Graphical Abstract
Keywords: Echinococcus equinus, Taeniidae, Zebra, Lion, Black-backed jackal, Etosha National Park
Highlights
-
•
A wildlife cycle of Echinococcus equinus exists in the Etosha National Park.
-
•
Lions, jackals and plain zebras are host of E. equinus.
-
•
Haplotype identity and diversity was similar to samples from Europe.
Abstract
Various species of Echinococcus have been described in the past from wild mammals of sub-Saharan Africa. However, it is only recently, that a few isolates have become available for molecular identification; therefore, the involvement of wildlife in the lifecycles of the various cryptic species within Echinococcus granulosus sensu lato is still only partially known. A preliminary survey was undertaken in Etosha National Park, Namibia, from August to October 2012. Faecal samples were obtained from 34 individual wild carnivores, and metacestodes were collected from carcasses of 18 culled herbivores. Single eggs and metacestode tissue were lysed and identified from sequences of the mitochondrial nad1 gene. In case of metacestodes, the cox1 gene was additionally sequenced and haplotype networks were constructed.
Echinococcus equinus was found in lions (4 of 6), black-backed jackals (2 of 7) and Burchell's zebras (11 of 12). The frequency of this parasite in the absence of domestic dogs, horses and donkeys strongly indicates its transmission in a wildlife cycle. Further, a variety of sequences were obtained from eggs and cysticerci from lions, cheetahs, caracals, spotted hyenas and oryx, which most closely clustered with species of Taenia. Only 3 of them, 2 of lion and 1 of hyena origin, could be allocated to Hydatigera (=Taenia) taeniaeformis (lion), Taenia regis (lions and oryx) and Taenia cf. crocutae (spotted hyena and oryx).
1. Introduction
Numerous records of Echinococcus adult and metacestode stages were reported during the second half of the 20th century from a wide range of wild African carnivore and herbivore species. In various surveys, mainly from eastern and southern Africa, two species of jackal, cape fox, African wild dog, spotted hyena, wild cat and lion were identified as definitive hosts, while cysts were found mainly in zebra, bush pig, warthog, hippopotamus, giraffe, buffalo and at least ten species of other bovids (“antelopes”) (lit. in Macpherson and Wachira, 1997; Hüttner and Romig, 2009). High prevalence levels are known for buffalo (Woodford and Sachs, 1973), hippopotamus (McCully et al., 1967), warthog (Dinnik and Sachs, 1969; Woodford and Sachs, 1973; Eugster, 1978), wildebeest (Eugster, 1978) and zebra (Young, 1975a), while sample sizes from carnivores were generally too small to determine reliable prevalences. Exceptions are golden and black-backed jackals (Canis aureus, Canis mesomelas) with prevalences of up to 30%, whose infections were assumed to have been obtained from scavenging livestock carcasses or discarded offal because they were obtained in areas where large wild herbivores are now rare or extinct (Macpherson et al., 1986). Following the description of Echinococcus felidis Ortlepp, 1937 from South African lions, an original sylvatic lifecycle involving wild animals was proposed, involving lions (and possibly other wild carnivores) and large herbivore species. Even after being synonymized with E. granulosus (Nelson and Rausch, 1963), this parasite continued to be recognized as a subspecies, or the ‘lion strain’, mainly due to its presence in a member of the Felidae. Subsequently, most records from African wild mammals were tentatively associated with this strain (Macpherson and Wachira, 1997), although the diagnostic validity of adult worm morphology has been questioned, and identification of the cyst stage was not possible at that time. Only recently could E. felidis be reinstated as a distinct species, based on molecular characterization of deposited worm material from South Africa and recent parasite eggs of lion origin from Uganda (Hüttner et al., 2008). Currently, the presence of E. felidis is confirmed in lions (Panthera leo) and spotted hyenas (Crocuta crocuta) from various conservation areas of Uganda and Kenya (Hüttner et al., 2009; Kagendo et al., 2014), but to date only one metacestode, from a Ugandan warthog (Phacochoerus africanus), has been identified as E. felidis. Therefore, the host range of this species is far from clear, particularly concerning intermediate hosts. In addition, lions and spotted hyenas, long thought to be exceptional hosts for Echinococcus spp. and specifically associated with E. felidis only, are now known to be frequently infected with E. granulosus sensu stricto as well (Kagendo et al., 2014). Apart from E. felidis and E. granulosus s.s., there are other candidates that may infect African wildlife. Echinococcus ortleppi had been identified in an unspecified species of zebra from Namibia (Obwaller et al., 2004), and E. equinus (the “horse strain”) was morphologically identified from dogs that had been fed with cysts from mountain zebra (Equus zebra) originating from Namibia (Kumaratilake et al., 1986). The situation is further complicated by the observation, that cysts from plains zebra (Equus quagga) originating from the Kruger National Park, South Africa were found to be infective for lions in experimental infection (Young, 1975a, 1975b). Therefore the identity of Echinococcus spp. in wildlife from different African regions is certainly in need of clarification. Following the recent revision of the genus Echinococcus, which is largely based on molecular data (Nakao et al., 2013a), five species are currently recognized as agents of cystic echinococcosis. Four of these are now confirmed or suspected to occur in African wildlife. However, additional taxa may exist. Two further species from South African wild dogs (Lycaon pictus) have been described as Echinococcus longimanubrius Cameron, 1926 and Echinococcus lycaontis Ortlepp, 1934. These species were later thought to be E. granulosus but their place in the currently recognized system is unclear after the split of this taxon. The same applies to the identity of the subspecies E. granulosus africanus Verster, 1965, which had been reported from a South African wild cat (Felis silvestris lybica) and from experimentally infected cape foxes (Vulpes chama) (Verster, 1965; Verster and Collins, 1966).
