Abstract
The traditional transvenous defibrillator has been one of the greatest advancement in Cardiology in the last 30 years and has demonstrated to reduce arrhythmic and total mortality in selected patients. However the traditional defibrillator can have a high price to pay in terms of complications, the “weakest link” being the transvenous/endocardial leads. The entirely subcutaneous defibrillator (S-ICD) has recently entered into the clinical scenario and represents a valid alternative to the transvenous device. S-ICD can provide substantial advantages, especially among some subgroups of patients (i.e. after device infection, in young patients and arrhythmogenic syndromes). However, given its characteristics, it is fundamental to choose patients that can benefit the most. In this review we will describe advantages and limitations of the S-ICD and point-out how to select the “ideal candidate” for the implantation.
Keywords: Subcutaneous defibrillator, sudden cardiac death, ventricular arrhythmias.
INTRODUCTION
The implantable cardioverter-defibrillator (ICD) has been a major advancement in the hard battle against sudden cardiac death (SCD), with extensive and secure evidence supporting its use [1, 2]. Since the first human implantation in 1980 we have observed a striking evolution of these devices, from surgically-applied epicardial patches with a big abdominally-placed generator to miniaturized pectoral systems capable not only of defibrillation but also of pacing and cardiac resynchronization and with leads inserted into venous circulation through cephalic, axillary or subclavian veins. During the years from nineties to our days several randomized trials have demonstrated a reduction in total mortality with the use of ICD both in primary and secondary prevention [3]. However several issues with conventional transvenous devices have emerged, blunting enthusiasm with their extensive use. The most obvious problem is the need to insert the defibrillation electrode into the central venous circulation and to place it inside cardiac chambers: so a number of complications can occur (vascular obstruction, thrombosis, infection, cardiac perforation), sometimes with catastrophic consequences [4, 5]. Transvenous leads are the “weakest link” in ICD therapy [6, 7] and their performance over time is a serious concern, with some leads showing reduced survival that has led to recalls and withdrawal from the market. The potential for serious adverse events with transvenous lead system is substantial: lead failure has been estimated 0.58%/year and up to 20% at 10 years [8]. “When lead failures occur, transvenous lead extraction may be necessary: this procedure is highly challenging with major complications rates of about 1% and a mortality risk of 0.3% also in experienced centers” [6-9]. In the context of this clinical background the entirely subcutaneous defibrillator (S-ICD) has entered into the clinical scenario [10, 11].
S-ICD: AN OVERVIEW
Description of the System, Developments and Approval
S-ICD system consists of a 3-mm tripolar parasternal lead (12 French, 45 cm length) which is connected to an electrically active pulse generator. “The lead is vertically positioned in the subcutaneous tissue of the chest, parallel to and 1-2 cm to the left sternal midline and then makes a curve followed by a horizontal segment, at the level of the 6th rib, until it reaches the left anterior axillary line. The electrode has a 8-cm shock coil, flanked by two sensing electrodes: the distal sensing electrode is positioned adjacent to manubriosternal junction and the proximal sensing electrode is adjacent to the xiphoid process” [9]. The pulse generator is bigger (about the double in size) than “traditional” ICD: 78x 65x 15 mm with a volume of 69 cc and a mass of 145 grams; estimated longevity is 5 years (defined as 3 full-energy capacitor charges per year). The generator is positioned in the subcutaneous tissue of the chest over the 6th rib between the left midaxillary and left anterior axillary line (Figs.1 and 2).
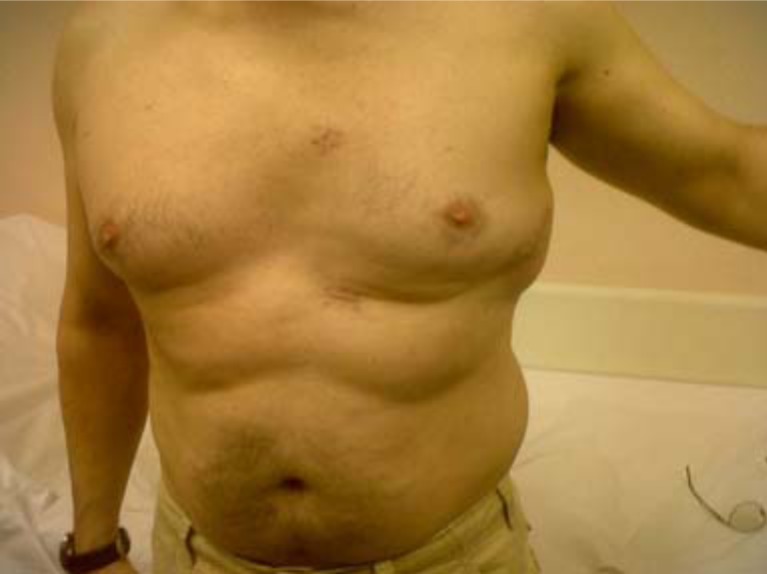
Fig. (1).
