Abstract
Radiofrequency ablation (RFA) of the cavo-tricuspid isthmus (CTI) is one of the most frequently performed procedures in electrophysiology. Despite a high success rate, ablation of the CTI can be unusually difficult in some cases. Multiple tools like angiography, 3D mapping, remote navigation and intracardiac echocardiography (ICE) have been introduced to facilitate typical flutter ablation. This review article summarizes the clinical value of different strategies and tools used for CTI ablation focusing on the importance of approaches utilizing ICE.
Keywords: Atrial flutter, cavo-tricuspid isthmus, fluoroscopy time, intracardiac echocardiography, procedure time, radiofrequency abla-tion.
INTRODUCTION
“The incidence of atrial flutter (Afl) in the general population is 88/100,000 person/years” [1]. The incidence of Afl in men is 2.5 times higher than that in women and the incidence increases exponentially with age. Clinical risk factors associated with Afl are heart failure and chronic pulmonary disease [1]. Afl is often (~60%) associated with atrial fibrillation and can lead to significant symptoms, tachycardiomyopathy, thromboembolic stroke and myocardial ischemia. The most frequent type of Afl is a cavo-tricuspid isthmus (CTI)-dependent flutter”, which is a macroreentrant atrial tachycardia with anatomically defined pathway. Its reentrant circuit rotates around the tricuspid valve annulus (TV), with the predominant area of slow conduction in the CTI. The shortcut activation towards the posterior right atrium is protected by the crista terminalis (CT). Pharmacological treatment is rarely effective for conversion of Afl and insufficient to control the high ventricular rate during ongoing arrhythmia [2, 3]. Direct current shock on CTI as a definitive treatment for Afl was introduced by Saoudi et al. in 1990. The acute success rate was high but the Afl recurred in 37.5% of the patients during follow up [4]. Two years later, working groups led by Feld GK and Cosio FG developed a new method with high success and low recurrence rate using radiofrequency ablation (RFA). The endpoint of the procedure is a permanent and continuous line of block along the CTI [5, 6]. “This method has been accepted as a first-line therapeutic option in the treatment of CTI-dependent Afl and one of the most commonly performed procedures in electrophysiology” [5, 6]. RFA of CTI-dependent Afl is a highly successful intervention, but in about 1–12% of the patients the RFA remains unsuccessful [7-10]”. The principal obstacle to successful ablation is the complex and variable anatomy of the CTI which has been demonstrated in anatomical and angiographic studies” [5, 6]. “Intracardiac echocardiography (ICE) has the potential role and capability to reveal anatomy of the isthmus and guide ablation in difficult cases” [5, 3]. This review article describes the importance of ICE in the ablation of typical flutter and presents available clinical data related to this issue.
INTRACARDIAC ECHOCARDIOGRAPHY IN ELECTROPHYSIOLOGY
In current practice, ablation catheter positioning is mainly guided by fluoroscopy and electrograms, although visualization by fluoroscopy is unable to depict the endocardial surfaces and the anatomic details required for precise navigation. Moreover, the electrode tip-endocardial contact and lesion formation cannot be assessed directly with fluoroscopy and radiation exposure can potentially increase the risk of malignancy to the patient and the operator. Transthoracic echocardiography (TTE) is not suitable to guide the ablation procedures: the optimal acoustic window cannot be maintained by positioning of the patients during the procedure along with the protected sterile field. Transesophageal echocardiography (TEE) is highly uncomfortable for patient and both TTE and TEE require a second operator. Almost two decades ago, Chu E. and colleagues demonstrated that ICE can be used in dogs to accurately guide radiofrequency lesion placement within the right atrium. In 97% of ablation points, ICE confirmed stable endocardial contact and the pathological examination proved that lesions were accurately placed less than 2 mm from an anatomically based target in the majority of cases [11]. Same year Chu E. and colleagues demonstrated the utility and feasibility of ICE in humans. They successfully identified anatomical structures in the right atrium and confirmed electrode tip-endocardial contact during ablation. In three cases of left atrial arrhythmias experienced operators were not able to cross the interatrial septum with a Brockenbrough needle. In these patients, the ICE was able to depict the septum, the needle and to guide the successful transseptal puncture [12]. Later on, ICE emerged as a widely used tool in the everyday practice of interventional electrophysiology. Beside real-time visualization of endocardial structures and catheters, it allows monitoring of ablation lesions, detection of intracardiac thrombi, facilitates transseptal puncture and allows early detection of complications. ICE also provides real-time anatomic data on the left atrium and pulmonary veins, allows precise positioning of circular mapping catheters in the ostium of pulmonary veins, prevents ablation in the pulmonary veins, promotes the transmurality of ablation lesions and prevents esophageal injury by monitoring the lesions caused by RFA on the left atrial posterior wall. ICE can provide additional information about arrhythmogenic substrate (scars, aneurysms) and the course of the coronary arteries. Its use has progressively increased, mainly during the ablation of atrial fibrillation and ventricular arrhythmias [13]. Recent publications using 2D and 3D ICE with sophisticated details proved that anatomical characteristics of the ablation targets strongly correlate with the procedural success [14-17].