The lifecycles and identities of Echinococcus spp. in African wildlife are far from clear, and every additional record that includes molecular data is of great scientific value. While a survey of wild definitive hosts has been recently conducted in Kenya (Kagendo et al., 2014), the general lack of data is particularly evident from the southern part of Africa, where no molecular characterization of Echinococcus has been undertaken except for some isolates of human origin (Mogoye et al., 2013). Here we report on a survey of Echinococcus in all species of large carnivores that occur in the Etosha National Park ecosystem, together with two species of large herbivore.
2. Materials and methods
2.1. Study area
The study was undertaken from August to October 2012 in Etosha National Park in northern Namibia. The area has been under protection as a game reserve since 1907, and was proclaimed a national park in 1967. Today, it covers more than 22,000 km2 and includes a variety of vegetation types (predominantly saline desert, grasslands and savannah woodlands). The park was entirely fenced in the 1970s. Since animal migrations to external water sources in the dry season are prevented, a number of artificial waterholes have been constructed. Most species of the large mammal fauna of southern Africa are present in the park, notable exceptions being African wild dogs (L. pictus) and buffaloes (Syncerus caffer).
2.2. Collection of parasites
Parasite samples were obtained in the context of a relocation programme, where 6 lions (P. leo), 6 leopards (Panthera pardus), 4 cheetahs (Acinonyx jubatus), 6 spotted hyenas (Crocuta crocuta), 7 black-backed jackals (C. mesomelas) and 5 caracals (Caracal caracal) were caught and kept temporarily in individual confinement for quarantine purposes. All handling of animals was performed by or under direct supervision of the wildlife veterinarian responsible for Etosha National Park, ensuring compliance with animal welfare regulations.
A faecal sample was obtained from each animal, prior to de-worming. Faecal samples were frozen at −80 °C for one week for safety reasons and thereafter stored at −20 °C until used. During the same period 12 plains zebras (Equus quagga burchellii) and 6 oryx (Oryx gazella) were culled and examined for larval stages of Echinococcus spp. and other metacestodes in lungs, liver, kidneys, spleen and skeletal musculature. Metacestode size (diameter) was measured and suspected Echinococcus cysts were examined microscopically for the presence of protoscolices. Subsequently, samples were stored in 70% ethanol at room temperature.
Three additional cyst samples of E. equinus from horses, one from Germany (Blutke et al., 2010) and two from Italy (kindly provided by Antonio Varcasia), were available for comparative analysis.
2.3. DNA amplification and sequencing
Taeniid eggs were isolated from faeces via zinc chloride flotation (Mathis et al., 1996). Single eggs were separated with a pipette and transferred within 1 µl H2O into 10 µl of 0.02 M NaOH. The larval stage material was treated similarly, single protoscolices or approximately equal sized pieces of cyst material were transferred into 10 µl of 0.02 M NaOH. The taeniid eggs or larval material were lysed at 95 °C for 10 min and used directly in the PCR (Nakao et al., 2003a).
DNA amplifications were performed by nested PCR. The target of the PCR was a 1073–1078 bp long fragment including the complete nad1 (NADH dehydrogenase subunit 1) gene. Used primers for the first amplification were forward 5′-TGG AAC TCA GTT TGA GCT TTA CTA-3′, reverse 5′-ATA TCA AAG TAA CCT GCT ATG CAG-3′ and for the second forward PCR 5′-TAT TAA AAA TAT TGA GTT TGC GTC-3′ and reverse 5′-TCT TGA AGT TAA CAG CAT CAC GAT-3′ as described previously (Hüttner et al., 2008). For the first PCR, a 50 µl reaction mixture containing 10 mM Tris–HCl (pH 8.3), 50 mM KCl, 2 mM MgCl2, 200 µM of each dNTP, 12.5 pmol of each external primer, 1.25 U Ampli-Taq Polymerase (Applied Biosystems) and 1 µl of the egg or larval lysate. Subsequent to the first PCR a nested PCR was performed using 1 µl of the first amplification product as template. The second PCR reaction mixture was identical to the first, except for the primers. All thermal reactions were performed for 35 cycles of denaturation (94 °C for 30 s), annealing (55 °C for 30 s) and extension (72 °C for 60 s).
