Abstract
Purpose
To conduct analyses exploring trial-level and patient-level associations between overall response rate (ORR), progression-free survival (PFS), and overall survival (OS) in advanced non–small-cell lung cancer (NSCLC) trials.
Methods
We identified 14 trials (N = 12,567) submitted to US Food and Drug Administration since 2003 of treatments for advanced NSCLC. Only randomized, active-controlled trials with more than 150 patients were included. Associations between trial-level PFS hazard ratio (HR), OS HR, and ORR odds ratio were analyzed using a weighted linear regression model. Patient-level responder analyses comparing PFS and OS between patients with and without an objective response were performed using pooled data from all studies.
Results
In the trial-level analysis, the association between PFS and ORR was strong (R2 = 0.89; 95% CI, 0.80 to 0.98). There was no association between OS and ORR (R2 = 0.09; 95% CI, 0 to 0.33) and OS and PFS (R2 = 0.08; 95% CI, 0 to 0.31). In the patient-level responder analyses, patients who achieved a response had better PFS and OS compared with nonresponders (PFS: HR, 0.40; 95% CI, 0.38 to 0.42; OS: HR, 0.40; 95% CI, 0.38 to 0.43).
Conclusion
On a trial level, there is a strong association between ORR and PFS. An association between ORR and OS and between PFS and OS was not established, possibly because of cross-over and longer survival after progression in the targeted therapy and first-line trials. The patient-level analysis showed that responders have a better PFS and OS compared with nonresponders. A therapy in advanced NSCLC with a large magnitude of effect on ORR may have a large PFS effect.
INTRODUCTION
Lung cancer is the leading cause of cancer death in men and women in the United States.1 Most patients are diagnosed at advanced stages and have a poor prognosis. New therapies are needed to cure patients, prolong survival, substantially delay progression, or improve lung cancer symptoms.
Over the last decade, there has been a paradigm shift in the classification and treatment of lung cancer. Traditionally, lung cancer had been classified based on histology. With the evolution of technologies to sequence the cancer genome and an improved understanding of the functional consequences of genetic aberrations, lung cancer is increasingly subclassified by underlying oncogenic driver mutation subset.2–4
In recent years, targeted therapies have been developed to inhibit aberrant oncogenic pathways. There are now several epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) inhibitors approved that demonstrate a large magnitude of durable overall response rate (ORR) in patients with non–small-cell lung cancer (NSCLC) who harbor certain EGFR mutations and ALK rearrangements.5–8
In the last decade, US Food and Drug Administration (FDA) approved several products for the treatment of advanced NSCLC.9 Regular approval can be granted based on an improvement in patient symptoms, function, or overall survival (OS), or on a large, clinically meaningful improvement in progression-free survival (PFS).10 Accelerated approval can be granted based on improvement in a surrogate end point reasonably likely to predict clinical benefit, such as ORR of large magnitude and long duration.11 The relationship between ORR and PFS or ORR and OS in advanced NSCLC has not been established, and validation of ORR as a surrogate for PFS or OS can be accomplished by a meta-analysis. Therefore, we conducted an analysis of trials submitted to the FDA between 2003 and 2013, including three trials testing targeted therapies in molecularly enriched populations where high ORRs were observed in early clinical development.
METHODS
Selection Criteria
We searched for trials evaluating treatments for advanced NSCLC submitted to the FDA as initial or supplemental New Drug or Biologics License Applications between 2003 and 2013. Studies include at least 150 patients with advanced NSCLC and have a randomized, multicenter, and active-controlled design (either head to head or add on).
Outcome Measures
OS was defined as the time from random assignment to death. For patients alive at the data cutoff date, OS was censored at the last follow-up date. PFS was defined as the time from random assignment to progression or death. Patients alive who had not experienced progression as of the analysis cutoff date were censored at the last disease assessment. In a majority of trials, PFS was determined by RECIST. Of 11 studies, three used RECIST version 1.1, whereas the remainder used RECIST version 1.0. WHO criteria were used to determine PFS in three trials. ORR was defined as the proportion of patients who achieve a complete or partial response per RECIST or WHO criteria. Patients with unevaluable or unknown response status were considered nonresponders. All analyses used the intent-to-treat population, defined as all patients who were randomly assigned.
Statistical Analysis
Trial-level analysis.
The association between treatment effects on ORR, PFS, and OS was evaluated using weighted linear regression models. Weighted linear regression analyses were performed on a logarithmic scale, with weights equal to sample size of each randomized comparison. We calculated the coefficient of determination (R2) and the associated 95% CIs from the weighted linear regression model to measure the association between ORR, PFS, and OS by treatment effect. Treatment effects on PFS and OS were presented as hazard ratios (HRs) estimated from Cox proportional hazards regression models, and treatment effects on ORR were presented as odds ratios (ORs) estimated from logistic regression models. An HR (experimental v control) of less than 1 denotes a favorable result for PFS and OS in the experimental group, and an OR (control v experimental) of less than 1 denotes a favorable result for ORR in the experimental group.
