Abstract
Atrial tachycardias are common after open heart surgery. Most commonly these are macro-reentrant including cavotricuspid isthmus dependent atrial flutter, incisional right atrial flutter and left atrial flutter. Focal atrial tachycardias occur less frequently. The specific type of atrial tachycardia highly depends on the type of surgical incision. Catheter ablation can be very effective, however requires a thorough understanding of anatomy and surgical technique.
Keywords: Atrial tachycardia, complex congenital syndromes, electroanatomical mapping, entrainment, re-entrant tachycardia, post-operative period.
INTRODUCTION
Atrial tachycardia (AT) occurs frequently after surgical repair of acquired and congenital heart disease [1]. Operations for acquired valvular and ischemic heart disease, tumors of the heart or congenital abnormalities, like transposition of the great arteries (TGA), ventricular septal defect (VSD), atrial septal defect (ASD), tetralogy of Fallot (TOF) and Ebstein’s anomaly involve atrial incisions to establish extracorporeal circulation and approach the intracardiac structures. Together with structural and hemodynamic changes resulting from the underlying heart disease, these atriotomies create a substrate for AT. Early postoperative atrial arrhythmias often occur due to the acute effect of cardiac surgery, such as myocardial ischemia, pericarditis and incisional trauma [2, 3]. On the other hand, late arrhytmias relate to chronic, post-operative myocardial processes. However in most reports, there is no clear distinction between regular AT and atrial fibrillation (AF), although their pathophysiology and therapy are fundamentally different.
METHODS
This review is based on a nonsystematic literature search using the Medline database, on the incidence, mechanism and therapy of ATs occurring late after open heart surgery.
INCIDENCE OF POSTOPERATIVE ATRIAL TACHYCARDIAS
Atrial fibrillation is the most common atrial arrhythmia after open heart surgery, but the literature on the incidence of atrial arrhythmias in the postoperative period does not exactly distinguish between AF and AT. The reported incidence of these arrhythmias has varied very widely between 10%-80%, in part because of the different modes of arrhythmia detection and different definitions of these arrhythmias [1]. Most commonly, the early postoperative atrial arrhythmias are transient and often less symptomatic, although in some patients may cause hypotension, congestive heart failure or lengthen the hospitalization. Individual reports trying to identify the incidence of early post-operative AT (and not AF) have shown an incidence of 17-35% [4, 5]. However data are even more scarce on AT occurring years or even decades after surgery, such long follow up being rarely available. Nevertheless AF or AT occurred in 33% of patients during a follow up of three decades after ASD repair [6] and in more than 50% of survivors to 20 years after the Senning operation for TGA [7] and up to 30% of patients after Mustard operation [8]. Atrial flutter (AFL) occurred in up to 30% of patients after the Fontan operation [9].
MECHANISM OF ATRIAL TACHYCARDIAS RELATED TO OPEN HEART SURGERY
Surgical incisions applied to the atria create an anatomical substrate for arrhythmias. Besides the iatrogenic scar, many authors tried to evaluate other risk factors for post-operative arrhythmias. The most commonly identified risk factor has been increasing patient age [1]. With aging the fibrous and adipose tissue increases and this process may have a role in the development of atrial arrhythmias during the post-operative period. Structural heart diseases, such as rheumatic heart disease and also chronic obstructive pulmonary disease increase the risk of developing post-operative ATs [1].
TYPE OF AT/AFL
The arrhytmogenic substrate in patients with prior surgical lesions can be very complex and the location of these tachycardias very diverse due to individual differences in anatomy, surgical correction and effects of atrial fibrosis. The understanding of the mechanism of atrial arrhythmias is essential for successful curative therapy. Atrial tachycardias can be grouped according to the pattern of atrial activation as macroreentrant or focal, although the underlying electrophysiological mechanism is probably reentry in the latter also. Focal ATs are characterized by an atrial activation spreading radially from a small focus. The mechanism of macroreentrant ATs is re-entrant activation around a large central obstacle. Atrial macro-reentry (flutter) is far more common than focal AT late after surgery. In a series of 100 patients, we found that 93% of late postoperative ATs were diagnosed to be flutter and 7% focal AT [5]. The central obstacle of macroreentry can be an anatomical structure (eg. atrioventricular valves, pulmonary veins) or scar tissue created surgically (eg. atriotomy, prosthetic patch) or by the underlying disease (eg. ischemia). Complex and multiple reentry circuits can develop after placement of an intra-atrial baffle, such as in the Mustard and Senning procedure and in a very dilated right atrium, such as after Fontan or Maze procedure. With Senning operation, an atrial conduit, atrial baffle is created from atrial tissue within the atrium which reroutes the systemic venous blood from the venae cavae towards the mitral valve. In the Mustard procedure this atrial baffle is created from pericardial or artificial tissue. The Fontan procedure is a palliative treatment for tricuspid or pulmonary atresia, hypoplastic left or right heart syndrome where intra- or extracardiac cavopulmonary connection is established.