External appearance of an implanted device.
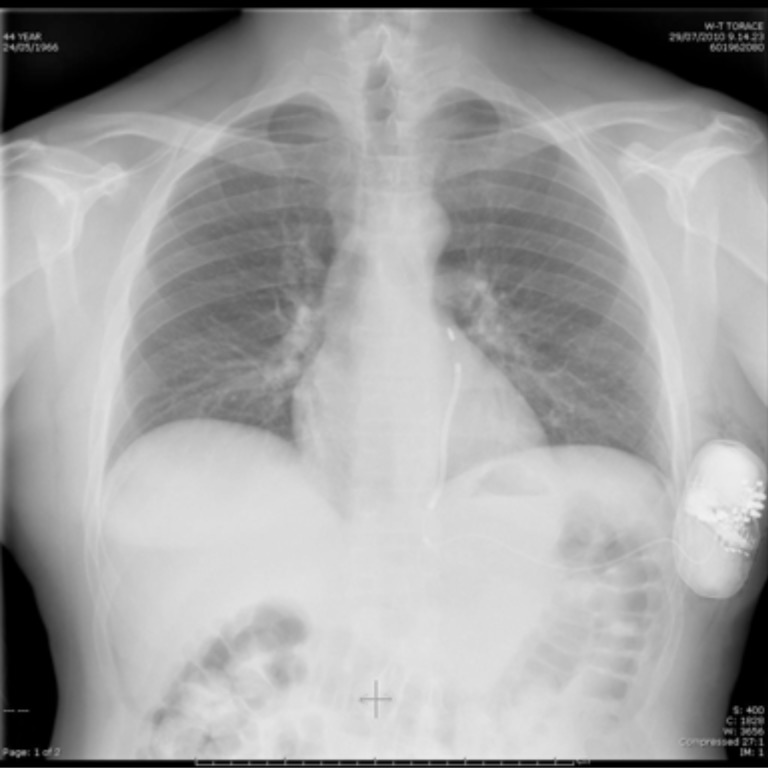
Fig. (2).
Radiographic appearance of an implanted device.
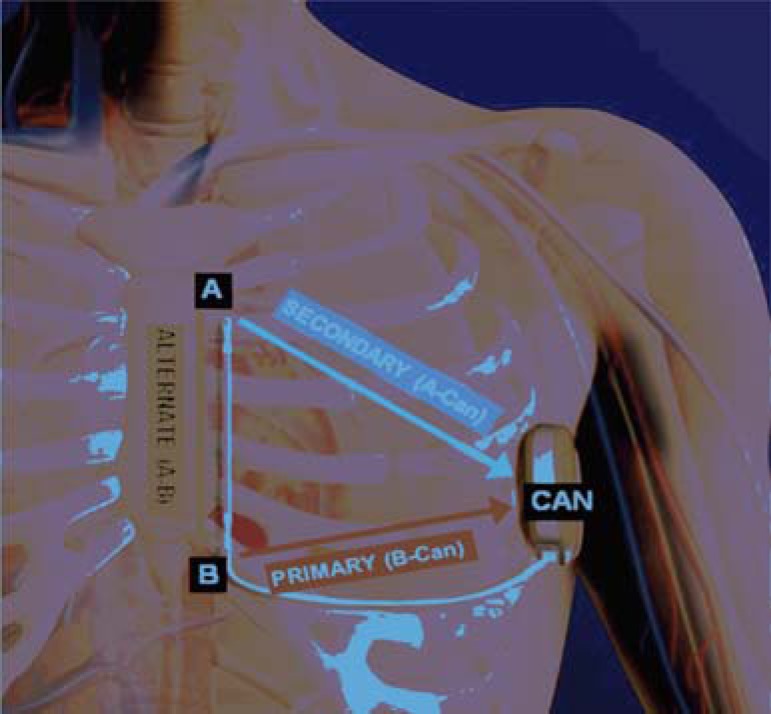
The most important issue of the S-ICD is the sensing and detection function. “The device senses subcutaneous signals (subcutaneous ECG) and detects cardiac rhythm from the two sensing electrodes or from either electrodes and pulse generator. There are three available sensing vectors: primary (from proximal electrode ring to can), secondary (from distal electrode ring to can) and alternate (from distal to proximal electrode)” [9] (Fig. 3): the system automatically selects the optimal vector for detection to prevent inappropriate therapy due to myopotentials, noise or multiple counting (in particular double QRS and T-wave oversensing). A dedicated algorithm is used for the discrimination of supraventricular arrhythmias: the approach incorporates template matching that is conceptually similar to transvenous devices, but the S-ICD evaluates a greater number of points (up to 41) of ventricular complex to improve signal resolution. In a recent head-to-head comparison (the START study), S-ICD discrimination criteria proved to be superior to the transvenous ICD with regard to specificity for supraventricular arrhythmia discrimination [12]. In this context earlier use of morphology discriminators in the S-ICD (compared to late stage morphology discriminators in transvenous ICD) may represent an advantage [12, 13]. Moreover, the ultra far field signal recorded by S-ICD lead mimics more closely the surface ECG, theoretically improving diagnostic capability. For arrhythmia termination “a Shock Zone is programmed (therapy is delivered only according to heart rate i.e above 240 beats per minute) and a Conditional Shock Zone can be used i.e. heart rate between 170 and 240 beats per minute to distinguish supraventricular from ventricular tachyarrhythmias” [9]. The device testing during implantation is performed with a 65-J shock on induced VF, however after implantation the device delivers only up to five consecutive biphasic 80-J shocks per episode (but does not deliver Anti Tachycardia Pacing- ATP-) and can automatically reverse shock polarity if initial shock is unsuccessful. Charge time is approximately 15 seconds to maximum output (longer than transvenous systems). Demand transthoracic pacing at a fixed rate of 50 beats per minute is available for 30 seconds after a shock, with 200-mA biphasic pulse; pacing is activated only after more than 3500 msec of post-shock asystole. Programming is simple because all device settings are automated except for shock therapy (on/off), pacing after a shock (on/off), conditional discrimination of supraventricular tachyarrhythmias (on/off) and upper-rate cut off for the conditional shock zone (170 to 240 beats per minute). The implantation of the system is relatively simple and guided only by anatomical landmarks, no fluoroscopy is required; however a learning curve, consisting at least of 3-4 cases, is necessary for an operator to become familiar with the procedure [9, 13].
Fig. (3).
The three available sensing vectors of S-ICD: primary (from proximal electrode ring to can), secondary (from distal electrode ring to can) and alternate (from distal to proximal electrode).