Patient-level responder analysis.
A responder analysis was performed to compare PFS and OS between responders and nonresponders, irrespective of treatment assignment using the pooled data set. We estimated HRs of PFS and OS from Cox proportional hazards models stratified by study and obtained Kaplan-Meier estimates of PFS and OS by response status. In addition, we conducted multivariable analyses using Cox regression models including baseline factors (age, race, smoking status, histology, performance status, and number of prior lines of therapy) and response status. Patients with missing factors were excluded from multivariable analyses.
In addition, the analysis method of Burzkowski was used to estimate patient-level associations between PFS, OS, and ORR by θ, which represents the (constant) ratio of odds for surviving beyond any time t in responders versus nonresponders.12 A θ with a lower 95% CI greater than 1 indicates that a patient-level association may exist. As supportive analyses, we also performed landmark analyses at different time points (2.5, 3, 4, and 5 months) to account for possible length bias in the responder analysis.
RESULTS
We identified 14 trials (N = 12,567) submitted between 2003 and 2013 in support of initial or supplemental New Drug or Biologics License Applications for treatments of advanced NSCLC (Table 1). Due to a three-arm trial with two comparisons and a shared control, there were 15 randomized comparisons included in the trial-level analysis (Fig 1). Three of the 14 trials tested targeted therapies in molecularly enriched populations (EGFR mutation positive, n = 2; ALK rearranged, n = 1). Eight trials were head-to-head comparisons against an active control, whereas seven were add-on comparisons to a standard-of-care backbone. Of the 15 randomized comparisons, the primary end point was PFS in nine, OS in five, and ORR in one.
Table 1.
Summary of Trials Analyzed
| Experimental Drug | Control | Design | No. of Patients | Patient Population | Primary EP | ORR OR | ORR (%) |
PFS HR* | Median PFS (months) |
OS HR* | Median OS (months) |
Study | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental Drug | Control | Experimental Drug | Control | Experimental Drug | Control | ||||||||||
| Crizotinib | Pem (or doc) | H-H | 347 | 2L ALK+ | PFS (IRC) | 0.13 | 65 | 20 | 0.49 | 7.7 | 3 | 1.04 | 20.3 | 22.8 | Shaw et al13 |
| Afatinib | Cis + pem | H-H | 345 | 1L EGFRm | PFS (IRC) | 0.21 | 56 | 23 | 0.58 | 11.1 | 6.9 | 0.91 | 28.1 | 28.2 | Sequist et al14 |
| Erlotinib | Cis (car) + doc (gem) | H-H | 174 | 1L EGFRm | PFS (INV) | 0.10 | 65 | 16 | 0.34 | 10.4 | 5.2 | 0.93 | 22.9 | 19.5 | Rosell et al15 |
| Nab-paclitaxel + car | Car + pac | H-H | 1,052 | 1L | ORR (IRC) | 0.68 | 33 | 25 | 0.93 | 6.3 | 5.8 | 0.93 | 12.1 | 11.2 | Socinski et al16 |
| Cetuximab | Car + tax | A-O | 676 | 1L | PFS (IRC) | 0.60 | 26 | 17 | 0.89 | 4.4 | 4.2 | 0.95 | 9.7 | 8.4 | Lynch et al17 |
| Cetuximab | Cis + vin | A-O | 1,125 | 1L | OS | 0.72 | 36 | 29 | 0.99 | 4.7 | 4.9 | 0.90 | 11.3 | 10.1 | Pirker et al18 |
| Vandetanib | Erl | H-H | 1,240 | 2L+ | PFS (INV) | 1.0 | 12 | 12 | 0.98 | 2.6 | 2.1 | 1.01 | 6.9 | 7.8 | Natale et al19 |
| Vandetanib | Pem | A-O | 534 | 2L+ | PFS (INV) | 0.36 | 19 | 8 | 0.86 | 4.0 | 2.7 | 0.86 | 10.5 | 9.2 | de Boer et al20 |
| Vandetanib | Doc | A-O | 1,391 | 2L+ | PFS (INV) | 0.54 | 17 | 10 | 0.79 | 4.0 | 3.2 | 0.91 | 10.6 | 10 | Herbst et al21 |