In addition to the nad1 gene, the cox1 (cytochrome c oxidase subunit 1) gene was amplified via nested PCR and sequenced from 19 cyst samples with zebra origin. The ingredients for the PCR reaction mixture were identical to the nad1 PCR except for the primer. The primers for the first PCR were forward 5′-GTG GAG TTA CTG CTA ATA ATT TTG-3′ and reverse 5′-TAC GAC TYA CTT ATC AC-3′. The primer pair of the nested PCR was forward 5′-TTA CTG CTA ATA ATT TTG TGT CAT-3′ and reverse 5′-GCA TGA TGC AAA AGG CAA ATA AAC-3′ as published previously (Hüttner et al., 2008). The condition during amplification was 35 cycles of denaturation (94 °C for 30 s), annealing (55 °C for 30 s) and extension (72 °C for 90 s).
Amplification products were purified by using High Pure PCR Product Purification Kit (Roche) according to the manufacturer's instructions. The purified PCR products were sequenced (GATC, Konstanz, Germany).
2.4. Data processing
Complete mitochondrial genomes, that are available for 11 taxa of Echinococcus spp. (Le et al., 2002; Nakao et al., 2002, 2007) and for Taenia solium (Nakao et al., 2003b), served as comparative sequences (accession nos. AB018440, AB086256, AB208063-4, AB208545-6, AB235846-8, AF297617, AB732958, AF346403 and NC020374).
Multiple alignments of nucleotide sequences of the cox1 and nad1 were made by using the ClustalX program (V 2.0.12) and used for phylogenetic analyses. The PAUPv4b10 program was employed for maximum likelihood (ML) analysis using the DNA data set. The substitution model HKY + G + I and its parameters were determined by Akaike Information Criterion (AIC) implemented in MODELTEST 3.7 (Posada and Crandall, 1998; Swofford, 2002). A full heuristic search algorithm was run to estimate the ML tree. The robustness of the inferred tree was tested by bootstrapping with 1000 replicates. The calculated trees were rooted with T. solium as an outgroup taxon.
Haplotype networks of the E. equinus sequences of nad1 and cox1 genes were calculated by using the TCS 1.2 software (Clement et al., 2000). The network estimation was run at 95% connection limit.
3. Results
3.1. Carnivores
In Table 1, the number of taeniid eggs is listed for each individual carnivore, as well as the number of eggs, which yielded sequences of sufficient length to allow species identification. Eggs of E. equinus were found in faeces of four out of six lions and two out of seven jackals. No other Echinococcus spp. sequence was obtained from any other taeniid egg. Taenia regis and Hydatigera (=Taenia) taeniaeformis were identified in two and one lion, respectively. One hyena harboured a species whose sequence conformed to ‘lineage II’ of an Ethiopian spotted hyena, which had tentatively been assigned to Taenia crocutae (Terefe et al., 2014). Additional sequences from taeniid eggs of lion, hyena, cheetah and caracal origin clustered with taxa of Taenia, but were different from any other sequences deposited in GenBank. In addition, faeces from all jackals contained eggs of Trichuris and unidentified nematodes, and leopard faeces contained eggs of Spirometra sp. and unidentified nematodes as well as coccidia.
Table 1.
Presence and identity of taeniid eggs in carnivore faeces.
| Host species | Number of isolated taeniid eggs | Number of successful amplified and sequenced eggs | Identified species (n eggs) |
|---|---|---|---|
| Panthera leo 1 | 18 | 11 | E. equinus (11) |
| Panthera leo 2 | 1 | 0 | – |
| Panthera leo 3 | 49 | 10 | E. equinus (7), H. taeniaeformis (1), T. regis (2) |
| Panthera leo 4 | 36 | 7 | E. equinus (5), T. regis (2) |
| Panthera leo 5 | 5 | 2 | E. equinus (2) |
| Panthera leo 6 | 18 | 2 | Taenia spp. (2) |
| Canis mesomelas 1 | 1 | 1 | E. equinus (1) |
| Canis mesomelas 2 | 6 | 2 | E. equinus (2) |
| Canis mesomelas 3 | – | – | – |
| Canis mesomelas 4 | – | – | – |
| Canis mesomelas 5 | – | – | – |
| Canis mesomelas 6 | – | – | – |
| Canis mesomelas 7 | – | – | – |
| Acinonyx jubatus 1 | 26 | 8 | Taenia spp. (8) |
| Acinonyx jubatus 2 | 10 | 4 | Taenia spp. (4) |
| Acinonyx jubatus 3 | – | – | – |
| Acinonyx jubatus 4 | – | – | – |
| Crocuta crocuta 1 | 12 | 3 | T. cf. crocutae (2), Taenia sp. (1) |
| Crocuta crocuta 2 | 2 | 0 | – |
| Crocuta crocuta 3 | – | – | – |
| Crocuta crocuta 4 | – | – | – |
| Crocuta crocuta 5 | – | – | – |
| Crocuta crocuta 6 | – | – | – |
| Caracal caracal 1 | 18 | 6 | Taenia sp. (6) |
| Caracal caracal 2 | 24 | 5 | Taenia sp. (5) |
| Caracal caracal 3 | – | – | – |
| Caracal caracal 4 | – | – | – |
| Caracal caracal 5 | – | – | – |
| Panthera pardus 1 | – | – | – |
| Panthera pardus 2 | – | – | – |
| Panthera pardus 3 | – | – | – |
| Panthera pardus 4 | – | – | – |
| Panthera pardus 5 | – | – | – |
| Panthera pardus 6 | – | – | – |
Due to the small amount of DNA in individual eggs, nad1 sequences were mostly incomplete, and the material was insufficient to amplify cox1. Therefore the isolates from carnivores were not included in the haplotype analysis.