TOOLS FACILITATING RFA OF THE CTI
Catheter design: Studies comparing 4- with 8-mm tip electrodes for RFA of typical Afl [18-20] demonstrated higher success rate (67% vs. 92%) with a lower number of RFA application, shorter procedure and radiation time required for creation of bidirectional block with 8-mm tip electrodes. Feld et al. showed reduction in recurrence rate (from 43% to 10%) by using 8-mm tip catheters [19]. Studies evaluating the efficacy of RFA of typical Afl by using an 8-mm tip catheter (with 100 W power) reported 88%, 90% and 99% acute success rates, respectively [21-23]. In the prospective, multicenter clinical trial (Atakr) with 8-mm electrode catheter, Calkins et al. reported low complication rate (2.7%) but a significant number of recurrences (at 6 months 13% and at 12 months 20%). “Procedure time was the only predictor of recurrence of atrial flutter in this study” [21]. After introduction of irrigated-tip catheters, studies have found that these catheters are more effective and as safe as conventional 4-mm-tip catheters [23] but results comparing irrigated-tip and 8-mm-tip catheters were controversial. A metaanalysis by Da Costa et al. [24] which included seven trials and 603 patients concluded that irrigated-tip and 8-mm-tip catheters are equally effective for isthmus ablation with similar success rates (84% vs.85%) and procedural characteristics (RFA time, fluoroscopy and total procedure time). “In the review article by Da Costa et al., the authors concluded that 8-mm tip catheters seem to be more effective in patients with straight isthmus morphology and irrigated catheters appear to be more effective in cases of complex CTI anatomy” [25]. In a recent prospective, randomized, multicentre study comparing conventional, 8-mm platinum–iridium-tip (Pt-Ir) catheters with 8-mm gold-tip catheters, the authors found that procedure and fluoroscopy time, complication and recurrence rate were similar between the two catheters [26]. “The gold-tip catheter was, however, associated with a higher ablation success rate (94.3 vs. 89.0%, P= 0.042) and a lower incidence of char and coagulum formation” [26]. In a study by Feld GK et al. [27] cryoablation of the CTI demonstrated similar acute (87.5%) and long-term efficacy and safety as RFA, but the total procedure time, ablation time and fluoroscopy time were significantly higher than usual. In some difficult cases, the reason for failure to achieve block on the isthmus is a lack of stability and contact. Using a conventional long or a steerable sheath can overcome this obstacle. In a study conducted by Matsuo et al., the use of a steerable sheath decreased the procedure time and amount of RFA energy needed to achieve block on the isthmus in comparison to procedures performed with a non-steerable sheath [28]. As the procedural success is mainly defined by the preexistent anatomy of the CTI preprocedural (by TTE, CT or MRI) or intraprocedural (by angiography or ICE) imaging of CTI anatomy can facilitate the appropriate catheter selection [29-31].
Angiography: Heidbüchel et al. introduced biplane right atrial angiography [32] to reveal highly variable CTI anatomy before ablation (isthmogram). They adapted the ablation approach to these anatomic findings. In patients with a long isthmus (≥40mm), they inserted a 3-in catheter and in cases with deep pouches ablation catheters with a very short curve were used. Angiographic findings corresponded well with a previous anatomic descriptions of the CTI [33, 34] regarding width and shape and showed a prominent, muscular-type Eustachian valve (EVR) in 24% of cases. An angiographic study by Da Costa et al. [35] proved that both CTI length and isthmus characteristics such as concavity and the presence of pouch-like recesses are factors that significantly increase procedure duration, x-ray exposure and require RF applications. The same group [36] demonstrated that pre-ablation isthmogram can predict the success of RFA (the 8 mm-tip catheter is more suitable in patients with straight isthmus morphology) and may prevent the crossover to another ablation catheter. Right atrial angiography is performed with a pigtail catheter located at the inferior vena cava-right atrial junction using multiple contrast injections from different fluoroscopic projections. Thereby this “diagnostic” step increases x-ray exposure and can result in contrast induced complications such as nephropathy. Angiography may be useful for determining contact with the wall in the right anterior oblique projection but provides little information for the positioning in the left anterior oblique view. Information gathered by isthmogram is only two-dimensional and not real-time. Finally, there is no randomized study showing that CTI ablation with angiography is superior to the conventional approach.
3D mapping systems: The use of 3D mapping systems for CTI ablation was introduced by Kottkamp et al. [37]. The Euroflutter study demonstrated that using 3D mapping system for CTI ablation the fluoroscopy exposure time can be reduced by almost 50% [38]. In 90% of all cases, fluoroscopy was not required (zero-fluoroscopy) for successful ablation of typical flutter using impedance-based 3D mapping [39]. Using 3D mapping systems about 1.2-6% of procedures remained unsuccessful and the total procedure time was longer than that in the conventional group although statistically it was not significant. The cost of procedure in a group with 3D mapping was remarkably higher than in the conventional group. “Introduction of 3D mapping systems for ablation of CTI did not result in a reduction of procedure (and RFA) time and was not able to maximize the success rate which clearly proves that the main obstacle to successful isthmus ablation is the high anatomical variability of the CTI that can be overcome only with real time visualization” [5, 6].
Remote navigation: The first human study with remote magnetic navigation system (MNS) for isthmus ablation was reported by Arya et al. in 2008 [40]. This study demonstrated that using MNS for CTI ablation is safe, feasible and effective. Total procedure time, fluoroscopy time and RFA time were comparable with procedural parameters using a conventional approach in other studies, but not shorter. There was a short learning curve after which procedure time and fluoroscopy time shortened significantly, but RFA time remained the same. 4% of cases remained unsuccessful. A prospective, randomized trial conducted by Vollmann [41] et al. compared procedural parameters of typical flutter ablation using conventional approach versus MNS. The number of radiofrequency applications, the RFA time and the total procedure time were found to be higher in the group utilizing MNS, on the other hand the fluoroscopy time was shorter than that in the conventional group. The acute success rate was found to be lower than that in the conventional group (73 vs.89%) and interestingly the long-term recurrence rate was higher in the MNS group (13 vs.2%). A prospective, randomized study by Steven et al. [42] compared procedural parameters of CTI ablation using a conventional approach versus robotic navigation system (RNS). X-ray exposure and RFA time as well as the amount of radiofrequency energy were significantly smaller in the RNS group, but “the total procedure and preparation time was significantly higher in the group” using RNS. In summary, CTI ablation by MNS can result in decreased fluoroscopy times but the procedure times and RFA times are comparable or even longer than those used by the conventional approach. Concerns can be raised related to acute and long term success rate of CTI ablation by MNS.