CAVOTRICUSPID-ISTHMUS DEPENDENT POST-OPERATIVE ATRIAL FLUTTER
Functional or fixed block created by the underlying heart disease or the surgical lesions is responsible for this arrhythmia after heart surgery. Many studies have found the major role of CTI in postoperative AFL mechanism, however some studies have shown that incisional tachycardia is the most frequent depending on a scar created by an atrial incision. In one study [10], CTI-dependent AFL was the most common in the congenital heart disease group, while right atrial (RA) incisional tachycardia was more frequent in a group of patients with acquired heart disease [11, 12]. In contrast in another study, reentry related to the lateral atriotomy was the most common circuit in patients with congenital heart disease [13]. According to our results, CTI dependent AFL is the most common AT after open heart surgery both in patients with acquired and congenital heart disease [5]. It is also the leading AT mechanism after Mustard or Senning atrial switch operations [14].
RIGHT ATRIAL INCISIONAL FLUTTER
While a barrier between the venae cavae supports the development of CTI dependent AFL, a longer lesion on the RA free wall may also predispose to incisional tachycardia [11, 12]. The extent and position of the lesion seem to be some of the important factors. This AT is less common among patients who undergo venous cannulation only (at the RA appendage) compared to those who have long free wall atriotomy (15% vs 43%) [5] (Fig. 1). According to our and other studies’ results, incisional AT was even more common when the RA free wall atriotomy was prolonged to the septum during transseptal access to the left atrium (LA) [5, 13]. The longer incision may support peri-lesional AFL by a long path length of reentry and/or the development of slow conduction at sites of reconnection along the long lesion or in the corridor between the incision and the tricuspid annulus [4]. The wavefront propagates through the isthmus between the incision and the inferior caval vein, or a channel of reconnection along the lesion, but always uses at least some part of the corridor between the incision and the tricuspid annulus. Reconnection could be occurring anywhere along the line thereby connecting the two sides of the incision and closing the reentrant circuit.
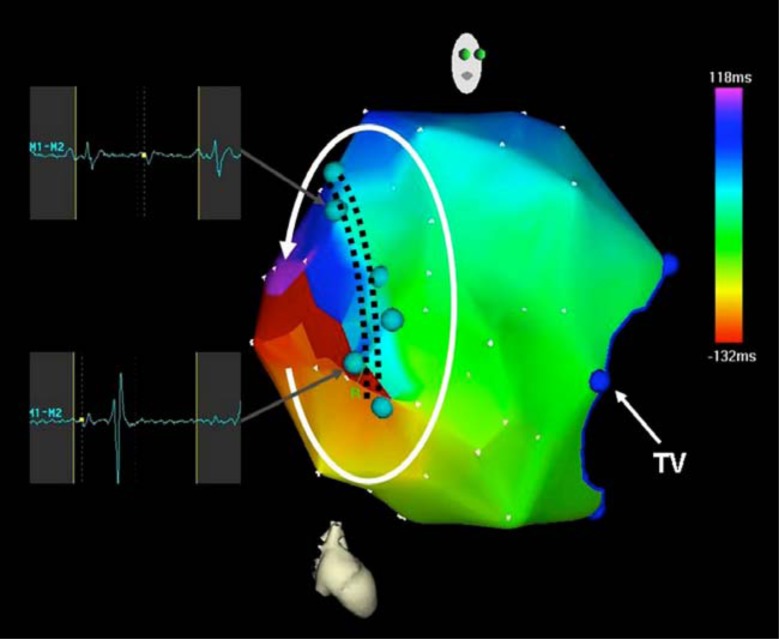
Fig. (1).
Electroanatomic activation map of the right atrium during incisional tachycardia. The atriotomy is marked by light blue dots. Activation proceeds in the counterclockwise direction around the atriotomy. Dark blue dots mark the tricuspid valve.