3.2. Herbivores
All of the twelve zebras examined contained cysts morphologically suspected to be Echinococcus metacestodes; 34 cysts were recovered for further examination. For eleven of the zebras (27 cysts), infection with E. equinus could be molecularly confirmed (no amplification product could be obtained from two calcified cysts of the remaining animal) (Table 2). Of the 27 molecularly characterized cysts, 23 were located in the liver and four in the lungs. All lung cysts were small (1–3 cm) and sterile, while liver cysts ranged in diameter from 1 to 8 cm (mean 3.3 cm), eight of them sterile (mean 3.0 cm), eleven fertile (mean 4.2 cm) and four calcified. The size of calcified lesions ranged from 0.5 to 8 cm, but most of these lesions were not collected or gave no amplification product. In four of the twelve zebras, liver cysts were fertile. Severe pathology was not apparent in any of the zebras, and even in case of multiple infections cysts occupied only a small proportion of the liver (Fig. 1). Spontaneous death of the cysts (with subsequent calcification) seems to be common and occurs at a comparatively small cyst size.
Table 2.
Location, size, fertility status and E. equinus haplotype variants of individual cysts.
| Sample | Organ, cyst diameter, fertility status | Haplotype | |||
|---|---|---|---|---|---|
| nad1 | cox1 | Combined | |||
| Zebra 1 | Cyst 1 | Liver, 2.5 cm, sterile | I | I | A |
| Cyst 2 | Liver, 1.5 cm, sterile | III | I | B | |
| Cyst 3 | Liver, 1.3 cm, sterile | I | I | A | |
| Zebra 2 | Cyst 1 | Liver, 6.0 cm, sterile | II | II | C |
| Cyst 2 | Lung, 3.0 cm, sterile | II | – | – | |
| Cyst 3 | Lung, 1.1 cm, sterile | II | – | – | |
| Zebra 3 | Cyst 1 | Lung, 3.0 cm, sterile | III | I | B |
| Cyst 2 | Liver, 8.0 cm, fertile | II | II | C | |
| Cyst 3 | Liver, 8.0 cm, fertile | II | II | C | |
| Cyst 4 | Liver, 6.0 cm, sterile | III | I | B | |
| Cyst 5 | Lung, 3.0 cm, sterile | III | – | – | |
| Cyst 6 | Liver, 3.0 cm, fertile | III | – | – | |
| Zebra 4 | Cyst 1 | Liver, 2.5 cm, fertile | IV | III | E |
| Cyst 2 | Liver, 2.0 cm, fertile | II | II | C | |
| Cyst 3 | Liver, 2.0 cm, fertile | V | IV | G | |
| Zebra 5 | Cyst 1 | Liver, 1.5 cm, calcified | – | – | – |
| Cyst 2 | Liver, 1.5 cm, calcified | IV | II | D | |
| Cyst 3 | Liver, 1.0 cm, sterile | – | – | – | |
| Cyst 4 | Liver, 1.0 cm, calcified | III | – | – | |
| Cyst 5 | Liver, 1.0 cm, sterile | – | – | – | |
| Cyst 6 | Liver, 1.5 cm, sterile | IV | II | D | |
| Zebra 6 | Cyst 1 | Liver, 5.0 cm, calcified | II | – | – |
| Zebra 7 | Cyst 1 | Liver, 1.0 cm, calcified | III | – | – |
| Cyst 2 | Liver, 1.0 cm, calcified | – | – | – | |
| Zebra 8 | Cyst 1 | Liver, 8.0 cm, calcified | – | – | – |
| Cyst 2 | Liver, 6.0 cm, calcified | – | – | – | |
| Zebra 9 | Cyst 1 | Liver, 4.0 cm, fertile | III | I | B |
| Cyst 2 | Liver, –, fertile | IV | III | E | |
| Cyst 3 | Liver, –, fertile | III | – | – | |
| Zebra 10 | Cyst 1 | Liver, 2.0 cm, sterile | III | I | B |
| Zebra 11 | Cyst 1 | Liver, 4.0 cm, fertile | II | II | C |
| Cyst 2 | Liver, –, fertile | II | II | C | |
| Cyst 3 | Liver, –, sterile | – | – | – | |
| Zebra 12 | Cyst 1 | Liver, –, sterile | III | I | B |
| Germany | Cyst 1 | III | I | B | |
| Italy | Cyst 1 | VI | III | F | |
| Cyst 2 | VI | III | F | ||
| UK | Ref. seq. | IV | UK | UK | |
Fig. 1.