Real-time 3D TEE: A study by Regoli et al. [43] demonstrated that real-time 3-dimensional transesophageal echocardiography (RT3DTEE)–guided ablation of the CTI is feasible and safe and enables continuous visualization of the ablation catheter during RFA. Total procedure time, fluoroscopy time, fluoroscopy dose and number of radiofrequency applications were significantly decreased in comparison with the conventional approach but RFA time was in the same range in both groups. RT3DTEE requires general anesthesia with its all possible drawbacks.
Voltage-guided ablation of CTI: In 2006, Redfearn et al [44] introduced a new ablation strategy targeting large voltage “bundles” without producing a continuous line on the CTI. “This ablation approach operates on the hypothesis that the muscle bundles in CTI act like a variably thick sheet of tissue and block can be obtained without ablation of the entire isthmus by targeting only the functionally important muscle bundles” [44]. The same group in a prospective, randomized trial demonstrated that using the voltage-guided technique for CTI ablation significantly reduces RFA time and the number of ablation lesions in comparison with the anatomically-guided strategy although without “a significant reduction in procedure or fluoroscopy time” [45]. Other studies have supported these results [46-48]. In the post-hoc analysis of AURUM 8 trial, Lewalter et al. confirmed that all procedural parameters are remarkably improved with voltage-guided technique, including fluoroscopy and total procedure times [49].
INTRACARDIAC ECHOCARDIOGRAPHY FOR CTI ABLATION
First ICE-guided RFA of typical flutter in humans was performed by Chu et al. [11] in 1994. ICU (10-MHz rotational ICE inserted via femoral vein to the right atrium) was used to visualize a corridor defined by the ostium of coronary sinus, the inferior vena cava and the TV in the right atrium. Anatomic structures such as TV, the coronary sinus ostium, the interatrial septum and EVR were identified with ICE in all patients. Additional manipulation with the ablation catheter was frequently required to establish adequate contact on the CTI during ablation [12]. In the majority of the cases they were able to directly visualize the electrode-endocardial contact, continuous movements (sliding) of the ablation catheter, evolution of thrombi and RFA lesions as well as microbubble formation. One year later, rotational ICE was used to facilitate endocardial activation and entrainment mapping of typical atrial flutter [50, 51]. Rotational ICE can promote endocardial mapping but is limited in visualization of other CTI details [52]. A study conducted by Morton et al. [53] in 2003 showed the feasibility and utility of phased-array ICE during ablation of typical Afl. They used 7.5-10 MHz phased-array ICE and visualized the CTI in its long axis. In this view, both the anterior and posterior borders of the isthmus were easily depicted. By clockwise rotation the septal part (coronary sinus ostium), by counterclockwise rotation the free wall of the right atrium were visualized. This observational study proved that ICE is a valid, high-definition real-time imaging modality for describing the complex morphology of the isthmus. ICE measurements (length and thickness of the isthmus) correlated well with previous anatomical descriptions. ICE was able to visualize trabeculations, pouches and recesses as well as dynamic changes of the isthmus during the cardiac cycle. Moreover, ICE was able to monitor the evolution of RFA lesions, like swelling and changes in echo-density. The study identified pouches in 73% of cases (5.9 ± 2.0 mm) and found them to be deeper in the septal isthmus. Previous anatomical studies described pouching of the isthmus in similar rates (two thirds of the patients) [33]. We reported the effectiveness of phased-array ICE imaging in two challenging cases of isthmus ablation [54, 55]. In the first case, ICE imaging depicted a prominent EVR as a main obstacle to reach the sub-Eustachian part of the isthmus. The ablation catheter was bended and pulled down to reach the sub-Eustachian isthmus (Fig. 1). After RFA at this point isthmus block was achieved. In the second case despite 150 minutes of procedure and 40 minutes of fluoroscopy time block on the CTI was not achieved. With introduction of ICE we found a highly prominent and muscular EVR (Fig. 2). With curving the ablation catheter (~320˚), we reached the edge of the thick and actively contracting EVR and recorded a high voltage atrial potential in that location. After ablation at this point, we confirmed bidirectional block on the isthmus. Furthermore, we reported other complex and variable isthmuses depicted by ICE (Fig. 3 and Fig. 4) [56]. In our prospective, randomized study 102 patients were included [56]. Patients were randomized in two groups: fluoroscopy (n=52) or ICE guided (n=50) g
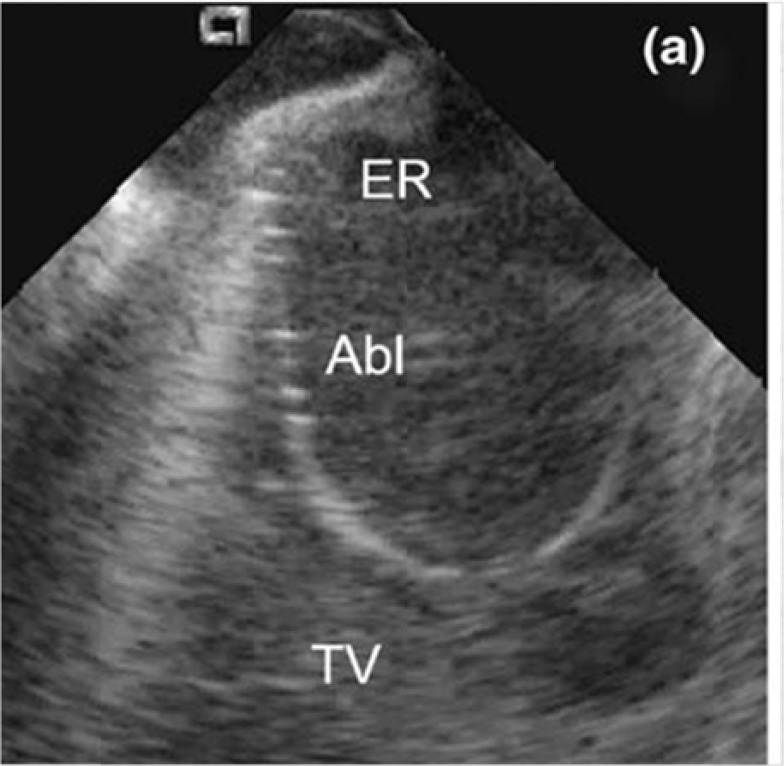
Fig. (1).