LEFT ATRIAL REENTRANT TACHYCARDIAS
Left atrial (LA) flutter is much less common than right atrial flutter, nevertheless LA AFL is more common in patients with, compared to those without a history of heart surgery [15]. Similarly to peritricuspid flutter after RA incision, perimitral flutter can occur after LA incision (Fig. 2). However according to our and other studies’ results, roof dependent LA AFL around pulmonary veins also occurred in patients with surgical LA approach [5, 15, 16].
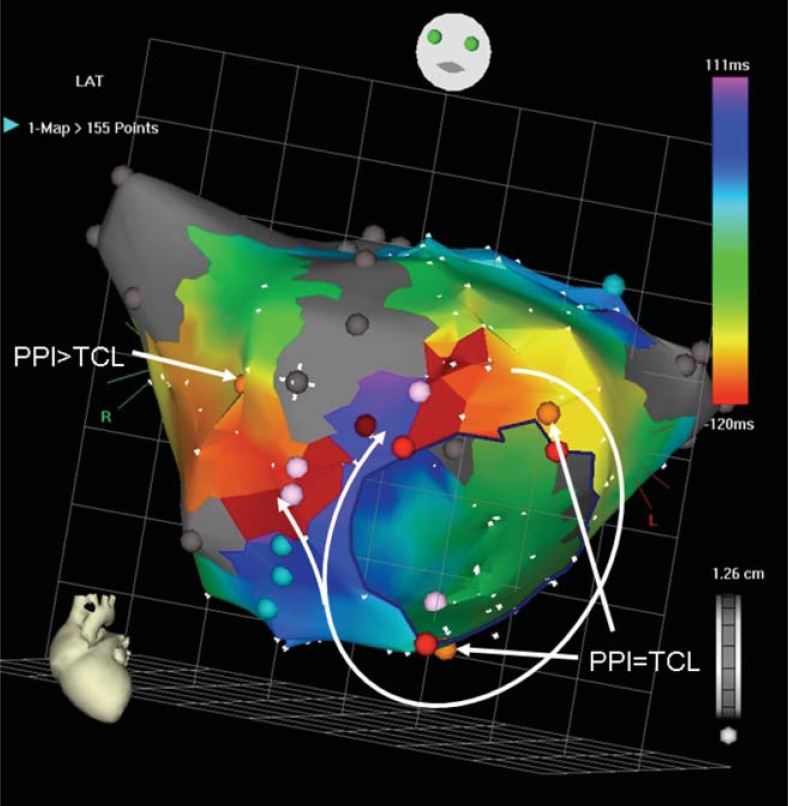
Fig. (2).
Perimitral macroreentry after direct left atriotomy. Activation proceeds in the clockwise direction around the mitral annulus and also through a reconnection in the atriotomy line. The circuit uses a narrow isthmus between the scar and the mitral annulus, where radiofrequency pulse (dark red dot) terminated the tachycardia. Orange dots represent pacing sites, light blue dots mark double potentials, grey dots electrically silent areas, and pink dots represent fragmented signals. Light red dots mark the mitral annulus. PPI post-pacing interval after entrainment, TCL tachycardia cycle length.
FOCAL ATRIAL TACHYCARDIAS
Focal atrial tachycardia is much less common later after heart surgery [5, 17, 18] Similar to patients without heart surgery it often originates from predilection sites, like the crista terminalis, the atrioventricular rings and pulmonary veins [19]. However focal AT originating from around surgical scar (eg. atritomy, patch) is also observed [5]. Localized (micro) reentry is the most probable mechanism of postoperative focal AT, as evidenced by long, fractionated electrograms usually recorded at the site of origin.
DETERMINANTS OF THE TYPE OF AT
In the largest reported series of postoperative AFL to date, Aktas et al. found that the indication for surgery (valve- or nonvalve-related) did not influence the type of AFL. We studied the association between atrial incisions and type of AFL and have found that the mode of atriotomy was the only independent predictor that determined the mechanism and location of postoperative atrial tachycardia. Demographic and clinical parameters, like hypertension, congenital heart disease, ischemic heart disease, valve surgery and preprocedural echocardiographic dimensions were not associated with the specific type of AT [5].