| Gefitinib | Doc | H-H | 1,466 | 2L+ | OS (NI) | 0.82 | 8 | 7 | 1.01 | 2.2 | 2.7 | 1.02 | 8.4 | 7.5 | Kim et al22 |
| Bevacizumab† | Cis + gem | A-O | 692 | 1L NSq | PFS (INV) | 0.45 | 37 | 22 | 0.75 | 6.7 | 6.1 | 0.93 | 13.6 | 13.1 | Reck et al23 |
| Bevacizumab† | Cis + gem | A-O | 698 | 1L NSq | PFS (INV) | 0.52 | 34 | 22 | 0.85 | 6.5 | 6.1 | 1.03 | 13.4 | 13.1 | Reck et al23 |
| Pemetrexed + cis | Cis + gem | H-H | 1,725 | 1L | OS (NI) | 0.88 | 27 | 25 | 1.06 | 4.8 | 5.1 | 0.93 | 10.3 | 10.3 | Scagliotti et al24 |
| Bevacizumab | Car + pac | A-O | 878 | 1L NSq | OS | 0.37 | 27 | 12 | 0.66 | 6.2 | 4.5 | 0.8 | 12.3 | 10.3 | Sandler et al25 |
| Pemetrexed | Doc | H-H | 571 | 2L | OS (NI) | 0.98 | 9 | 8 | 0.97 | 2.9 | 2.9 | 0.99 | 8.3 | 7.9 | Hanna et al26 |
Abbreviations: 1L, first line; 2L+, second or third line; ALK+, anaplastic lymphoma kinase rearranged; A-O, add on; car, carboplatin; cis, cisplatin; doc, docetaxel; EGFRm, epidermal growth factor receptor mutant; EP, end point; erl, erlotinib; gem, gemcitabine; H-H, head to head; HR, hazard ratio; INV, investigator; IRC, independent review committee; NI, noninferiority; NSq, nonsquamous; OR, odds ratio; ORR, overall response rate; OS, overall survival; pac, paclitaxel; pem, pemetrexed; PFS, progression-free survival; tax, taxane; vin, vinorelbine.
HRs estimated from unstratified Cox proportional hazards model.
Three-arm trial with shared control.
Fig 1.
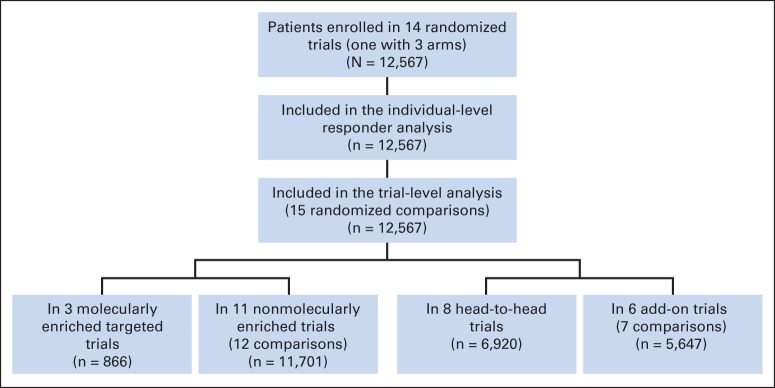
Study flow chart.
In the three molecularly enriched targeted therapy studies, the ORR, median PFS, and median OS were high. In the targeted therapy studies, ORR ranged from 56% to 65%, median PFS from 8 to 11 months, and median OS from 20 to 28 months. In addition, the effect sizes for ORR and PFS for the three targeted studies relative to control were large, with 79% to 90% relative improvements in ORR and 42% to 66% relative improvements in PFS. In the nontargeted therapy studies, the ORR ranged from 7% to 37%, median PFS from 2 to 7 months, and median OS from 7 to 14 months. The effect sizes versus control tended to be smaller in the non–molecularly enriched studies, ranging from 0% to 64% relative improvements in ORR and 0% to 34% relative improvements in PFS.
The key baseline patient demographics and disease characteristics are listed in Table 2. The median age was 60 years, younger than the average age at diagnosis of advanced NSCLC in the United States. Only 2% of the patients in these studies were black.
Table 2.
Key Baseline Patient Demographics and Disease Characteristics
| Demographic or Disease Characteristic | Total No. of Patients* | % of Patients |
|---|---|---|
| Age, years | 12,564 | |
| Mean | 60 | |
| Range | 18-92 | |
| Sex | 12,567 | |
| Male | 64 | |
| Female | 36 | |
| Race | 12,567 | |
| White | 76 | |
| Black | 2 | |
| Asian | 20 | |
| Other | 2 | |
| Region | 10,271 | |
| United States | 20 | |
| Not United States | 80 | |
| Smoking status | 10,820 | |
| Never | 25 | |
| Former or current | 75 | |
| Histology | 12,562 | |
| Squamous | 21 | |
| Nonsquamous | 79 | |
| Performance status† | 12,492 | |
| 0 | 32 | |
| 1 | 63 | |
| 2+ | 5 | |
| Tumor stage | 11,534 | |
| IIIB | 18 | |
| IV | 77 | |
| Other | 4 | |
| No. of prior lines of therapy | 12,554 | |
| 0 | 56 | |
| 1 | 38 | |
| ≥ 2 | 6 |
Numbers may vary if variable is not defined or located within the data set.
Eastern Cooperative Oncology Group or WHO performance status.