A number of pivotal studies (initiated more than 15 years ago) demonstrated the ability of the subcutaneous system to terminate ventricular arrhythmias using different shock vectors and with variable energy requirements. The first human study then provided information regarding optimal lead configuration, lead and generator placement, shock efficacy on both induced and spontaneous arrhythmias [14]. After obtaining the CE marking in 2009, several single-center and multicenter experiences across Europe demonstrated safety and efficacy of the S-ICD in a “real world” scenario [15-18]. Finally the first prospective, single-arm, non-randomized, multicenter trial was completed in U.S. with an objective performance criterion: both effectiveness and safety endpoints were met compared with traditional devices [19]; in this study ventricular fibrillation (VF) was detected without delay in >99% of cases and all spontaneous VT/VF episodes were terminated by the device with a first shock efficacy >92% (comparable to transvenous ICD); these results led to the approval by US Food and Drug Administration late in 2012.
The EFFORTLESS is the first large, international, non-randomized, multicentre Registry designed to collect long-term, system-related, clinical, and patient reported outcome data from S-ICD implanted patients since June 2009 [20]. Recently preliminary data have been published [21]: study population included 472 patients with a mean follow-up of 558 days, 72% male, mean age of 49 ± 18 years (9-88 years), 42% mean left ventricular ejection fraction. Complication-free rates were 97 and 94%, at 30 and 360 days, respectively. Three hundred and seventeen spontaneous episodes were recorded in 85 patients during the follow-up period. Of these episodes, 169 (53%) received therapy, 93 being for VT/VF. One patient died of recurrent VF and severe bradycardia. First shock conversion efficacy was 88% with 100% overall successful clinical conversion after a maximum of five shocks. The 360-day inappropriate shock rate was 7% with the vast majority occurring for oversensing (62/73 episodes), primarily of cardiac signals (94% of oversensed episodes).
Advantages
The most obvious advantage of the S-ICD is that it avoids the implantation of leads within the heart and preserves the central venous circulation. The development of this device was initially motivated by peculiar cases, such as pediatric population with congenital heart diseases or patients with no venous access who were unsuitable for transvenous ICD [9, 10, 11, 13].
However several advantages of the S-ICD may make it a first line approach for some patients, rather than simply an alternative to traditional devices. First of all, due to the absence of leads within the body, there is no risk of vascular injury or pneumothorax; moreover the risk of systemic infections seems very low making S-ICD a possible first line approach for patients at high risk (i.e. previous device infection, hemodialysis, chronic immunosuppression therapy, immunodeficiencies, artificial heart valves etc) [9, 13]. Pocket infections can occur with the S-ICD (between 5 and 10%, not so different compared to transvenous devices), but a striking observation is that the resolution of the infection with antibiotic therapy is possible in the majority of cases, without explantation of the system [9, 10, 19]. Even when explantation becomes necessary, it represents a much more simple and safer procedure compared to endovascular lead extraction; this is particularly important in young patients with a long life expectancy and high lead failure rate due to active lifestyles [9, 10, 11].
The simplified implant procedure, without the need for fluoroscopy, is another advantage: it could be a reason for a more widespread use of a life-saving therapy, even in those centers without a great experience in traditional surgical procedure, with less risk of serious complications [9, 10, 11].