THERAPY
Drug Therapy
The value of pharmacological therapy for the treatment of AT is limited and accompanied by side effects. Some studies compared the effectiveness of ablative versus pharmacological therapy and showed that treating post-operative ATs with drugs is less successful than catheter ablation [21]. Beta-blockers, digoxin and calcium- channel blockers might be used to slow the ventricular rate, Class III antiarrhytmic drugs might be used to convert arrhythmias to sinus rhythm; however the majority of these studies have evaluated the effect of these drugs in the early post-operative period [22, 23].
Ablative Therapy
Recently, catheter ablation has evolved as a feasible curative treatment modality for post-operative tachycardias. Various right and left atrial macro-reentrant circuits and focal ATs can be treated by radiofrequency catheter ablation.
Conventional activation and entrainment mapping with or without electroanatomic mapping are used to determine the mechanism of spontaneous or induced ATs [20]. Lines of block due to surgical incision are reflected by recording double potentials due to sequential activation on both sides of the line of block. Fractionated electrograms may be recorded at sites of local conduction disturbances or slow conduction. Voltage mapping is a useful tool to determine the substrate in operated patients. Areas of low amplitude or absent electrograms represent scar.
One study showed the incremental value of an electroanatomical mapping system over conventional mapping on the outcome of ablation of post-operative atrial tachycardias [3]. However in some studies, especially in patients who underwent atrial baffle operation, almost one third of the ATs could not be terminated despite using a 3D mapping system. The complexity of the reentrant circuit is associated with the complexity of the congenital heart disease and corresponding extensiveness of surgical lesions.
Cavotricuspid isthmus (CTI) dependent atrial flutter is the most common circuit in all patients with late postoperative AT. Therefore the first step of the procedure should be to test for this flutter by entrainment from the CTI. This arrhythmia can be treated by conventional linear ablation of the CTI, with the aim of termination of the arrhythmia and establishment of bidirectional conduction block of the CTI. In case of a non-CTI dependent circuit limited entrainment at sites accessible from the RA (coronary sinus, septum, free wall of RA) can determine the chamber of origin of the tachycardia [24]. However it should be carefully executed since termination or transformation of the AT is common. Activation mapping of the chamber of origin using an electroanatomic mapping system, combined with entrainment is useful in non-CTI dependent AT. In macroreentrant AT it is usually possible to map more than 80% of the cycle length in the chamber of origin, and an “early-meets-late” circular activation. An “in circuit” response to entrainment from remote sites further supports macroreentry. Focal AT on the other hand is characterized by radial activation and shorter atrial activation time, but significant conduction slowing in the atria is a caveat.
In the RA the most common non-CTI dependent circuit is related to the free wall atriotomy (Fig. 1) [5]. During the transseptal surgical approach to the left atrium this atriotomy is prolonged onto the septum and therefore part of the reentrant circuit can involve the septum also [25]. Over the years reconnection can appear in the atriotomy line bridging the two sites therefore the exact location of the circuit can vary from low RA free wall to the septum. Catheter ablation of isolated channels of reconnection or a narrow corridor between the atriotomy and the annulus allows for focal ablation [2, 25]. Otherwise the arrhythmia can be terminated by a linear lesion connecting the incision to another barrier: the inferior vena cava or tricuspid annulus. [26] Demonstration of bidirectional conduction block over this linear lesion is key to long term success [26]. Dual loop or figure-of-eight AT is quite common after RA atriotomy according to some reports [27, 28] (Fig. 3). In such a case there is a peritricuspid circuit in either clockwise or anticlockwise direction and simultaneously a peri-incisional circuit in the reverse direction. During ablation of the CTI the tachycardia continues with the same cycle length, but different activation sequence despite achievement of CTI block. The peri-incisional loop is then ablated separately as discussed above.