Intracardiac echocardiography (ICE) image of the CTI. The ablation catheter (Abl) is curved around the prominent Eustachian ridge (EVR). TV: tricuspid valve.
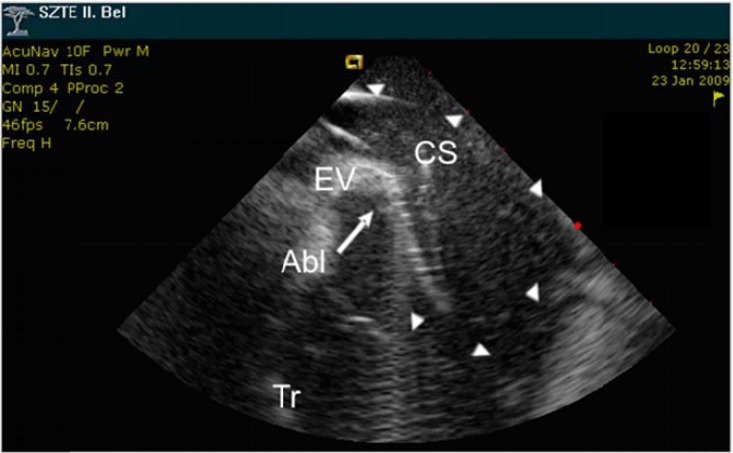
Fig. (2).
ICE image presenting a prominent, muscular and actively contracting EVR, which could only be engaged by curving the ablation catheter (Abl) into full circle (arrowheads) to touch the anterior surface of the valve. CS: coronary sinus, Tr: tricuspid valve.
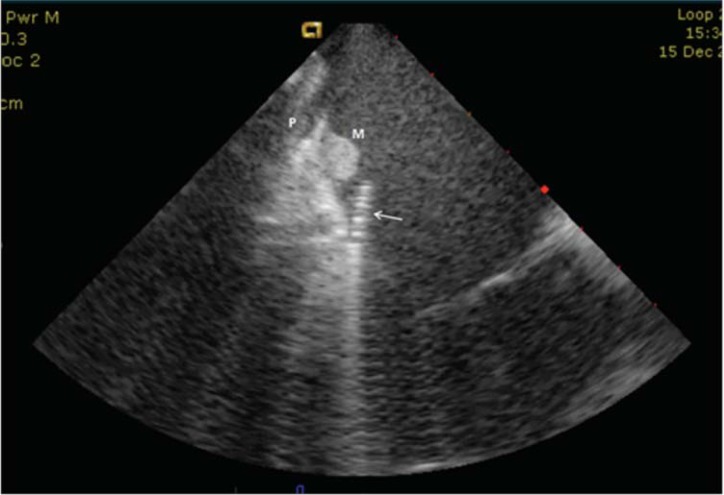
Fig. (3).
CTI with a muscular bundle on the ventricular side and a deep and narrow pouch on the atrial side of the isthmus. Targeted ablation of both structures was needed to establish a block on the isthmus. Arrow: ablation catheter, P: pouch, M: muscular bundle.
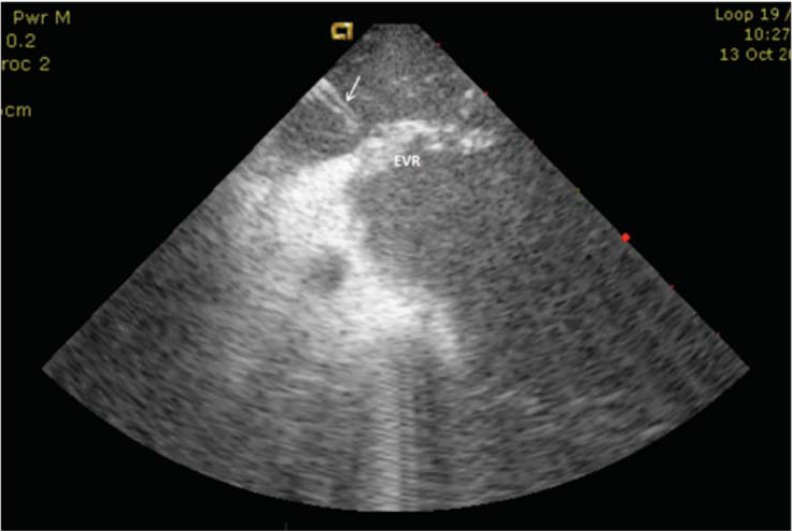
Fig. (4).