Despite its larger size the S-ICD can have some cosmetic advantages, due to the anatomic location in the lateral axilla: it is anecdotally reported that this location is particularly appreciated by women, rather than the anterior infraclavicular position [9, 10, 11, 13]. It is also noteworthy that the device is well tolerated by patients: up to now no case of explantation because of discomfort is known [10].
Another benefit with the S-ICD, is the lack of apparent myocardial damage despite a greater shock size (80 J) [9, 11, 13]. In fact energy is distributed more evenly throughout the myocardium, with only 10% of delivered energy reaching cardiac myocytes: elevation of serum bio-markers of myocardial damage is less when compared to transvenous systems [22, 23], and this may be of utmost importance in patients with already impaired ventricular function.
Problems and Limitations
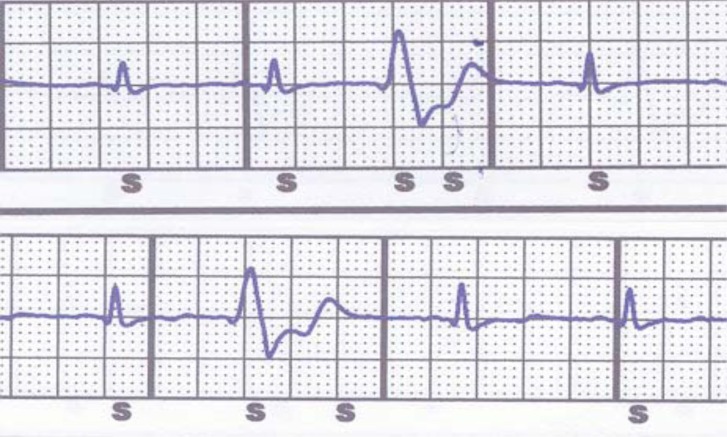
The first drawback with S-ICD is that not every patient is suitable for the implantation; in fact it is necessary to ensure an appropriate device performance by identifying signal characteristics that may lead to unsatisfactory detection. For this reason a pre-implantation screening is mandatory, using a customized transparent plastic tool provided by the manufacturer and modified “ad hoc” ECG skin electrode position [9]: the aim is to verify an adequate sensing of subcutaneous signals, avoiding in particular double counting of QRS or T wave oversensing (TWOS) (Fig. 4). It has been estimated that up to 7.4% of possible candidates would not be suitable for the implant: in particular hypertrophic cardiomyopathy, heavy weight, prolonged QRS duration and a R/T ratio<3 were independently associated with screening failure [24].
Fig. (4).
Two examples of oversensing during premature ventricular contractions. Top: double QRS counting. Bottom: T wave oversensing. S indicates a sensed event.
Rate of inappropriate shocks ranges between 4 and 25%, that is not so different from transvenous ICD, but with a different mechanism: up to 80% is caused by TWOS, expecially in some patient population (i.e. congenital heart disease, Brugada and long QT syndromes), while in transvenous ICD TWOS is involved in about 20% of cases [15-19]. This aspect is important because it underlies the importance of an accurate screening with acquisition of templates not only in resting conditions: inappropriate shocks due to TWOS can be managed and prevented by reprogramming the sensing vector and/or the therapy zones using a template acquired during exercise; so exercise testing shortly after the implantation may be considered in patients at high risk for TWOS [25].
Moreover about 5 to 10% of inappropriate shocks can be caused by muscular noise due to myopotentials (lead is placed over intercostal and pectoral muscles) but this problem may be overcome by reprogramming sensing vector. Explantation can be necessary (5% in a study) when repeated shocks due to TWOS or noise occur despite different vectors programming [18].
Other concerns that have been raised are: risk of undersensing of true arrhythmias (i.e. VF with very low amplitude waves); prolonged time to therapy compared with transvenous ICD (14-18” vs. 7-8”); shock efficacy on spontaneous clinical VT/VF [22, 26]. Until now, there are still relatively few data regarding long term performance of the S-ICD in a “real world” scenario so larger experience and longer follow up are required, but initial results are encouraging [19-21].
The S-ICD has a number of other important limitations: no pacing capability (with the exception of 30” post-shock backup pacing) that also means no possibility to deliver ATP and resynchronization therapy (CRT); remote monitoring is not available in current generation S-ICD; atrial tachyarrhythmias monitoring is not yet available; pulse generator is larger, with anticipated life battery shorter than traditional devices (about 5 years); costs are still high (about three-fold compared to a single chamber transvenous ICD in Italy).
CHOOSING THE RIGHT PATIENT FOR THE S-ICD
S-ICD as a First Choice
There are patients for whom a traditional transvenous ICD is not feasible, so that S-ICD represents the only way to protect them against SCD. First of all we can consider pediatric or GUCH (Grown-Up Congenital Heart disease) population with no venous access due to congenital anomalies. In children with preserved venous access others factors favoring S-ICD are the effect of growth on endocardial leads and the possibility of saving venous vasculature [9, 10, 22].