Zebra (Equus quagga burchellii) liver infected with Echinococcus equinus. Arrows point to three cysts of different sizes.
No Echinococcus metacestodes were found in six oryx examined. In contrast to the zebras, which harboured no other taeniid metacestodes, cysticerci of three Taenia spp. were found in five of the six animals. Two species could be identified as T. regis (in three oryx) and T. cf. crocutae (in one oryx), the third species did not conform to any deposited sequence and to any of the sequences obtained from carnivores.
3.3. Phylogenetic and haplotype analyses of Echinococcus equinus
Comparative analysis of the E. equinus sequences (nad1 and cox1 genes) showed only a small number of polymorphic sites. Five haplotypes could be identified within the 894 bp long sequence of the complete nad1 gene of the Namibian samples (submitted to GenBank under accession numbers KP161211–KP161216). A sixth haplotype was represented by the horse samples from Italy, whereas the German haplotype (and the sequence of the UK reference) was also present among our Namibian samples. The 1608 bp long cox1 gene showed less variance with only 4 haplotypes (accession numbers KP161207–KP161210), including the Italian and German isolates (Tables 2 and 3, Fig. 2). Cox1 and nad1 haplotypes showed a high degree of correlation, so the combination of both did not result in a drastic increase of haplotype numbers (six variants from Namibia and one additional from each Italy and UK) (Table 2). The combined (concatenated) haplotype sequences were used for the phylogenetic ML analysis (Fig. 3).
Table 3.
Positions of nucleotide substitutions within the nad1 and cox1 gene of E. equinus.
| nad1 gene | cox1 gene | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| bp position | 321 | 381 | 818 | 695 | 888 | bp position | 393 | 1165 | 1455 | 1572 |
| NC_020374UK | G | T | A | T | C | NC_020374UK | G | G | T | A |
| Haplotype IN | A | T | A | T | T | Haplotype IN,G | G | A | C | G |
| Haplotype IIN | G | T | A | C | C | Haplotype IIN | G | A | C | A |
| Haplotype IIIN,G | G | T | A | T | T | Haplotype IIIN,I | G | A | T | A |
| Haplotype IVN,* | G | T | A | T | C | Haplotype IVN | T | A | C | G |
| Haplotype VN | G | C | A | T | C | |||||
| Haplotype VII | G | T | G | T | C |
Numbers indicate the position beginning from the start codon.
N = Namibia, G = Germany, I = Italy, UK = United Kingdom.
Identical to reference sequence NC_020374.
Fig. 2.
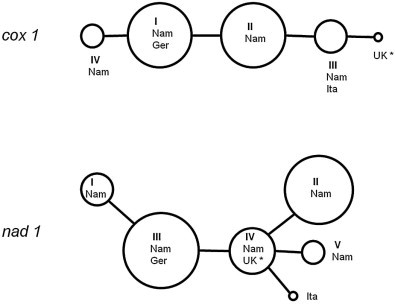
Haplotype networks of the complete mitochondrial cox1 (n = 19) and nad1 (n = 27) sequences of E. equinus isolates obtained in this study. Circle sizes are proportional to the number of Namibian zebra isolates, non-Namibian haplotypes are added as dots only (Nam = Namibia, Ger = Germany, Ita = Italy; * = reference isolate, accession number NC020374). For haplotype numbers (I–VI) see Table 2.
Fig. 3.
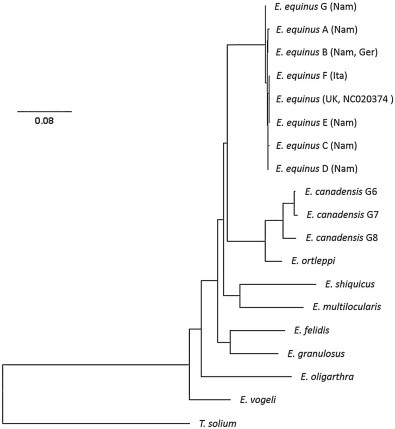
Phylogenetic tree of the genus Echinococcus obtained by maximum likelihood analysis. Phylogram inferred from the concatenated nucleotide sequences of the mitochondrial cox1 and nad1 genes. The scale bar represents the estimated number of nucleotide substitutions per nucleotide site. Nam = Namibia, Ita = Italy, Ger = Germany, UK = United Kingdom. For haplotype letters (A–F) see Table 2. For accession numbers of sequences used for species other than E. equinus see text.