Figures 2 and 3 are scatterplots of the treatment effects on the log-scale, illustrating trial-level association among the end points. As shown in Figure 2A, treatment effects on PFS and ORR were strongly associated (R2 = 0.89; 95% CI, 0.80 to 0.98). When excluding the three targeted therapy trials with a sample size less than 500 in the linear model analysis, the R2 was 0.77 (95% CI, 0.58 to 0.96) between the treatment effect on PFS and ORR. The trial-level analysis between PFS and ORR was further analyzed by trial type (add on or head to head), as depicted in Figure 2B. The head-to-head trials seemed to have a stronger ORR to PFS association (R2 = 0.94; 95% CI, 0.88 to 1.00) compared with add-on trials (R2 = 0.65; 95% CI, 0.32 to 0.98). There was no association between treatment effects on OS and ORR (Fig 3A), with an R2 of 0.09 (95% CI, 0 to 0.33). When excluding the three targeted therapy trials with a sample size less than 500 in the linear model analysis, there was an improved but still weak association between OS and ORR (R2 = 0.44; 95% CI, 0.08 to 0.80). Figure 3B shows no association between treatment effects on OS and PFS (R2 = 0.08; 95% CI, 0 to 0.31); when excluding the three targeted therapy trials, the association was weak (R2 = 0.35; 95% CI, 0 to 0.72).
Fig 2.
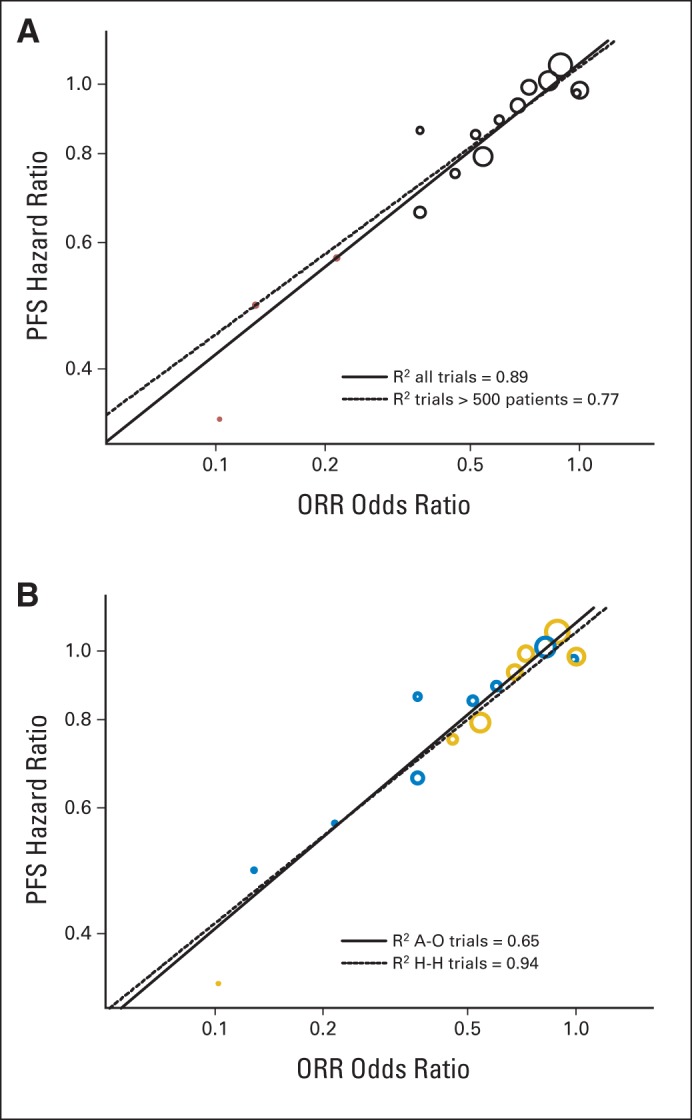
(A) Scatter plot of trial-level association between treatment effects on progression-free survival (PFS) and overall response rate (ORR). Trials with targeted treatments in molecularly enriched populations (n ≤ 500 patients per trial) are represented by red circles. (B) Scatter plot of trial-level association between treatment effects on PFS and ORR by study design. Add-on (A-O) trials are represented by blue circles and head-to-head (H-H) trials are denoted by gold circles.
Fig 3.