Another strong indication for S-ICD is the presence of stenosis or obstruction of central veins (in absence of congenital anomalies) that may make the placement of a lead difficult or even impossible; it has been reported that such venous anomalies can be found in up to 7% of cases during pre-implant intravenous contrast venography [27].
S-ICD should be a first line option also in patients with a history of previous endocarditis or device infections: the risk of relapse is very high in these cases, with potential catastrophic consequences. Other situations with a very high risk of infection of chronic endovascular leads are: dialysis, immunodeficiencies, cancer with the need of a chronic indwelling catheter for drug infusion.
Additionally S-ICD should be considered in patients anticipating cardiac transplantation by avoiding endovascular fibrosis that may render surgical lead extraction very difficult at the time of transplantation [9, 22].
S-ICD as a Reasonable Choice
Due to its characteristics S-ICD appears suited to young patients with an active lifestyle and a long life expectancy. This is particularly true for inherited genetic arrhythmogenic syndromes (Brugada, Long and Short QT, Early Repolarization) where clinical arrhythmias are polymorphic VT or VF (not treatable with ATP) and the risk of bradycardia and monomorphic VT is very low.
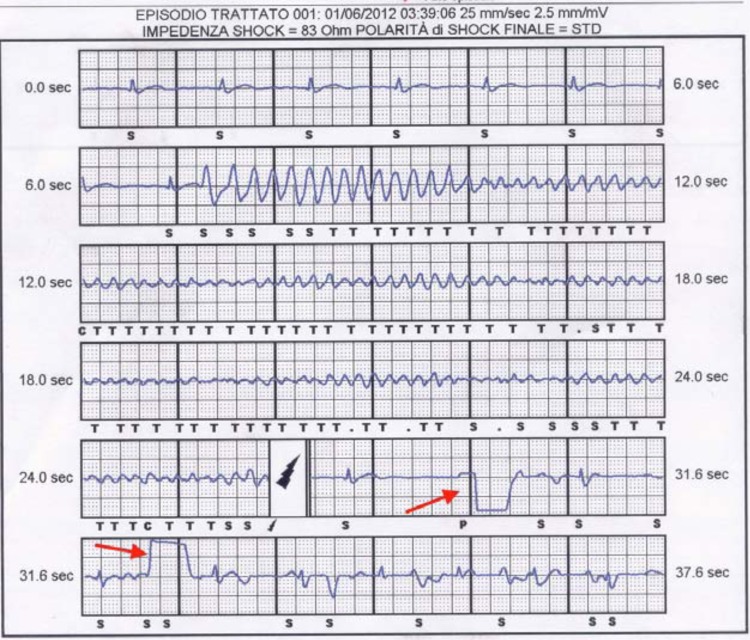
In Brugada patients the incidence of endocardial lead dysfunction was reported to be as high as 2%/year versus 0.58%/year of the general ICD population and the overall risk of device-related complications can be as high as 8-9%/year, so they would benefit from a therapeutic option not requiring endocardial lead implantation [1, 2, 28]. However the issues of S-ICD therapy efficacy and detection accuracy are particularly relevant in Brugada Syndrome: indeed, the unique QRS to T wave ratio and morphology, carries the risk of double QRS/T wave counting and inappropriate therapy. Moreover low amplitude wave VF may result in tachyarrhythmias undersensing, with delayed detection and intervention [9, 28]. Until now very few patients with Brugada syndrome were included in clinical trials and published series, but initial reports are encouraging with evidence of correct detection and shock efficacy even when very low amplitude waves VF occurred (Fig. 5) [28]. A prospective registry (PRELUDE-2) is going to start that will enroll patients with Brugada Syndrome implanted with a S-ICD: this study will give important informations about safety and efficacy of this device in a long term follow up (up to 4 years).
Fig. (5).
S-ICD electrogram of spontaneous ventricular polymorphic tachycardia degenerating into ventricular fibrillation and treated with a shock in a patient with Brugada Syndrome. C= capacitor charging; S= sensing of an event not classified as tachycardia; T= sensing of an event classified as tachycardia. A dot indicates sensing of an unclassifiable event that is discarded. Black lightning symbol indicates a shock. Red arrows indicate post shock transthoracic pacing (reproduced with permission from reference 24).
The S-ICD could represent a valuable option also in hypertrophic cardiomyopathy, as traditional transvenous ICD is associated with a high complication rate [1, 2].
Other potential indications for this new device are: patients with prosthetic heart valves (infection risk) and in women (“cosmetic” issue).