4. Discussion
Cystic echinococcosis, a zoonosis of worldwide distribution, is typically associated with domesticated animals. Dogs are the principal definitive host, and a wide range of livestock species (cattle, yak, water buffalo, sheep, goats, pigs, camels, horses and donkeys) carry the cystic metacestode stage (Cardona and Carmena, 2013). In addition to this, a large number of wild mammal species are known to be suitable hosts, either being involved in primary or secondary “sylvatic” lifecycles, or as accidental hosts due to spill-over from domestic transmission (Carmena and Cardona, 2014). Occasional spill-over may occur wherever the parasite is present in dogs and livestock, and in some cases secondary cycles, involving wild mammals only, became established after anthropogenic introduction of the parasite, e.g. in Australia and possibly in eastern Africa (Jenkins and Morris, 2003; Kagendo et al., 2014). However, sylvatic lifecycles, which are assumed to be primary, i.e. having existed before the domestication of livestock and the subsequent dispersal of their parasites, appear to be restricted to two regions. One is the temperate to subarctic part of the northern hemisphere, with wolves and various cervids (particularly moose) as hosts (Rausch, 1995; Nakao et al., 2013b; Schurer et al., 2013), the other is sub-Saharan Africa, from where many species of large wild carnivores and ungulates have been recorded as hosts (Macpherson and Wachira, 1997; Hüttner and Romig, 2009).
Here we report the presence of E. equinus in lions, black-backed jackals and plains zebras in northern Namibia. We think the transmission of the parasite in this region to be a genuine sylvatic lifecycle – without any involvement of domesticated animals – because domestic dogs, horses or donkeys are not present in the park or in the vicinity, and movement of animals in or out of the park is restricted by fencing. The small sample size of each animal species examined does not allow reliable prevalence estimates, but the high proportions of infection confirmed in lions (four of six) and zebras (eleven of twelve) strongly suggest a stable endemic presence of E. equinus in this ecosystem. Four of the twelve zebras harboured fertile cysts, but these cysts were small (mean diameter 4.2 cm). A large number of cysts were sterile (n = 12), and calcified cysts were also present, ranging in size from <1 cm to 8 cm. Thus, our data on cyst size and condition contrast with the description of E. equinus cysts from three Italian horses, all of which contained large, fertile cysts; small sterile or degenerated cysts were present in an additional three horses, but these cysts were identified as E. granulosus s.s. (Varcasia et al., 2008). Our cyst size and fertility data also contrast with infection data from Tunisian donkeys, whose cysts were invariably fertile whether belonging to E. equinus (n = 22) or E. granulosus s.s. (n = 13) (Boufana et al., 2014).
The parasite was completely absent in six oryx from the same area, a species, which is often seen grazing in mixed groups with zebras. A basic lifecycle between lions and zebras would therefore be highly plausible, with the jackals to become infected through scavenging on zebra carcasses killed by larger predators or by diseases (e.g. anthrax – Beyer et al., 2012). While a large number of other taeniid eggs were found in the carnivores examined, the absence of other Echinococcus spp. was conspicuous. Unfortunately, our small sample does not allow definite conclusions on the absence of other Echinococcus taxa from Etosha National Park. However, even based on these limited data, the epidemiological situation appears fundamentally different from that in eastern Africa, where lions and spotted hyenas from Ugandan and Kenyan national parks and reserves were found to be frequently infected with E. felidis and E. granulosus s.s., but not with E. equinus (Hüttner et al., 2009; Kagendo et al., 2014). Previous records of echinococcosis in the Etosha National Park are restricted to one out of six examined giraffes (Giraffa camelopardalis) having cyst(s) of unreported fertility status and location (Krecek et al., 1990). The identity of this specimen cannot be established, but it raises the question of infectivity of E. equinus for giraffes. As the large mammal faunas of eastern and southern Africa are extremely similar, the differences of Echinococcus spp. lifecycles between Etosha National Park and East African conservation areas cannot be explained. Whether the situation in the Etosha ecosystem is representative for the region, or artificial due to a rather recent population bottleneck of host animals and their inhibited migratory movements (see below), is currently under investigation in similar studies in other parts of Namibia and southern Africa.
Black-backed jackals have not previously been reported before as host for E. equinus, but their host competence – as canids – is not surprising; numerous records of Echinococcus infections of this and other species of jackals are reported from Africa (Macpherson and Wachira, 1997). Lions, however, have long been considered suitable hosts for E. felidis only, and the specific identity of E. felidis (as species, subspecies or strain) had for some time been justified by its adaptation to a felid as definitive host. Records of gravid infections with E. granulosus s.s. in Kenya have already shown that lions are competent host for a wider range of Echinococcus species, and our findings of E. equinus support this conclusion. Interestingly, leopards (P. pardus) and cheetahs (A. jubatus), whose prey range overlaps with that of lions, have neither in this nor in previous studies been positively identified as carriers of Echinococcus spp. (Macpherson and Wachira, 1997; Hüttner et al., 2009; Kagendo et al., 2014). Therefore susceptibility to Echinococcus may be a specific feature of lions rather than other species of large cats.