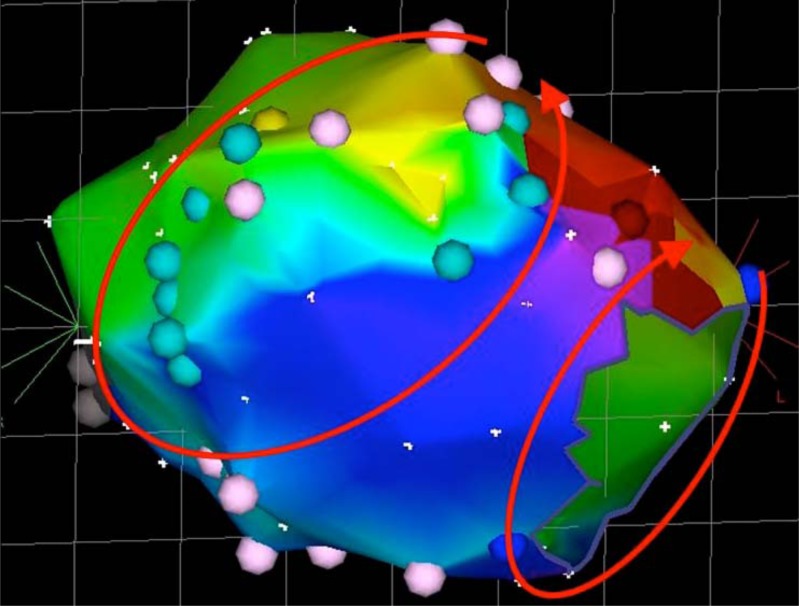
Fig. (3).
Dual loop reentry tachycardia. Both circuits (peritricuspid and peri-incisional) share a common isthmus, with the two wavefronts merging and forming a common wavefront that propagates between the atriotomy scar and the tricuspid annulus.
Various flutter circuits can develop in the left atrium (LA) after heart surgery. Surgical or spontaneous scar - the former involving mostly the septum, the latter the posterior wall – participates in the mechanism. The most common circuit in the LA is that of a perimitral flutter. This can be approached by ablation of the mitral isthmus, between left sided pulmonary veins (PVs) and the mitral annulus, posterior to the LA appendage. However this is a difficult line to complete, up to 50% of cases requiring epicardial ablation from the coronary sinus [5]. An alternative line can therefore be recommended in patients with anteroseptal scar from the surgical approach to the LA. Sometimes only a narrow channel is conducting between this scar and the mitral annulus allowing for minimal, or even focal ablation of the flutter circuit (Fig. 2) [5]. Roof dependent LA flutter circuits around right or left PVs usually incorporating surgical or spontaneous scar can be ablated by creating a roof line between right and left PVs, or closing small channels in scar (Fig. 4). Focal AT after heart surgery can be highly successfully ablated by mapping earliest endocardial activation in the relevant chamber [5].
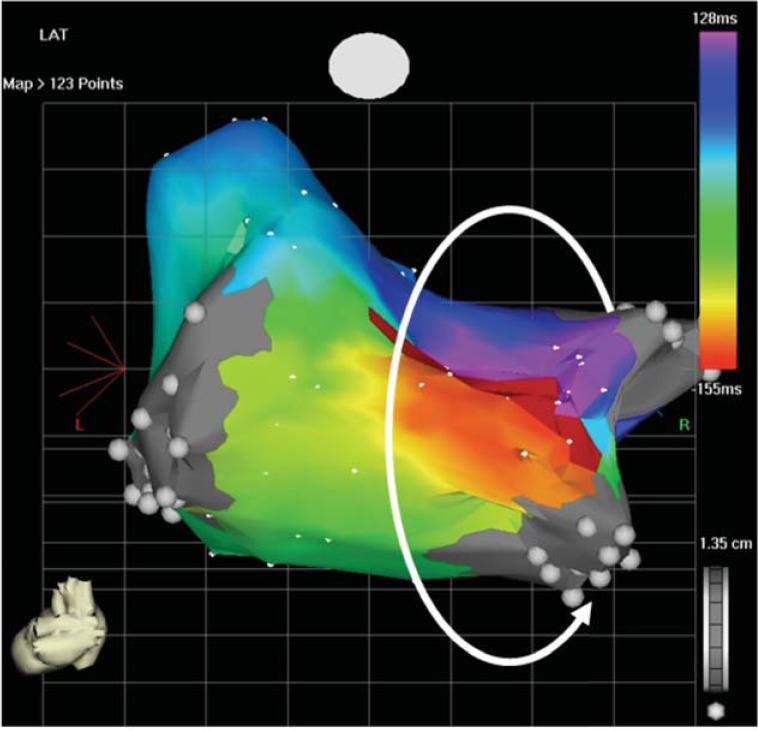
Fig. (4).
Roof dependent left atrial tachycardia after direct left atriotomy. The wavefront propagates around the right pulmonary veins.