Regarding primary prevention in patients with ischemic/non ischemic dilated cardiomyopathy the S-ICD can be a reasonable choice for two main reasons: 1) pacing indication for bradycardia is relatively rare when ICD is implanted (less than 10% of patients); 2) the likelihood of developing first time monomorphic VT is not so high (only 15-20% of patients experience high rate monomorphic VT during the first year after the implant) and subsequent risk is 1.8%/year [22]. However a cause for caution exists in patients with left ventricular dysfunction following myocardial infarction; a long term study (2 years follow up) recording cardiac rhythm with implantable loop monitor [29] showed a non negligible incidence of bradycardic events in patients without previous pacing indication: 10% high-degree AV block, 7% sinus bradycardia<30 bpm and 5% sinus arrest.
Finally, regarding secondary prevention indicated patients a S-ICD can be considered if they are survivors of an out-of-hospital VF episode (with no evidence of monomorphic VT as index arrhythmia).
When to Avoid the S-ICD
Obviously S-ICD should not be implanted when the pre-implant screening test has failed (as mentioned above in up to 7% of cases). Also, S-ICD is contraindicated in patients with: symptomatic bradycardia requiring permanent pacing; previously implanted unipolar pacemaker (because of sensing/detection pitfalls); systolic heart failure and left bundle branch block indicated for CRT; recurrent sustained monomorphic VT treatable with ATP.
Moreover there are patients with some anatomic characteristics that may pose them at risk of complications following the implant: for example very thin patients with poor subcutaneous tissue (risk of skin erosion over the generator) or patients with abnormalities of chest wall like “pectus excavatum” (risk of skin erosion along the lead).
A summary of proposed recommendations for patient selection is shown in Table 1.
Table 1.
The choice of the candidates for a S-ICD.
| S-ICD as a first choice |
| -Pediatric or GUCH patients with no venous access. |
| -Acquired stenosis or obstruction of central veins. |
| -Previous endocarditis or device infection. |
| -Patients at very high risk of infection of endovascular leads: dialysis, immunodeficiencies, cancer, need of a chronic indwelling catheter. |
| -Patients candidates to cardiac transplantation. |
| S-ICD as a reasonable choice |
| -Young patients with an active lifestyle and a long life expectancy. |
| -Inherited genetic arrhythmogenic syndromes (Brugada, Long and Short QT, Early Repolarization). |
| -Hypertrophic cardiomyopathy. |
| -Prosthetic heart valves (infection risk). |
| -Women (“cosmetic” issue). |
| -Primary prevention patients with ischemic/non ischemic dilated cardiomyopathy. |
| -Secondary prevention patients survivors of out-of-hospital VF. |
| When to avoid the S-ICD |
| -Failed pre-implant screening (up to 7% of cases). |
| -Symptomatic bradycardia requiring permanent pacing. |
| -Previously implanted unipolar pacemaker (sensing/detection pitfalls). |
| -Systolic heart failure and left bundle branch block indicated for CRT. |
| -Recurrent sustained monomorphic VT treatable with ATP. |
| -Anatomic characteristics: thin patients with poor subcutaneous tissue, “pectus excavatum”. |
Table 2 compares characteristics, advantages and disadvantages of S-ICD versus traditional ICD.
Table 2.
Head-to-head comparison of S-ICD versus transvenous (TV) ICD.
| S-ICD | TV-ICD | |
|---|---|---|
| Leads within heart and vessels | NO | YES |
| Implant complications | Low/Negligible | Significant |
| X-Ray exposure | NO | YES |
| Infections | Lower risk More simple to manage | Higher risk Difficult to manage |
| Shock induced myocardial damage | Negligible | Significant |
| Patients suitable for implant | About 90% | Virtually all |
| Inappropriate shocks | 4-25% (TWOS or external noise) | 20-30% (supraventricular tachycardia) |
| Time to shock delivery | 14-20” | 7-9” |
| Pacing capability | NO | YES |
| Antitachycardia pacing | NO | YES |
| Pulse generator size | 69 cc/145 grams | 30 cc/70 grams |
| Battery life span | Up to 5 years | Up to 10 years |
| Costs | High | Medium/Low |
| Home Monitoring | NO | YES |
| Atrial arrhythmias monitoring | NO | YES |
FUTURE DIRECTIONS AND CONCLUSIONS
The S-ICD represents a major advancement in ICD technology in the last 10 years [9, 10, 12]. Despite current limitations, up to 55% of patients in routine clinical practice needing an ICD are potential candidates for a subcutaneous device [9, 10, 30]: however, to maximize clinical outcome and cost/benefit ratio, it is fundamental to choose candidates that can benefit the most, taking into account both patient’s and device’s characteristics.
For the next generation devices many of the current limitations will be addressed: improvement of battery technology; downsizing of the generator; further improvements in algorithm design; remote monitoring capability; pacing capability (leadless ultrasound-based cardiac stimulation).
Currently there are still no clinical data that demonstrate S-ICD can prevent sudden death or improve survival in the same way as traditional ICD. The possible use of S-ICD as a first-line strategy or as an alternative approach for specific populations will require the confirmation of large clinical trials and registries that are currently in progress [20, 21, 31].