E. equinus is considered to be globally distributed and specific for Equidae as intermediate hosts (although a captive primate has recently been found infected in the UK – Boufana et al., 2012). However, Echinococcus cysts in equids may also belong to other species, which makes molecular confirmation necessary (Varcasia et al., 2008; Boufana et al., 2014). So far, E. equinus has only been confirmed from domestic horses or donkeys in the United Kingdom (UK), Ireland, Germany, Italy, Spain, Tunisia and Egypt, and a dog in Kyrgyzstan (Bowles et al., 1992; Mwambete et al., 2004; Ziadinov et al., 2008; Blutke et al., 2010; Aboelhadid et al., 2013). With the exception of a captive zebra (E. quagga), born and raised in a UK zoo (Boufana et al., 2012), no molecular confirmation of E. equinus infection for any wildlife host has been previously reported. However, E. equinus was morphologically identified from worm material of dogs that had been fed cysts from mountain zebras (E. zebra) of unknown origin in Namibia (Kumaratilake et al., 1986). This latter record raises the question of the geographic extent of this sylvatic lifecycle in southern Africa. Etosha National Park has been entirely fenced for 40 years, so migration of larger mammals is restricted. The fence is not impenetrable, however, and a lion population to the west of Etosha, coexisting with mountain zebra, is believed to have originated from the park. Also, dispersal of the parasite could occur with smaller hosts (e.g. jackals). While the Etosha region is the southern and western limit of the plains zebra's distribution, the northern subspecies of mountain zebras (E. zebra hartmannae) is much more widespread in Namibia, suggesting a wider distribution of E. equinus. In addition, 60% of plains zebras of the Kruger National Park in northern South Africa were found infected with Echinococcus cysts, which were successfully used in experimental infections of lions (Young, 1975a, 1975b). While this does not prove the existence of E. equinus in the Kruger National Park, it is certainly suggestive that this sylvatic lifecycle of E. equinus could be widespread at least in the northern parts of southern Africa.
There is no evidence to indicate this lifecycle is primary, i.e. having existed before the introduction of domestic animals into the region, or is the result of secondary establishment due to host switching from domestic dog–horse (or dog–donkey) transmission into wildlife. Horses, mules and donkeys, imported from Europe, were kept in large numbers in southern Africa until the early 20th century, and an occasional transmission from dogs to zebras or from horses to lions would not be improbable. Also, when considering the present distribution of the parasite, dynamic changes in the presence of wildlife over time has to be taken into account. Due to excessive hunting, the Etosha region was depleted of larger wild animals from the 1880s until after 1907, when the area became a game reserve and when animals (and their parasites) migrated back from elsewhere (Berry, 1997).
Our panel of Namibian isolates showed some polymorphic sites on the mitochondrial cox1 and nad1 genes. Only a few samples from Italy (2), Germany (1) and UK (reference sequence) were available for comparison of the complete gene sequences used. The Italian cox1 haplotype, but not the nad1 haplotype, was represented in our Namibian panel. The reverse situation was found for the UK isolate, while the German haplotypes of both genes were present in the same combination in Namibia. The latter finding is intriguing (given the colonial history), but any conclusions on possible introduction routes cannot be drawn based on these few samples. In the only other study with a good sized panel of E. equinus samples (22 isolates of donkey origin from Tunisia) no polymorphism of partial cox1 and ef1a gene sequences was found (Boufana et al., 2014). The partial cox1 sequence of the Tunisian samples (acc. No. KM014645) is present in our haplotypes I, II and III. In summary, our samples do not provide evidence that E. equinus in Namibian wildlife shows unique haplotype patterns when compared to “domestic” isolates worldwide, or show differences in levels of diversity. To place the Etosha samples in a global context, a far larger number of isolates will have to be examined from other parts of southern Africa and of the world.
While this study focussed on Echinococcus, eggs or metacestodes of other taeniid species were found in lions, cheetahs, caracals, spotted hyenas and oryx. Of twelve taxa distinguished based on nad1 sequences, only T. regis (lions and oryx), H. taeniaeformis (lion) and T. cf. crocutae (“lineage II” of Terefe et al., 2014, in spotted hyena and oryx) could be identified to species level. The sequences of 9 other taxa clustered with Taenia spp., but did not give sufficient degrees of homology to allocate them to any molecularly characterized species. Only very few sequences are known from any sub-Saharan wildlife Taenia, and both molecular and morphological examinations indicate that the species diversity is far higher than previously assumed (Loos-Frank, 2000; Terefe et al., 2014). We have not attempted to discuss these preliminary Taenia data here, as a more comprehensive study on this genus in African wildlife is in progress.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgements
We are grateful to Antonio Varcasia (Sassari) for kindly providing us with the E. equinus isolates from Italy, and to David Jenkins (Wagga Wagga) for translating the manuscript into a more conventional form of English. The study was undertaken within the framework of the CESSARi consortium (Cystic Echinococcosis in sub-Saharan Africa Research Initiative) and was financially supported by the Deutsche Forschungsgemeinschaft (Projects RO 3753/1-1 and RO 3753/2-1). Permission for undertaking this study was provided by the Ministry of Environment and Tourism, Namibia (Research/Collecting Permit no. 1740/2012).