ICE image of case where ablation of the muscular EVR was performed from the venous side to complete the block on the CTI. Arrow: ablation catheter, EVR: Eustachian-valve/ridge.
roup. Seven patients (13%) from the fluoroscopy group crossed over to ICE group because of failed RFA and in all of these patients block on the isthmus was obtained using the ICE (100% acute success rate). Regarding patients characteristics we found no significant differences between the two groups. Moreover, the complication and recurrence rates were identical in both groups [56]. We have shown that procedure and fluoroscopy time, the time spent for ablation were significantly shorter, radiation (dose-area product-DAP) and the sum of applied radiofrequency energy were significantly lower in the ICE-group (Table 1). ICE depicted individual characteristics of the isthmus and allowed real time navigation making the procedure faster and more effective.
Table 1.
Procedural parameters of CTI ablation in ICE-guided versus fluoro-only guided groups.
| ICE-group (n:50) | Fluoro-only group (n:52) | P-value | |
|---|---|---|---|
| Procedure time (min) | 68.06±15.09 | 105.94±36.51 | P<0.001 |
| Fluoroscopy time (min) | 5.54±3.77 | 18.63±10.60 | P<0.001 |
| Radiation exposure-DAP (cGycm2 ) | 397.62±380.81 | 1312.92±1129.28 | P<0.000 |
| RFA time (sec) | 482.80±534.12 | 779.76±620.82 | P=0.001 |
| Delivered radiofrequency energy (Ws) | 10866.84±6930.84 | 16393.56±13995.78 | P=0.048 |
Our study has shown that ICE-guidance during isthmus ablation reduces overall procedure time, radiation exposure and decreases needless radiofrequency delivery [56]. “We concluded that ICE-guided ablation of the CTI is not indicated routinely but in selected patients when the conventional approach has failed additional ICE-guidance permits 100% procedural success and becomes justified” [56].
CONCLUSIONS
ICE-guided ablation of CTI permits 100% acute success rate and low recurrence rate (1.96%). In comparison with angiography, 3D mapping systems, remote navigation tools and RT3DTEE, ICE-navigated ablation of CTI guarantees the best procedural parameters, namely: procedure time, fluoroscopy time, radiation exposure, RFA time and delivered radiofrequency energy. Different navigation tools have been introduced to facilitate ablation of typical Afl, to reduce the radiation exposure and procedure time [56]. ICE-guided ablation allowed greater reduction in radiation exposure in comparison to RNS or MNS and 3D mapping [8, 41, 42, 57]. In contrast to these systems, ICE-guided ablation of CTI resulted in a significant reduction of procedure and ablation time and markedly decreased the sum of delivered energy [56]. Radiation exposure and procedure time reduction were similar to that achieved by 3DTEE [43] but ICE was better in eliminating needless RF delivery [56]. We can conclude that ICE is an optimal imaging tool in cases of difficult CTI ablation.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
REFERENCES
- 1.Granada J, Uribe W, Chyou PH , et al. Incidence and predictors of atrial flutter in the general population. J Am Coll Cardiol. 2000;36:2242–6. doi: 10.1016/s0735-1097(00)00982-7. [DOI] [PubMed] [Google Scholar]
- 2.Babaev A, Suma V, Tita C, Steinberg JS. Recurrence rate of atrial flutter after initial presentation in patients on drug treatment. Am J Cardiol. 2003;92(9):1122–4. doi: 10.1016/j.amjcard.2003.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Natale A, Newby KH, Pisanó E , et al. Prospective randomized comparison of antiarrhythmic therapy versus first-line radiofrequency ablation in patients with atrial flutter. J Am Coll Cardiol. 2000;35(7):1898–904. doi: 10.1016/s0735-1097(00)00635-5. [DOI] [PubMed] [Google Scholar]
- 4.Saoudi N, Atallah G, Kirkorian G, Touboul P. Catheter ablation of the atrial myocardium in human type I atrial flutter. Circulation. 1990;81(3):762–71. doi: 10.1161/01.cir.81.3.762. [DOI] [PubMed] [Google Scholar]
- 5.Feld GK, Fleck RP, Chen PS , et al. Radiofrequency catheter ablation for the treatment of human type 1 atrial flutter.Identification of a critical zone in the reentrant circuit by endocardial mapping techniques. Circulation. 1992;86(4):1233–40. doi: 10.1161/01.cir.86.4.1233. [DOI] [PubMed] [Google Scholar]
- 6.Cosio FG, López-Gil M, Goicolea A, Arribas F, Barroso JL. Radiofrequency ablation of the inferior vena cava-tricuspid valve isthmus in common atrial flutter. Am J Cardiol. 1993;71(8):705–9. doi: 10.1016/0002-9149(93)91014-9. [DOI] [PubMed] [Google Scholar]
- 7.Da Costa A, Cucherat M, Pichon N , et al. Comparison of the efficacy of cooled-tip and 8 mm-tip catheters for radiofrequency catheter ablation of the cavo- tricuspid isthmus Meta-analysis. Pace. 2005;28:1081–7. doi: 10.1111/j.1540-8159.2005.00231.x. [DOI] [PubMed] [Google Scholar]