ACKNOWLEDGEMENTS
Declared none.
DISCLOSURES
Part of this article has been presented by De Maria E, Olaru A and Cappelli S on the E-Journal of Cardiology Practice of European Society of Cardiology. “The subcutaneous defibrillator: who stands to benefit”. 2014, March. Available from: www.escardio.org/communities/ councils/ccp/e-journal/volume12/Pages/subcutaneous defibrillator-who-may- benefit-demaria-olaru-cappeli.aspx
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Hohnloser SH, Israel CW. Current evidence base for use of the implantable cardioverter-defibrillator. Circulation. 2013;128(2):172–83. doi: 10.1161/CIRCULATIONAHA.112.000547. [DOI] [PubMed] [Google Scholar]
- 2.John RM, Tedrow UB, Koplan BA , et al. Ventricular arrhythmias and sudden cardiac death. Lancet. 2012;380(9852):1520–9. doi: 10.1016/S0140-6736(12)61413-5. [DOI] [PubMed] [Google Scholar]
- 3.Katritsis DG, Josephson ME. Sudden cardiac death and implantable cardioverter defibrillators two modern epidemicsκ. Europace. 2012;14(6):787–94. doi: 10.1093/europace/eus001. [DOI] [PubMed] [Google Scholar]
- 4.Gasparini M, Nisam S. Implantable cardioverter defibrillator harmκ. Europace. 2012;14(8):1087–93. doi: 10.1093/europace/eus025. [DOI] [PubMed] [Google Scholar]
- 5.Atwater BD, Daubert JP. Implantable cardioverter defibrillators risks accompany the life-saving benefits. Heart. 2012;98(10):764–72. doi: 10.1136/heartjnl-2012-301853. [DOI] [PubMed] [Google Scholar]
- 6.Maisel WH. Transvenous implantable cardioverter-defibrillator leads the weakest link. Circulation. 2007;115(19):2461–3. doi: 10.1161/CIRCULATIONAHA.107.698597. [DOI] [PubMed] [Google Scholar]
- 7.Maisel WH, Kramer DB. Implantable cardioverter-defibrillator lead performance. Circulation. 2008;117(21):2721–3. doi: 10.1161/CIRCULATIONAHA.108.776807. [DOI] [PubMed] [Google Scholar]
- 8.Kalahasty G, Ellenbogen KA. Management of the patient with implantable cardioverter-defibrillator lead failure. Circulation. 2011;123(12):1352–4. doi: 10.1161/CIRCULATIONAHA.110.986828. [DOI] [PubMed] [Google Scholar]
- 9.De Maria E, Bonetti L, Patrizi G , et al. Implantation of a completely subcutaneous ICD system case report of a patient with Brugada syndrome and state of the art. J Interv Card Electrophysiol. 2012;34(1):105–13. doi: 10.1007/s10840-011-9626-5. [DOI] [PubMed] [Google Scholar]
- 10.Grace A. The subcutaneous implantable cardioverter-defibrillator. Curr Opin Cardiol. 2014;29(1):10–9. doi: 10.1097/HCO.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 11.Akerström F, Arias MA, Pachón M, Puchol A, Jiménez-López J. Subcutaneous implantable defibrillator State-of-the art 2013,. World J Cardiol. 2013;5(9):347–54. doi: 10.4330/wjc.v5.i9.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold MR, Theuns DA, Knight BP , et al. Head-to-head comparison of arrhythmia discrimination performance of subcutaneous and transvenous ICD arrhythmia detection algorithms the START study. J Cardiovasc Electrophysiol. 2012;23(4):359–66. doi: 10.1111/j.1540-8167.2011.02199.x. [DOI] [PubMed] [Google Scholar]
- 13.Rowley CP, Gold MR. Subcutaneous implantable cardioverter defibrillator. Circ Arrhythm Electrophysiol. 2012;5(3):587–93. doi: 10.1161/CIRCEP.111.964676. [DOI] [PubMed] [Google Scholar]
- 14.Bardy GH, Smith WM, Hood MA , et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363(1):36–44. doi: 10.1056/NEJMoa0909545. [DOI] [PubMed] [Google Scholar]
- 15.Aydin A, Hartel F, Schlüter M , et al. Shock efficacy of subcutaneous implantable cardioverter-defibrillator for prevention of sudden cardiac death initial multicenter experience. Circ Arrhythm Electrophysiol. 2012;5(5):913–9. doi: 10.1161/CIRCEP.112.973339. [DOI] [PubMed] [Google Scholar]
- 16.Köbe J, Reinke F, Meyer C , et al. Implantation and follow-up of totally subcutaneous versus conventional implantable cardioverter-defibrillators a multicenter case-control study. Heart Rhythm. 2013;10(1):29–36. doi: 10.1016/j.hrthm.2012.09.126. [DOI] [PubMed] [Google Scholar]
- 17.Olde Nordkamp LR, Dabiri Abkenari L, Boersma LV , et al. The entirely subcutaneous implantable cardioverter-defibrillator initial clinical experience in a large Dutch cohort. J Am Coll Cardiol. 2012;60(19):1933,–9. doi: 10.1016/j.jacc.2012.06.053. [DOI] [PubMed] [Google Scholar]