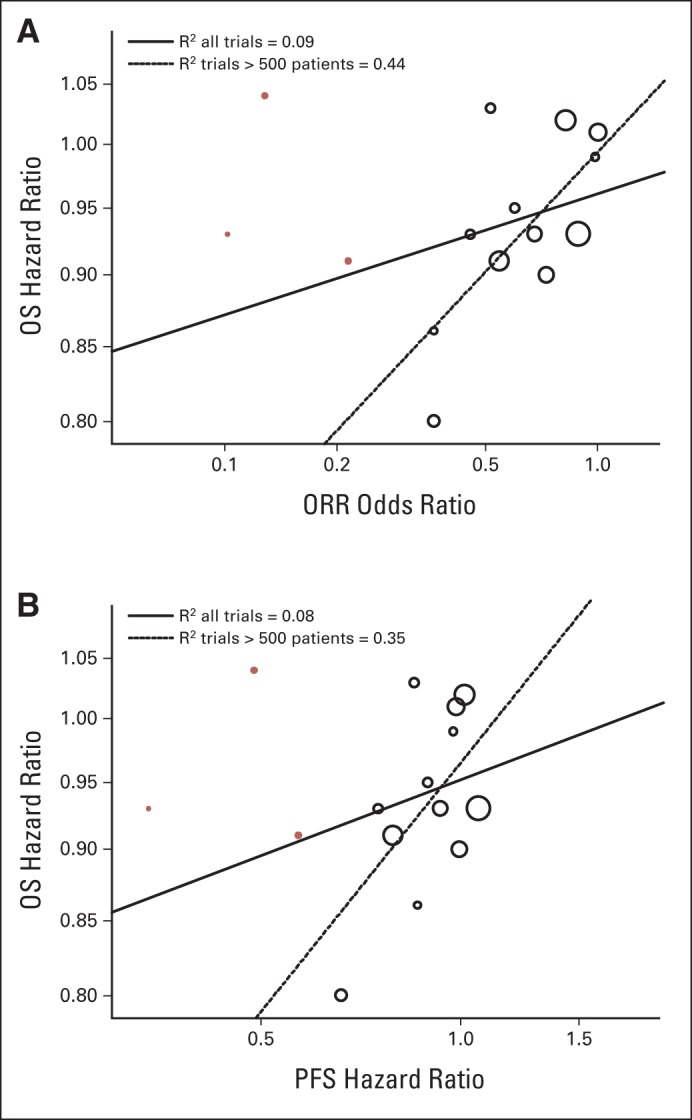
(A) Scatter plot of trial-level association between treatment effects on overall survival (OS) and overall response rate (ORR). (B) Scatter plot of trial-level association between treatment effects on OS and progression-free survival (PFS). Trials with targeted treatments in molecularly enriched populations (n ≤ 500 patients per trial) are represented by red circles.
On the basis of pooled data from the 14 trials, responders (n = 2,694, 21%) were associated with better PFS (HR, 0.40; 95% CI, 0.38 to 0.42) and OS (HR, 0.40; 95% CI, 0.38 to 0.43) compared with nonresponders (n = 9,873, 79%) irrespective of treatment assigned, as shown in Figure 4. In addition, from multivariable Cox models adjusted by baseline factors (age, race, smoking status, histology, performance status, and number of prior lines of therapy), associations were consistent with the findings from the unadjusted analysis.
Fig 4.
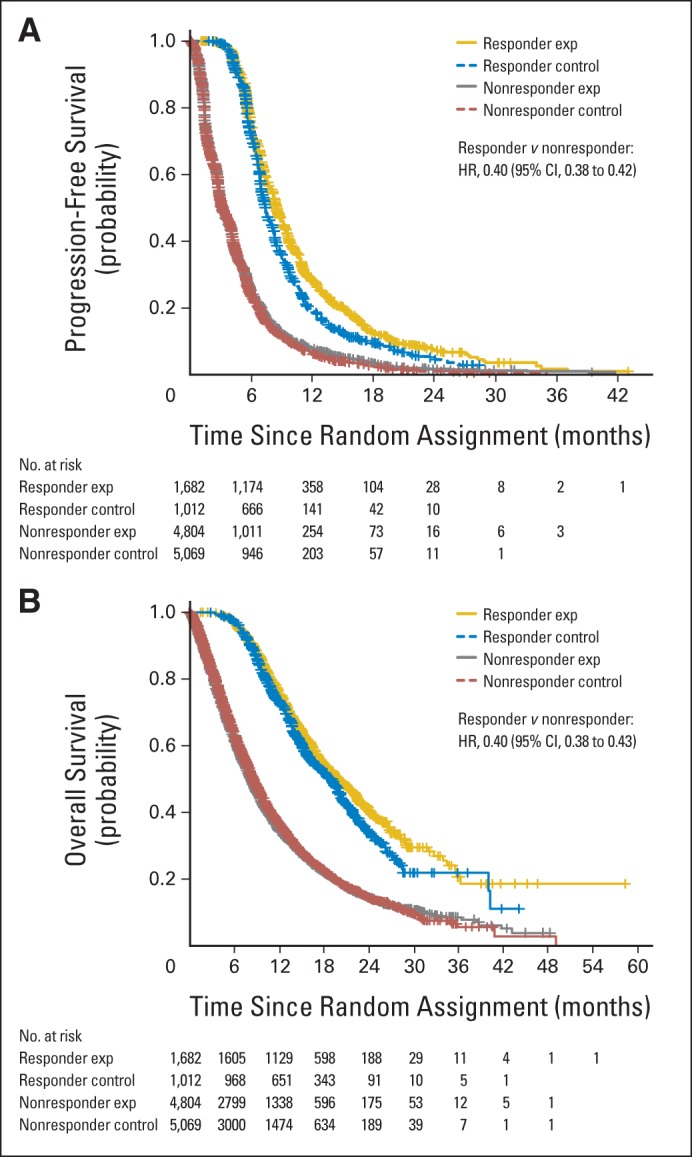
Kaplan-Meier estimates of (A) progression-free survival and (B) overall survival between responders and nonresponders. Exp, experimental; HR, hazard ratio.