MAPPING AND ABLATION OF AT IN COMPLEX CONGENITAL HEART DISEASE
Preprocedural planning in patients undergoing catheter ablation of AT after complex corrective surgery due to congenital heart disease requires accurate understanding of the anatomy. After the Senning or Mustard procedure the systemic venous atrium is approachable from the femoral venous site, except in cases with vascular obstruction from prior interventions or venous cut-down. In this scenario percutaneous hepatic venous access can be obtained under fluoroscopic and ultrasound guidance [29]. Ablation [30] of the most common peritricuspid flutter circuit is hampered by the tricuspid annulus located on the pulmonary venous side of the baffle. Thereby the CTI is divided into a systemic (at the inferior caval vein) and a pulmonary venous part. If the reentrant tachycardia is not terminated by ablation on the systemic venous portion of the isthmus - as usually is the case - it is recquired to access the pulmonary venous atrium. Rarely it can be achieved through spontaneous baffle leaks. Other options include a retrograde femoral approach or transbaffle puncture (Fig. 5). Similarly access to the RA is limited in patients after Fontan operation and a transconduit puncture technique has been described [31].
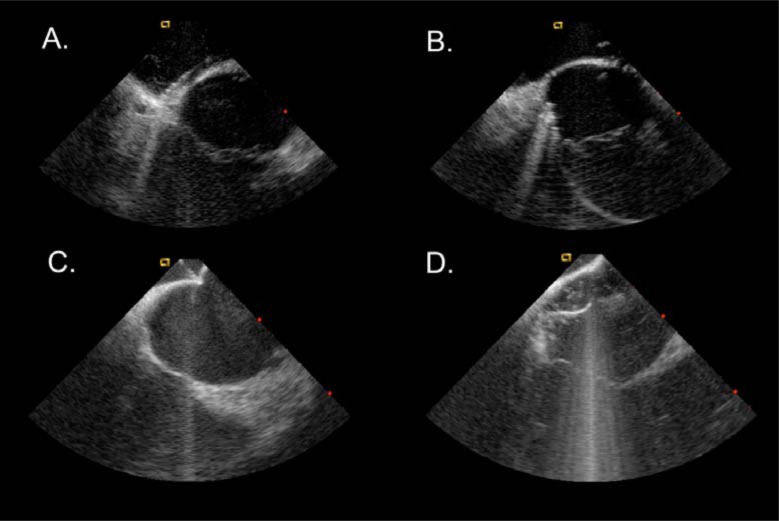
Fig. (5).
Intracardiac echocardiography images during electrophysiology study in patients after Senning procedures. A. mapping catheter on the systemic venous side of the cavotricuspid isthmus. B. mapping catheter on the pulmonary venous side of the isthmus. C. transbaffle puncture. D. mapping catheter in the pulmonary venous atrium after transbaffle puncture.
FUTURE PERSPECTIVE
Knowledge of the mechanisms leading to postoperative ATs should inspire modifications of the surgical technique that might be effective against the future occurrence of AT in these patients. Avoiding the long RA atriotomy, or on the contrary prolonging it down to the inferior vena cava could prevent RA incisional tachycardia. For example avoiding RA incision by using direct LA atriotomy instead of the transseptal approach has been shown to be associated with less frequent occurrence of AFL [13].
CONCLUSION
Atrial lesions applied during open heart surgery, together with patient specific factors, result in a high incidence of late postoperative AT. Cavotricuspid isthmus dependent AFL is the most common mechanism. The occurrence of right atrial incisional and left atrial AFL is determined by the specific surgical approach. Cardiac surgery due to congenital heart disease can result in a complex anatomic situation. We have to emphasize the importance of reviewing the surgical records and refining the specific anatomical approach, since different lesions can be the source of different ATs.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56(3):539–49. doi: 10.1016/0003-4975(93)90894-n. [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa H, Shah N, Matsudaira K , et al. Characterization of Reentrant Circuit in Macroreentrant Right Atrial Tachycardia After Surgical Repair of Congenital Heart Diseaseκ: Isolated Channels Between Scars Allow “Focal” Ablation. Circulation. 2001;103(5):699–709. doi: 10.1161/01.cir.103.5.699. [DOI] [PubMed] [Google Scholar]
- 3.Delacretaz E, Ganz LI, Soejima K , et al. Multiple atrial macro-re-entry circuits in adults with repaired congenital heart disease entrainment mapping combined with three-dimensional electroanatomic mapping. J Am Coll Cardiol. 2001;37(6):1665–76. doi: 10.1016/s0735-1097(01)01192-5. [DOI] [PubMed] [Google Scholar]
