [DOI] [PubMed] [Google Scholar]
- 54.Snider GL, Doctor L, Demas TA, Shaw AR. Obstructive airway disease in patients with treated pulmonary tuberculosis. Am Rev Respir Dis. 1971;103(5):625–640. doi: 10.1164/arrd.1971.103.5.625. [DOI] [PubMed] [Google Scholar]
- 55.Lee CH, Lee MC, Lin HH, et al. Pulmonary tuberculosis and delay in anti-tuberculous treatment are important risk factors for chronic obstructive pulmonary disease. PLoS One. 2012;7(5):e37978. doi: 10.1371/journal.pone.0037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Demography and severity of never-smoker COPD and smoker COPD
| Factors | Never-smoker COPD N (%) |
Smoker COPD N (%) |
P-value |
|---|---|---|---|
| Subjectsa | 258 | 562 | – |
| Sex, female | 187 (71.6) | 40 (7.5) | <0.0001 |
| Age, mean ± SE | 65.7±0.9 | 62.8±0.6 | 0.008 |
| COPD severity | 0.205 | ||
| Mild | 108 (44.8) | 247 (43.3) | |
| Moderate | 142 (53.5) | 282 (52.0) | |
| Severe | 6 (1.6) | 29 (4.4) | |
| Very severe | 0 | 3 (0.3) | |
Notes:
Patients with COPD were recruited from 5,784 subjects designated in Figure 1.
Abbreviations: COPD, chronic obstructive pulmonary disease; SE, standard error.
Table S2.
Multivariate analysis of risk factors for COPD in total subjects and ever-smokers
| Factors | Total
|
Ever-smoker
|
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Sex | ||||||
| Male | 5.9 | 4.5–7.8 | <0.0001 | 2.6 | 1.5–4.5 | 0.001 |
| Female | Reference | Reference | ||||
| Age (years) | ||||||
| 40–49 | Reference | Reference | ||||
| 50–59 | 2.4 | 1.7–3.5 | <0.0001 | 2.8 | 1.8–4.4 | <0.0001 |
| 60–69 | 5.9 | 4.0–8.7 | <0.0001 | 7.2 | 4.6–11.5 | <0.0001 |
| 70–79 | 13.4 | 8.8–20.5 | <0.0001 | 15.6 | 9.0–27.0 | <0.0001 |
| ≥80 | 16.2 | 7.3–36.3 | <0.0001 | 8.4 | 2.9–24.7 | <0.0001 |
| BMI (kg/m2) | ||||||
| <18.5 | 2.1 | 1.1–4.1 | 0.022 | 1.6 | 0.72–3.3 | 0.245 |
| 18.5–24.9 | Reference | Reference | ||||
| ≥25.0 | 0.7 | 0.5–0.9 | 0.006 | 0.7 | 0.5–1.0 | 0.049 |
| Secondhand smoking | ||||||
| No | Reference | Reference | ||||
| Yes | 1.2 | 0.9–1.5 | 0.266 | 1.1 | 0.8–1.6 | 0.458 |
| Education level | ||||||
| High school or more | Reference | Reference | ||||
| Middle school or less | 1.7 | 1.3–2.2 | <0.0001 | 1.6 | 1.2–2.2 | 0.005 |
| Occupation | ||||||
| Manager/professional/office worker/service/sales worker | Reference | Reference | ||||
| Agriculture/fishery worker | 1.4 | 0.9–2.1 | 0.156 | 1.2 | 0.7–1.9 | 0.535 |
| Skilled labor/machine operator | 1.0 | 0.7–1.6 | 0.909 | 0.9 | 0.6–1.5 | 0.769 |
| Manual laborer (construction, mining) | 2.2 | 1.5–3.1 | <0.0001 | 1.2 | 1.2–3.4 | 0.006 |
| No labor | 1.5 | 1.0–2.1 | 0.044 | 1.4 | 0.9–2.2 | 0.146 |
| Living with partner | ||||||
| Yes | Reference | Reference | ||||
| No | 1.3 | 1.0–1.7 | 0.077 | 1.2 | 0.8–1.4 | 0.441 |
| Family income | ||||||
| High | Reference | Reference | ||||
| Low | 1.0 | 0.8–1.3 | 0.929 | 1.0 | 0.81–1.54 | 0.764 |
| Diabetes mellitus | ||||||
| No | Reference | Reference | ||||
| Yes | 1.2 | 0.8–1.6 | 0.382 | 1.2 | 0.8–1.7 | 0.297 |
| Hypertension | ||||||
| No | Reference | Reference | ||||
| Yes | 0.7 | 0.6–0.9 | 0.003 | 0.6 | 0.5–0.8 | 0.002 |
| Pulmonary tuberculosis | ||||||
| No | Reference | Reference | ||||
| Yes | 2.9 | 1.9–4.3 | <0.0001 | 2.3 | 1.5–3.6 | <0.0001 |
| Bronchiectasis | ||||||
| No | Reference | Reference | ||||
| Yes | 2.7 | 1.0–7.6 | 0.06 | 1.0 | 0.4–2.7 | 0.966 |
Abbreviations: COPD, chronic obstructive pulmonary disease; OR, odds ratio; CI, confidence interval; BMI, body mass index.