- 18.Jarman JW, Todd DM. United Kingdom national experience of entirely subcutaneous implantable cardioverter-defibrillator technology important lessons to learn. Europace. 2013;15(8):1158–65. doi: 10.1093/europace/eut016. [DOI] [PubMed] [Google Scholar]
- 19.Weiss R, Knight BP, Gold MR , et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation. 2013;128(9):944–53. doi: 10.1161/CIRCULATIONAHA.113.003042. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen SS, Lambiase P, Boersma LV , et al. Evaluation oF FactORs ImpacTing CLinical Outcome and Cost EffectiveneSS of the S-ICD design and rationale of the EFFORTLESS S-ICD Registry. Pacing Clin Electrophysiol. 2012;35(5):574–9. doi: 10.1111/j.1540-8159.2012.03337.x. [DOI] [PubMed] [Google Scholar]
- 21.Lambiase PD, Barr C, Theuns DA , et al. on behalf of the EFFORTLESS Investigators.Worldwide experience with a totally subcutaneous implantable defibrillator early results from the EFFORTLESS S-ICD Registry. Eur Heart J. 2014;35(25):1657–65. doi: 10.1093/eurheartj/ehu112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poole JE, Gold MR. Who should receive the subcutaneous implanted defibrillatorκ: the subcutaneous implantable cardioverter defibrillator (icd) should be considered in all icd patients who do not require pacing. Circ Arrhythm Electrophysiol. 2013;6(6):1236–45. doi: 10.1161/CIRCEP.113.000481. [DOI] [PubMed] [Google Scholar]
- 23.Killingsworth CR, Melnick SB, Litovsky SH, Ideker RE, Walcott GP. Evaluation of acute cardiac and chest wall damage after shocks with a subcutaneous implantable cardioverter defibrillator in Swine. Pacing Clin Electrophysiol. 2013;36(10):1265–72. doi: 10.1111/pace.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olde Nordkamp LR, Warnaars JL, Kooiman KM , et al. Which Patients Are Not Suitable for a Subcutaneous ICD Incidence and Predictors of Failed QRS-T-Wave Morphology Screening. J Cardiovasc Electrophysiol. 2014;25(5):494–9. doi: 10.1111/jce.12343. [DOI] [PubMed] [Google Scholar]
- 25.Kooiman KM, Knops RE, Olde Nordkamp L, Wilde AA, de Groot Jr. Inappropriate subcutaneous implantable cardioverter-defibrillator shocks due to T-wave oversensing can be prevented implications for management. Heart Rhythm. 2014;11(3):426–34. doi: 10.1016/j.hrthm.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Acha MR, Milan D. Who should receive the subcutaneous implanted defibrillatorκ: timing is not right to replace the transvenous implantable cardioverter defibrillator. Circ Arrhythm Electrophysiol. 2013;6(6):1246–51. doi: 10.1161/CIRCEP.113.000445. [DOI] [PubMed] [Google Scholar]
- 27.Korkeila P, Nyman K, Ylitalo A , et al. Venous obstruction after pacemaker implantation. Pacing Clin Electrophysiol. 2007;30(2):199–206. doi: 10.1111/j.1540-8159.2007.00650.x. [DOI] [PubMed] [Google Scholar]
- 28.De Maria E, Cappelli S, Cappato R. Shock efficacy of the entirely subcutaneous defibrillator for termination of spontaneous ventricular fibrillation in Brugada syndrome. Heart Rhythm. 2013;10(12):1807–9. doi: 10.1016/j.hrthm.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Bloch Thomsen PE, Jons C, Raatikainen MJ , et al. Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) Study Group.Long-term recording of cardiac arrhythmias with an implantable cardiac monitor in patients with reduced ejection fraction after acute myocardial infarction the Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) study. . Circulation. 2010;122(13):1258–64. doi: 10.1161/CIRCULATIONAHA.109.902148. [DOI] [PubMed] [Google Scholar]
- 30.de Bie MK, Thijssen J, van Rees JB , et al. Suitability for subcutaneous defibrillator implantation results based on data from routine clinical practice. Heart. 2013;99(14):1018–23. doi: 10.1136/heartjnl-2012-303349. [DOI] [PubMed] [Google Scholar]
- 31.Olde Nordkamp LR, Knops RE, Bardy GH , et al. Rationale and design of the PRAETORIAN trial a Prospective, RAndomizEd comparison of subcuTaneOus and tRansvenous ImplANtable cardioverter-defibrillator therapy. Am Heart J. 2012;163(5):753–60. doi: 10.1016/j.ahj.2012.02.012. [DOI] [PubMed] [Google Scholar]