Using the method of Burzkowski, a patient-level association between PFS, OS, and ORR was estimated by θ, the (constant) ratio of odds for surviving beyond any time t in responders versus nonresponders.12 The estimated value of θ was 7.11 (95% CI, 6.52 to 7.70) for the association between PFS and ORR and was 4.66 (95% CI, 4.27 to 5.06) for the association between OS and ORR. In supportive analyses using a landmark at different time points (2.5, 3, 4, and 5 months), the lower 95% CI limits of θ for both PFS and ORR and OS and ORR were all greater than 1, which indicates that there still is an individual association between PFS and ORR and between OS and ORR after accounting for possible length bias.27
DISCUSSION
Although there has been considerable progress in the molecular classification of lung cancer and in the development of targeted therapies, many challenges remain. When studying a rare subset of patients, even in a common malignancy such as NSCLC, it may be difficult to screen patients and power a study for the gold standard end point of OS.28 This may be particularly challenging if a high ORR is observed early in clinical development, where allocation of patients to a toxic and marginally effective control may violate the principle of clinical equipoise.29,30 In the case of a targeted therapy with a large treatment effect, intermediate end points such as ORR and PFS may be indicated to characterize the benefit-risk profile and establish safety and efficacy.
To our knowledge, this is the first report of a strong association between ORR and PFS using trial-level and patient-level data in advanced NSCLC. Perhaps this association is not surprising because ORR and PFS are both tumor-based assessment end points. However, a trial-level association was difficult to discern before the era of targeted therapy, where ORR and PFS effect sizes in unselected NSCLC trials were modest. Other groups have performed responder analyses, showing that ORR with EGFR tyrosine kinase inhibitors was correlated with median survival time in advanced NSCLC and that week 8 tumor size change can predict OS and assist in early drug development decisions.31,32
The meta-analysis did not demonstrate a strong association between ORR and OS or between PFS and OS. When excluding the three smaller targeted therapy trials, the associations between ORR and OS and between PFS and OS were weak. The reasons for this weak association are unclear but could be because no relationship exists or a result of other factors confounding OS analysis, including cross-over, subsequent therapies, and long postprogression survival, particularly in the smaller targeted therapy studies and front-line studies.
Using ORR as a surrogate end point in oncology drug approval has a long history. One advantage of response, as opposed to time-to-event end points such as PFS and OS, is that a tumor response can be directly attributed to the therapy, because in the absence of treatment, spontaneous tumor regression is extremely rare. In addition, the more than 30-year experience of response criteria such as RECIST enables comparisons with historic controls.33 Therefore, ORR can be assessed in single-arm trials and has been used as the basis for accelerated approval in NSCLC, as well as other malignancies including lymphoma, GI stromal tumors, and multiple myeloma.7,8,34–36 There are several limitations to single-arm trials, including lack of controlled safety data, potential known and unknown biases in patient selection, and uncertain prognostic information of biomarker-defined subsets.
ORR may not be the optimal end point for hypothesis generation or expedited approval pathways for cytostatic therapies and immunotherapies, in which alternate end points may be needed to estimate activity and PFS or OS may be required to confirm clinical benefit.37,38 Further refinements to ORR or PFS by RECIST and novel means of measuring response may be indicated based on the disease or the mechanism of action of the therapy.39–42 Novel methods to assess drug activity such as depth of response, changes in tumor volume, and time to tumor growth warrant further investigation.43–45 In addition, incorporation of validated patient-reported outcome measures into future trials may assist in better alignment of radiographic responses to improvement in disease-related symptoms or patient function.
One limitation of this meta-analysis is that only trials that were submitted to the FDA were included, which enabled a patient-level analysis. However, not all of the studies included in the analysis reached a statistically or clinically positive result and not all studies led to a favorable regulatory action in terms of a new or expanded indication. Thus, there is a balance between so-called positive and negative studies within the meta-analysis.
In summary, the meta-analysis of 14 trials in 12,567 patients with advanced NSCLC submitted to the FDA between 2003 and 2013 demonstrated a strong patient-level association between response and PFS and OS and a strong trial-level association between ORR and PFS, but not OS. Therefore, a drug with a large magnitude of effect on ORR in patients with advanced NSCLC may also have a large effect on PFS.