- 8.Hindricks G, Willems S, Kautzner J , et al. Effect of electroanatomically guided versus conventional catheter ablation of typical atrial flutter on the fluoroscopy time and resource use A prospective randomized multicenter study. J Cardiovasc Electrophysiol. 2009;20:734–40. doi: 10.1111/j.1540-8167.2009.01439.x. [DOI] [PubMed] [Google Scholar]
- 9.Calkins H, Canby R, Weiss R , et al. for the 100W Atakr II Investigator Group Results of catheter ablation of Typical atrial flutter. Am J Cardiol. 2004;94:437–42. doi: 10.1016/j.amjcard.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez M, Tercedor L, Herrera N , et al. Cavotricuspid isthmus catheter ablation without the use of fluoroscopy as a first-line treatment. J Cardiovasc Electrophysiol. 2011;22:656–62. doi: 10.1111/j.1540-8167.2010.01962.x. [DOI] [PubMed] [Google Scholar]
- 11.Chu E, Fitzpatrick AP, Chin MC, Sudhir K, Yock PG, Lesh MD. Radiofrequency Catheter Ablation Guided by Intracardiac Echocardiography. Circulation. 1994;89:1301–5. doi: 10.1161/01.cir.89.3.1301. [DOI] [PubMed] [Google Scholar]
- 12.Chu E, Kalman JM, Kwasman MA , et al. Intracardiac echocardiography during radiofrequency catheter ablation of cardiac arrhythmias in humans. JACC. 1994;42:1351–7. doi: 10.1016/0735-1097(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 13.Dravid SG, Hope B, McKinnie JJ. Intracardiac echocardiography in electrophysiology a review of current applications in practice. Echocardiography. 2008;25(10):1172–5. doi: 10.1111/j.1540-8175.2008.00784.x. [DOI] [PubMed] [Google Scholar]
- 14.Okishige K, Kawabata M, Yamashiro K , et al. Clinical study regarding the anatomical structures of the right atrial isthmus using intra-cardiac echocardiography Implication for catheter ablation of common atrial flutter. J Interv Card Electrophysiol. 2005;12:9–12. doi: 10.1007/s10840-005-5835-0. [DOI] [PubMed] [Google Scholar]
- 15.Okumura Y, Watanabe I, Ashino S , et al. Anatomical characteristics of the cavotricuspid isthmus in patients with and without typical atrial flutter Analysis with two and three-dimensional intracardiac echocardiography. J Interv Cardiac Electrophysiol. 2006;17:11–9. doi: 10.1007/s10840-006-9054-0. [DOI] [PubMed] [Google Scholar]
- 16.Scaglione M, Caponi D, Di Donna P , et al. Typical atrial flutter ablation outcome Correlation with isthmus anatomy using intracardiac echo 3D reconstruction. Europace. 2004;6:407–17. doi: 10.1016/j.eupc.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Okumura Y, Watanabe I, Ashino S , et al. Electrophysiologic and anatomical characteristics of the right atrial posterior wall in patients with and without atrial flutter: Analysis by intracardiac echocardiography. Circ J. 2007;71:636–42. doi: 10.1253/circj.71.636. [DOI] [PubMed] [Google Scholar]
- 18.Tsai CF, Tai CT, Yu WC , et al. Is 8-mm more effective than 4-mm tip electrode catheter for ablation of typical atrial flutterκ. Circulation. 1999;100(7):768–71. doi: 10.1161/01.cir.100.7.768. [DOI] [PubMed] [Google Scholar]
- 19.Feld G, Fujimura O, Green U, Mazzola F. Radiofrequency catheter ablation of human type 1 atrial flutter comparison of results with 8 mm versus 4 mm tip ablation catheter. J Am Coll Cardiol. 1995;25:169A. [Google Scholar]
- 20.Iesaka Y, Takahashi A, Goya M , et al. High energy radiofrequency catheter ablation for common atrial flutter targeting the isthmus between the inferior vena cava and tricuspid valve annulus using a super long tip electrode. Pace. 1998;21:401–9. doi: 10.1111/j.1540-8159.1998.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 21.Calkins H, Canby R, Weiss R , et al. Results of catheter ablation of Typical atrial flutter. Am J Cardiol. 2004;94:437–42. doi: 10.1016/j.amjcard.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 22.Schmieder S, Ndrepepa G, Dong J , et al. Acute and long-term results of radiofrequency ablation of common atrial flutter and the influence of the right atrial isthmus ablation on the occurrence of atrial fibrillation. Eur Heart J. 2003;24:956–62. doi: 10.1016/s0195-668x(02)00846-1. [DOI] [PubMed] [Google Scholar]
- 23.Jais P, Shah DC, Haissaguerre M , et al. Prospective randomized comparison of irrigated-tip versus conventional-tip catheters for ablation of common flutter. Circulation. 2000;101:772–6. doi: 10.1161/01.cir.101.7.772. [DOI] [PubMed] [Google Scholar]
- 24.Da Costa A, Cucherat M, Pichon N , et al. K Comparison of the efficacy of cooled-tip and 8 mm-tip catheters for radiofrequency catheter ablation of the cavo-tricuspid isthmus Meta-analysis. Pace. 2005;28:1081–7. doi: 10.1111/j.1540-8159.2005.00231.x. [DOI] [PubMed] [Google Scholar]