- 4.Lukac P, Hjortdal VE, Pedersen AK, Mortensen PT, Jensen HK, Hansen PS. Atrial Incision Affects the Incidence of Atrial Tachycardia After Mitral Valve Surgery. Ann Thorac Surg. 2006;81:509–13. doi: 10.1016/j.athoracsur.2005.07.083. [DOI] [PubMed] [Google Scholar]
- 5.Pap R, Kohári M, Makai A , et al. Surgical technique and the mechanism of atrial tachycardia late after open heart surgery. J Interv Card Electr. 2012;35(2):127–35. doi: 10.1007/s10840-012-9705-2. [DOI] [PubMed] [Google Scholar]
- 6.Murphy JG, Gersch BJ, Phil D , et al. Long-term outcome after surgical repair of isolated atrial septal defect. N Engl J Med. 1990;323:1645–50. doi: 10.1056/NEJM199012133232401. [DOI] [PubMed] [Google Scholar]
- 7.Roubertie F, Thambo J-B, Bretonneau A , et al. Late outcome of 132 Senning procedures after 20 years of follow-up. Ann Thorac Surg. 2011;92(6):2206–13. doi: 10.1016/j.athoracsur.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Duster MC, Bink-Boelkens MT, Wampler D, Gillette PC, McNamara DG, Cooley DA. Long-term follow-up of dysrhythmias following the Mustard procedure. Am Heart J. 1985;109:1323. doi: 10.1016/0002-8703(85)90359-x. [DOI] [PubMed] [Google Scholar]
- 9.Garson A, Bink-Boelkens M, Hesslein PS , et al. Atrial flutter in the young a collaborative study of 380 cases. J Am Coll Cardiol. 1985;6(4):871–8. doi: 10.1016/s0735-1097(85)80497-6. [DOI] [PubMed] [Google Scholar]
- 10.Anné W, Van Rensburg H, Adams J, Ector H, Van de Werf F, Heidbüchel H. Ablation of post-surgical intra-atrial reentrant tachycardia.Predilection target sites and mapping approach. Eur Heart J. 2002;23(20):1609–16. doi: 10.1053/euhj.2002.3168. [DOI] [PubMed] [Google Scholar]
- 11.Tomita Y, Matsuo K, Sahadevan J, Khrestian CM, Waldo AL. Role of Functional Block Extension in Lesion-Related Atrial Flutter. Circulation. 2001;103:1025–30. doi: 10.1161/01.cir.103.7.1025. [DOI] [PubMed] [Google Scholar]
- 12.Waldo AL. Mechanisms of atrial flutter and atrial fibrillationκ: distinct entities or two sides of a coinκ. Cardiovasc Res. 2002;54:217–29. doi: 10.1016/s0008-6363(01)00549-1. [DOI] [PubMed] [Google Scholar]
- 13.Lukac P, Hjortdal V, Pedersen AK, Jensen HK, Mortensen PT, Hansen PS. The Superior Transseptal Surgical Approach to Mitral Valve Creates Slow Conduction. Pace. 2006;29:719–26. doi: 10.1111/j.1540-8159.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 14.Khairy P, Van Hare GF. Catheter ablation in transposition of the great arteries with Mustard or Senning baffles. Heart Rhythm. 2009;6(2):283–9. doi: 10.1016/j.hrthm.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 15.Aktas MK, Khan MN, Di Biase L , et al. Higher rate of recurrent atrial flutter and atrial fibrillation following atrial flutter ablation after cardiac surgery. J Cardiovasc Electr. 2010;21(7):760–5. doi: 10.1111/j.1540-8167.2009.01709.x. [DOI] [PubMed] [Google Scholar]
- 16.Jaïs P, Shah DC, Haïssaguerre M , et al. Mapping and Ablation of Left Atrial Flutters. Circulation. 2000;101:2928–34. doi: 10.1161/01.cir.101.25.2928. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang F, Ernst S, Vogtmann T , et al. Characterization of Reentrant Circuits in Left Atrial Macroreentrant Tachycardia Critical Isthmus Block Can Prevent Atrial Tachycardia Recurrence. Circulation. 2002;105:1934,–42. doi: 10.1161/01.cir.0000015077.12680.2e. [DOI] [PubMed] [Google Scholar]
- 18.De Groot NMS, Zeppenfeld K, Wijffels MC , et al. Ablation of focal atrial arrhythmia in patients with congenital heart defects after surgery role of circumscribed areas with heterogeneous conduction. Heart Rhythm. 2006;3(5):526–35. doi: 10.1016/j.hrthm.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Chen S, Tai C, Chiang C, Ding Y, Chang M. Focal Atrial Tachycardiaκ: Reanalysis of the Clinical and Electrophysiologic Characteristics and Prediction of Successful Radiofrequency Ablation. J Cardiovasc Electr. 1998;9:355–65. doi: 10.1111/j.1540-8167.1998.tb00924.x. [DOI] [PubMed] [Google Scholar]