Glossary Terms
- Cox proportional hazards regression model:
a statistical model for regression analysis of censored survival data, examining the relationship of censored survival distribution to one or more covariates. This model produces a baseline survival curve, covariate coefficient estimates with their standard errors, risk ratios, 95% CIs, and significance levels.
- hazard ratios:
the ratio of the hazard rate in one group (for example, a group of treated patients) to the hazard rate in another group (for example, an untreated control group of patients). The hazard rate is the probability of a specified event, such as death or cancer recurrence, occurring during a short time interval. The hazard ratio, therefore, is a measure of the relative probability of an event occurring at any given point in time.
- non–small-cell lung cancer (NSCLC):
a type of lung cancer that includes squamous cell carcinoma, adenocarcinoma, and large-cell carcinoma.
- progression-free survival:
time from random assignment until death or first documented relapse, categorized as either locoregional (primary site or regional nodes) failure or distant metastasis or death.
Footnotes
Presented in part at the 50th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2014, Chicago, IL.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
The opinions expressed in this article do not necessarily reflect those of the US Food and Drug Administration or the US Government. This is a US Government work. There are no restrictions on its use with the exception of any previously printed figures and tables.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Gideon M. Blumenthal, Sean Khozin, Dickran Kazandjian, Rajeshwari Sridhara, Patricia Keegan, Richard Pazdur
Collection and assembly of data: Gideon M. Blumenthal, Stella W. Karuri, Hui Zhang, Sean Khozin, Dickran Kazandjian
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Overall Response Rate, Progression-Free Survival, and Overall Survival With Targeted and Standard Therapies in Advanced Non–Small-Cell Lung Cancer: US Food and Drug Administration Trial-Level and Patient-Level Analyses
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Gideon M. Blumenthal
No relationship to disclose
Stella W. Karuri
No relationship to disclose
Hui Zhang
No relationship to disclose
Lijun Zhang
No relationship to disclose
Sean Khozin
No relationship to disclose
Dickran Kazandjian
No relationship to disclose
Shenghui Tang
No relationship to disclose
Rajeshwari Sridhara
No relationship to disclose
Patricia Keegan
No relationship to disclose
Richard Pazdur
No relationship to disclose
REFERENCES
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Clinical Lung Cancer Genome Project (CLCGP); Network Genomic Medicine (NGM) A genomics-based classification of human lung tumors. Sci Transl Med. 2013;5:209ra153. doi: 10.1126/scitranslmed.3006802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175–180. doi: 10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]
- 4.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khozin S, Blumenthal GM, Jiang X, et al. US Food and Drug Administration approval summary: Erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations. Oncologist. 2014;19:774–779. doi: 10.1634/theoncologist.2014-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dungo RT, Keating GM. Afatinib: First global approval. Drugs. 2013;73:1503–1515. doi: 10.1007/s40265-013-0111-6. [DOI] [PubMed] [Google Scholar]
- 7.Malik SM, Maher VE, Bijwaard KE, et al. US Food and Drug Administration approval: Crizotinib for treatment of advanced or metastatic non-small cell lung cancer that is anaplastic lymphoma kinase positive. Clin Cancer Res. 2014;20:2029–2034. doi: 10.1158/1078-0432.CCR-13-3077. [DOI] [PubMed] [Google Scholar]
- 8.Chabner BA. Approval after phase I: Ceritinib runs the three-minute mile. Oncologist. 2014;19:577–578. doi: 10.1634/theoncologist.2014-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration. Guidance for industry: Clinical trial endpoints for the approval of non-small cell lung cancer drugs and biologics. http://www.fda.gov/downloads/Drugs/Guidances/UCM259421.pdf.