- 25.Da Costa A, Jamon Y, Romeyer-Bouchard C, Thévenin J, Messier M, Isaaz K. Catheter selection for ablation of the cavotricuspid isthmus for treatment of typical atrial flutter. J Interv Card Electrophysiol. 2006;17(2):93–101. doi: 10.1007/s10840-006-9064-y. [DOI] [PubMed] [Google Scholar]
- 26.Lewalter T, Weiss C, Spencker S , et al. Gold vs.platinum-iridium tip catheter for cavotricuspid isthmus ablation the AURUM 8study. Europace. 2011;13(1):102–8. doi: 10.1093/europace/euq339. [DOI] [PubMed] [Google Scholar]
- 27.Feld GK, Daubert JP, Weiss R, Miles WM, Pelkey W. Cryoablation Atrial Flutter Efficacy Trial Investigators.Acute and long-term efficacy and safety of catheter cryoablation of the cavotricuspid isthmus for treatment of type 1 atrial flutter. Heart Rhythm. 2008;5(7):1009–14. doi: 10.1016/j.hrthm.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Matsuo S, Yamane T, Tokuda M , et al. Prospective randomized comparison of a steerable versus a non-steerable sheath for typical atrial flutter ablation. Europace. 2010;12(3):402–9. doi: 10.1093/europace/eup434. [DOI] [PubMed] [Google Scholar]
- 29.Kirchhof P, Ozgün M, Zellerhoff S , et al. Diastolic isthmus length and 'vertical' isthmus angulation identify patients with difficult catheter ablation of typical atrial flutter a pre-procedural MRI study. Europace. 2009;11(1):42–7. doi: 10.1093/europace/eun308. [DOI] [PubMed] [Google Scholar]
- 30.Chen JY, Lin KH, Liou YM, Chang KC, Huang SK. Usefulness of pre-procedure cavotricuspid isthmus imaging by modified transthoracic echocardiography for predicting outcome of isthmus- dependent atrial flutter ablation. J Am Soc Echocardiogr. 2011;24(10):1148–55. doi: 10.1016/j.echo.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Knecht S, Castro-Rodriguez J, Verbeet T , et al. Multidetector 16-slice CT scan evaluation of cavotricuspid isthmus anatomy before radiofrequency ablation. J Interv Card Electrophysiol. 2007;20(1-2):29–35. doi: 10.1007/s10840-007-9159-0. [DOI] [PubMed] [Google Scholar]
- 32.Heidbüchel H, Willems R, Van Rensburg H, Adams J, Ector H, Van de Werf F. Right atrial angiographic evaluation of the posterior isthmus.Relevance for ablation of typical atrial flutter. Circulation. 2000;101:2178–84. doi: 10.1161/01.cir.101.18.2178. [DOI] [PubMed] [Google Scholar]
- 33.Cabrera JA, Sanchez-Quintana D, Ho SY, Medina A, Anderson RH. The architecture of the atrial musculature between the orifice of the inferior caval vein and the tricuspid valve The anatomy of the isthmus. J Cardiovasc Electrophysiol. 1998;9:1186–95. doi: 10.1111/j.1540-8167.1998.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z, Jorge A, Jo W , et al. Anatomic variability of the human eustachian ridge. Pacing Clin Electrophysiol. 1996;19(part II):724. [Google Scholar]
- 35.Da Costa A, Faure E, Thevenin J , et al. Effect of isthmus anatomy and ablation catheter on radiofrequency catheter ablation of the cavotricuspid isthmus. Circulation. 2004;110:1030–5. doi: 10.1161/01.CIR.0000139845.40818.75. [DOI] [PubMed] [Google Scholar]
- 36.Da Costa A, Romeyer-Bouchard C, Dauphinot V , et al. Cavotricuspid isthmus angiography predicts atrial flutter ablation efficacy in 281 patients randomized between 8 mm- and externally irrigated-tip catheter. Eur Heart J. 2006;27(15):1833–40. doi: 10.1093/eurheartj/ehl121. [DOI] [PubMed] [Google Scholar]
- 37.Kottkamp H, Hugl B, Krauss B, Wetzel U, Fleck A, Schuler G. Electromagnetic versus fluoroscopic mapping of the inferior isthmus for ablation of typical atrial flutter A prospective randomised study. Circulation. 2000;102:2082–6. doi: 10.1161/01.cir.102.17.2082. [DOI] [PubMed] [Google Scholar]
- 38.Hindricks G, Willems S, Kautzner J , et al. Effect of electroanatomically guided versus conventional catheter ablation of typical atrial flutter on the fluoroscopy time and resource use A prospective randomized multicenter study. J Cardiovasc Electrophysiol. 2009;20:734–40. doi: 10.1111/j.1540-8167.2009.01439.x. [DOI] [PubMed] [Google Scholar]
- 39.Álvarez M, Tercedor L, Herrera N, Muñoz L, Galdeano RS, Valverde F. Cavotricuspid isthmus catheter ablation without the use of fluoroscopy as a first-line treatment. J Cardiovasc Electrophysiol. 22(6):656–62. doi: 10.1111/j.1540-8167.2010.01962.x. [DOI] [PubMed] [Google Scholar]
- 40.Arya A, Kottkamp H, Piorkowski C , et al. Initial clinical experience with a remote magnetic catheter navigation system for ablation of cavotricuspid isthmus-dependent right atrial flutter. Pacing Clin Electrophysiol. 2008;31(5):597–603. doi: 10.1111/j.1540-8159.2008.01047.x. [DOI] [PubMed] [Google Scholar]
- 41.Vollmann D, Lüthje L, Seegers J, Hasenfuss G, Zabel M. Remote magnetic catheter navigation for cavotricuspid isthmus ablation in patients with common-type atrial flutter. Circ Arrhythm Electrophysiol. 2009;2(6):603–10. doi: 10.1161/CIRCEP.109.884411. [DOI] [PubMed] [Google Scholar]