- 20.Lukac P, Pedersen AK, Mortensen PT, Jensen HK, Hjortdal V, Hansen PS. Ablation of atrial tachycardia after surgery for congenital and acquired heart disease using an electroanatomic mapping system Which circuits to expect in which substrateκ. Heart Rhythm. 2005;2(1):64–72. doi: 10.1016/j.hrthm.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 21.Natale A, Newby KH, Pisanó E , et al. Prospective randomized comparison of antiarrhythmic therapy versus first-line radiofrequency ablation in patients with atrial flutter. J Am Coll Cardiol. 2000;35(7):1898–904. doi: 10.1016/s0735-1097(00)00635-5. [DOI] [PubMed] [Google Scholar]
- 22.Babaev A, Suma V, Tita C, Steinberg JS. Recurrence Rate of Atrial Flutter After Initial Presentation in Patients on Drug Treatment. Am J Cardiol. 2003;92:1122–4. doi: 10.1016/j.amjcard.2003.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Gavaghan TP, Koegh AM, Kelly RP, Campbell TJ, Thorburn C, Morgan JJ. Flecainide compared with a combination of digoxin and disopyramide for acute atrial arrhythmias after cardiopulmonary bypass. Br Heart J. 1988;60(6):497–501. doi: 10.1136/hrt.60.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazaki H, Stevenson WG, Stephenson K, Soejima K, Epstein LM. Entrainment mapping for rapid distinction of left and right atrial tachycardias. Heart Rhythm. 2006;3:516–23. doi: 10.1016/j.hrthm.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Roberts-Thomson KC, Kalman JM. Right septal macroreentrant tachycardia late after mitral valve repair Importance of surgical access approach. Heart Rhythm. 2007;4:32–6. doi: 10.1016/j.hrthm.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 26.Snowdon RL, Balasubramaniam R, Teh AW , et al. Linear Ablation of Right Atrial Free Wall Flutterκ: Demonstration of Bidirectional Conduction Block as an Endpoint Associated With Long-Term Success. J Cardiovasc Electr. 2010;21:526–31. doi: 10.1111/j.1540-8167.2009.01660.x. [DOI] [PubMed] [Google Scholar]
- 27.Magnin-Poull I, Chillou CDe, Miljoen H, Andronache M, Aliot E. Mechanisms of Right Atrial Tachycardia Occurring Late After Surgical Closure of Atrial Septal Defects. J Cardiovasc Electr. 2005;16:681–7. doi: 10.1046/j.1540-8167.2005.30605.x. [DOI] [PubMed] [Google Scholar]
- 28.Seiler J, Schmid DK, Irtel TA , et al. Dual-loop circuits in postoperative atrial macro re-entrant tachycardias. Heart. 2007;93:325–30. doi: 10.1136/hrt.2006.094748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh SM, Neuzil P, Skoka J , et al. Percutaneous transhepatic venous access for catheter ablation procedures in patients with interruption of the inferior vena cava. Circ Arrhythm Electrophysiol. 2011;4:235–41. doi: 10.1161/CIRCEP.110.960856. [DOI] [PubMed] [Google Scholar]
- 30.De Groot NMS, Atary JZ, Blom NA, Schalij MJ. Long-term outcome after ablative therapy of postoperative atrial tachyarrhythmia in patients with congenital heart disease and characteristics of atrial tachyarrhythmia recurrences. Circ Arrhythm Electrophysiol. 2010;3:148–54. doi: 10.1161/CIRCEP.109.909838. [DOI] [PubMed] [Google Scholar]
- 31.Dave AS, Aboulhosn J, Child JS, Shivkumar K. Transconduit puncture for catheter ablation of atrial tachycardia in a patient with extra-cardiac Fontan palliation. Heart Rhythm. 2010;7(3):413–6. doi: 10.1016/j.hrthm.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]