- 10.Pazdur R. Endpoints for assessing drug activity in clinical trials. Oncologist. 2008;13:19–21. doi: 10.1634/theoncologist.13-S2-19. [DOI] [PubMed] [Google Scholar]
- 11.Johnson JR, Ning YM, Farrell A, et al. Accelerated approval of oncology products: The Food and Drug Administration experience. J Natl Cancer Inst. 2011;103:636–644. doi: 10.1093/jnci/djr062. [DOI] [PubMed] [Google Scholar]
- 12.Burzykowski T, Molenberghs G, Buyse M. The validation of surrogate end points by using data from randomized clinical trials: A case-study in advanced colorectal cancer. J R Stat Soc A. 2004;167:103–124. [Google Scholar]
- 13.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 14.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 15.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 16.Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: Final results of a phase III trial. J Clin Oncol. 2012;30:2055–2062. doi: 10.1200/JCO.2011.39.5848. [DOI] [PubMed] [Google Scholar]
- 17.Lynch TJ, Patel T, Dreisbach L, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: Results of the randomized multicenter phase III trial BMS099. J Clin Oncol. 2010;28:911–917. doi: 10.1200/JCO.2009.21.9618. [DOI] [PubMed] [Google Scholar]
- 18.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): An open-label randomised phase III trial. Lancet. 2009;373:1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 19.Natale RB, Thongprasert S, Greco FA, et al. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:1059–1066. doi: 10.1200/JCO.2010.28.5981. [DOI] [PubMed] [Google Scholar]
- 20.de Boer RH, Arrieta Ó, Yang CH, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: A randomized, double-blind phase III trial. J Clin Oncol. 2011;29:1067–1074. doi: 10.1200/JCO.2010.29.5717. [DOI] [PubMed] [Google Scholar]
- 21.Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): A double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11:619–626. doi: 10.1016/S1470-2045(10)70132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): A randomised phase III trial. Lancet. 2008;372:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 23.Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: Results from a randomised phase III trial (AVAiL) Ann Oncol. 2010;21:1804–1809. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 25.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 26.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 27.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 28.Sharma MR, Schilsky RL. Role of randomized phase III trials in an era of effective targeted therapies. Nat Rev Clin Oncol. 2011;9:208–214. doi: 10.1038/nrclinonc.2011.190. [DOI] [PubMed] [Google Scholar]
- 29.Miller FG, Joffe S. Equipoise and the dilemma of randomized clinical trials. N Engl J Med. 2011;364:476–480. doi: 10.1056/NEJMsb1011301. [DOI] [PubMed] [Google Scholar]
- 30.Kurzrock R, Stewart DJ. Equipoise abandoned? Randomization and clinical trials. Ann Oncol. 2013;24:2471–2474. doi: 10.1093/annonc/mdt358. [DOI] [PubMed] [Google Scholar]
- 31.Tsujino K, Kawaguchi T, Kubo A, et al. Response rate is associated with prolonged survival in patients with advanced non-small cell lung cancer treated with gefitinib or erlotinib. J Thorac Oncol. 2009;4:994–1001. doi: 10.1097/JTO.0b013e3181a94a2f. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Sung C, Dartois C, et al. Elucidation of relationship between tumor size and survival in non-small-cell lung cancer patients can aid early decision making in clinical drug development. Clin Pharmacol Ther. 2009;86:167–174. doi: 10.1038/clpt.2009.64. [DOI] [PubMed] [Google Scholar]
- 33.Oxnard GR, Morris MJ, Hodi FS, et al. When progressive disease does not mean treatment failure: Reconsidering the criteria for progression. J Natl Cancer Inst. 2012;104:1534–1541. doi: 10.1093/jnci/djs353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Claro RA, McGinn K, Kwitkowski V, et al. US Food and Drug Administration approval summary: Brentuximab vedotin for the treatment of relapsed Hodgkin lymphoma or relapsed systemic anaplastic large-cell lymphoma. Clin Cancer Res. 2012;18:5845–5849. doi: 10.1158/1078-0432.CCR-12-1803. [DOI] [PubMed] [Google Scholar]
- 35.Dagher R, Cohen M, Williams G, et al. Approval summary: Imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res. 2002;8:3034–3038. [PubMed] [Google Scholar]
- 36.Herndon TM, Deisseroth A, Kaminskas E, et al. US Food and Drug Administration approval: Carfilzomib for the treatment of multiple myeloma. Clin Cancer Res. 2013;19:4559–4563. doi: 10.1158/1078-0432.CCR-13-0755. [DOI] [PubMed] [Google Scholar]
- 37.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 38.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franz DN, Belousova E, Sparagana S, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381:125–132. doi: 10.1016/S0140-6736(12)61134-9. [DOI] [PubMed] [Google Scholar]
- 40.Zhao B, Oxnard GR, Moskowitz CS, et al. A pilot study of volume measurement as a method of tumor response evaluation to aid biomarker development. Clin Cancer Res. 2010;16:4647–4653. doi: 10.1158/1078-0432.CCR-10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith AD, Shah SN, Rini BI, et al. Morphology, attenuation, size, and structure (MASS) criteria: Assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. AJR Am J Roentgenol. 2010;194:1470–1478. doi: 10.2214/AJR.09.3456. [DOI] [PubMed] [Google Scholar]
- 42.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 43.Jain RK, Lee JJ, Ng C, et al. Change in tumor size by RECIST correlates linearly with overall survival in phase I oncology studies. J Clin Oncol. 2012;30:2684–2690. doi: 10.1200/JCO.2011.36.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venook AP, Tabernero J. Progression-free survival: Helpful biomarker or clinically meaningless end point? J Clin Oncol. doi: 10.1200/JCO.2014.57.9557. [epub ahead of print on November 3, 2014] [DOI] [PubMed] [Google Scholar]
- 45.Sharma MR, Gray E, Goldberg RM, et al. Resampling the N9741 trial to compare dynamic versus conventional end points in randomized phase II trials. J Clin Oncol. doi: 10.1200/JCO.2014.57.2826. [epub ahead of print on October 27, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]