- 42.Steven D, Rostock T, Servatius H , et al. Robotic versus conventional ablation for common-type atrial flutter a prospective randomized trial to evaluate the effectiveness of remote catheter navigation. Heart Rhythm. 2008;5(11):1556–60. doi: 10.1016/j.hrthm.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 43.Regoli F, Faletra FF, Nucifora G , et al. Feasibility and acute efficacy of radiofrequency ablation of cavotricuspid isthmus-dependent atrial flutter guided by real- time 3D TEE. JACC Cardiovasc Imaging. 2010;4:716–26. doi: 10.1016/j.jcmg.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 44.Redfearn DP, Skanes AC, Gula LJ, Krahn AD, Yee R, Klein GJ. Cavotricuspid isthmus conduction is dependent on underlying anatomic bundle architecture observations using a maximum voltage-guided ablation technique. J Cardiovasc Electrophysiol. 2006;17(8):832–8. doi: 10.1111/j.1540-8167.2006.00512.x. [DOI] [PubMed] [Google Scholar]
- 45.Gula LJ, Redfearn DP, Veenhuyzen GD , et al. Reduction in atrial flutter ablation time by targeting maximum voltage results of a prospective randomized clinical trial. J Cardiovasc Electrophysiol. 2009;20(10):1108–12. doi: 10.1111/j.1540-8167.2009.01511.x. [DOI] [PubMed] [Google Scholar]
- 46.Subbiah RN, Gula LJ, Krahn AD , et al. Rapid ablation for atrial flutter by targeting maximum voltagefactors associated with short ablation times. J Cardiovasc Electrophysiol. 2007;18:612–6. doi: 10.1111/j.1540-8167.2007.00804.x. [DOI] [PubMed] [Google Scholar]
- 47.Bauernfeind T, Kardos A, Foldesi C, Mihalcz A, Abraham P, Szili- Torok T. Assessment of the maximum voltage-guided technique for cavotricuspid isthmus ablation during ongoing atrial flutter. J Interv Card Electrophysiol. 2007;19:195–9. doi: 10.1007/s10840-007-9158-1. [DOI] [PubMed] [Google Scholar]
- 48.Posan E, Redfearn DP, Gula LJ , et al. Elimination of cavotricuspid isthmus conduction by a single ablation lesion Observations from a maximum voltage-guided ablation technique. Europace. 2007;9:208–11. doi: 10.1093/europace/eum014. [DOI] [PubMed] [Google Scholar]
- 49.Lewalter T, Lickfett L, Weiss C , et al. "Largest amplitude ablation" is the optimal approach for typical atrial flutter ablation a subanalysis from the AURUM 8 study. J Cardiovasc Electrophysiol. 2012;23(5):479–85. doi: 10.1111/j.1540-8167.2011.02252.x. [DOI] [PubMed] [Google Scholar]
- 50.Olgin JE, Kalman JM, Fitzpatrick AP, Lesh MD. Role of right atrial endocardial structures as barriers to conduction during human type I atrial flutter.Activation and entrainment mapping guided by intracardiac echocardiography. Circulation. 1995;92(7):1839–48. doi: 10.1161/01.cir.92.7.1839. [DOI] [PubMed] [Google Scholar]
- 51.Kalman JM, Olgin JE, Saxon LA, Fisher WG, Lee RJ, Lesh MD. Activation and entrainment mapping defines the tricuspid annulus as the anterior barrier in typical atrial flutter. Circulation. 1996;94(3):398–406. doi: 10.1161/01.cir.94.3.398. [DOI] [PubMed] [Google Scholar]
- 52.Darbar D, Olgin JE, Miller JM, Friedman PA. Localization of the origin of arrhythmias for ablation from Electrocardiography to advanced endocardial mapping systems. J Cardiovasc Electrophysiol. 2001;12(11):1309–25. doi: 10.1046/j.1540-8167.2001.01309.x. [DOI] [PubMed] [Google Scholar]
- 53.Morton JB, Sanders P, Davidson NC, Sparks PB, Vohra JK, Kalman JM. Phased- array intracardiac echocardiography for defining cavotricuspid isthmus anatomy during radiofrequency ablation of typical atrial flutter. J Cardiovasc Electrophysiol. 2003;14:591–7. doi: 10.1046/j.1540-8167.2003.02152.x. [DOI] [PubMed] [Google Scholar]
- 54.Pap R, Klausz G, Gallardo R, Sághy L. Intracardiac echocardiography in a case with previous failed cavotricuspid isthmus ablation. J Interv Card Electrophysiol. 2009;26:119–20. doi: 10.1007/s10840-009-9399-2. [DOI] [PubMed] [Google Scholar]
- 55.Bencsik G, Pap R, Sághy L. Intracardiac echocardiography for visualization of the Eustachian valve during radiofrequency ablation of typical atrial flutter. Europace. 2009;11:901. doi: 10.1093/europace/eup123. [DOI] [PubMed] [Google Scholar]
- 56.Bencsik G, Pap R, Makai A , et al. Randomized trial of intracardiac echocardiography during cavotricuspid isthmus ablation. J Cardiovasc Electrophysiol. 2012;23(9):996–1000. doi: 10.1111/j.1540-8167.2012.02331.x. [DOI] [PubMed] [Google Scholar]
- 57.Ilg KL, Kuhne M, Crawford T , et al. Randomized comparison of cavotricuspid isthmus ablation for atrial flutter using an open irrigationtip versus a large-tip radiofrequency ablation catheter. J Cardiovasc Electrophysiol. 2011;22:1007–12. doi: 10.1111/j.1540-8167.2011.02045.x. [DOI] [PubMed] [Google Scholar]