Footnotes
Nucleotide sequence data reported in this paper are available in GenBank/DDBJ/EMBL databases under the accession numbers KP161207–KP161216.
References
- Aboelhadid S.M., El-Dakhly K.M., Yanai T., Fukushi H., Hassanin K.M. Molecular characterization of Echinococcus granulosus in Egyptian donkeys. Vet. Parasitol. 2013;193:292–296. doi: 10.1016/j.vetpar.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Berry H.H. Historical review of the Etosha Region and its subsequent administration as a National Park. MADOQUA. 1997;20:3–12. [Google Scholar]
- Beyer W., Bellan S., Eberle G., Ganz H.H., Getz W.M., Haumacher R. Distribution and molecular evolution of Bacillus anthracis genotypes in Namibia. PLoS Negl. Trop. Dis. 2012;6:e1534. doi: 10.1371/journal.pntd.0001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blutke A., Hamel D., Hüttner M., Gehlen H., Romig T., Pfister K. Cystic echinococcosis due to Echinococcus equinus in a horse from southern Germany. J. Vet. Diagn. Invest. 2010;22:458–462. doi: 10.1177/104063871002200323. [DOI] [PubMed] [Google Scholar]
- Boufana B., Stidworthy M.F., Bell S., Chantrey J., Masters N., Unwin S. Echinococcus and Taenia spp. from captive mammals in the United Kingdom. Vet. Parasitol. 2012;190:95–103. doi: 10.1016/j.vetpar.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Boufana B., Lahmar S., Rebai W., Ben Safta Z., Jebabli L., Ammar A. Genetic variability and haplotypes of Echinococcus isolates from Tunisia. Trans. R. Soc. Trop. Med. Hyg. 2014;108:706–714. doi: 10.1093/trstmh/tru138. [DOI] [PubMed] [Google Scholar]
- Bowles J., Blair D., McManus D.P. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992;54:165–173. doi: 10.1016/0166-6851(92)90109-w. [DOI] [PubMed] [Google Scholar]
- Cardona G.A., Carmena D. A review of the global prevalence, molecular epidemiology and economics of cystic echinococcosis in production animals. Vet. Parasitol. 2013;192:10–32. doi: 10.1016/j.vetpar.2012.09.027. [DOI] [PubMed] [Google Scholar]
- Carmena D., Cardona G.A. Echinococcosis in wild carnivorous species: epidemiology, genotypic diversity, and implications for veterinary public health. Vet. Parasitol. 2014;202:69–94. doi: 10.1016/j.vetpar.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Clement M., Posada D., Crandall K.A. TCS: a computer program to estimate gene genealogies. Mol. Ecol. 2000;9:1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- Dinnik J.A., Sachs R. Cysticercosis, echinococcosis and sparganosis in wild herbivores in East Africa. Vet. Med. Rev. 1969;2:104–114. [Google Scholar]
- Eugster, R.O., 1978. A contribution to the epidemiology of echinococcosis/hydatidosis in Kenya (East Africa) with special reference to Kajiado District. DVM thesis. University of Zurich.
- Hüttner M., Romig T. Echinococcus species in African wildlife. Parasitology. 2009;136:1089–1095. doi: 10.1017/S0031182009990461. [DOI] [PubMed] [Google Scholar]
- Hüttner M., Nakao M., Wassermann T., Siefert L., Boomker J.D., Dinkel A. Genetic characterization and phylogenetic position of Echinococcus felidis (Cestoda: Taeniidae) from the African lion. Int. J. Parasitol. 2008;38:861–868. doi: 10.1016/j.ijpara.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Hüttner M., Siefert L., Mackenstedt U., Romig T. A survey of Echinococcus species in wild carnivores and livestock in East Africa. Int. J. Parasitol. 2009;39:1269–1276. doi: 10.1016/j.ijpara.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Jenkins D.J., Morris B. Echinococcus granulosus in wildlife in and around the Kosciuszko National Park, south-eastern Australia. Aust. Vet. J. 2003;81:81–85. doi: 10.1111/j.1751-0813.2003.tb11440.x. [DOI] [PubMed] [Google Scholar]
- Kagendo D., Magambo J.K., Agola E.L., Njenga S.M., Zeyhle E., Kakundi E.M. A survey for Echinococcus spp. of carnivores in six wildlife conservation areas in Kenya. Parasitol. Int. 2014;63:604–611. doi: 10.1016/j.parint.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Krecek R.C., Boomker J., Penzhorn B.L., Scheepers L. Internal parasites of giraffes (Giraffa camelopardalis angolensis) from Etosha National Park, Namibia. J. Wildl. Dis. 1990;26:395–397. doi: 10.7589/0090-3558-26.3.395. [DOI] [PubMed] [Google Scholar]
- Kumaratilake L.M., Thompson R.C., Eckert J. Echinococcus granulosus of equine origin from different countries possess uniform morphological characteristics. Int. J. Parasitol. 1986;16:529–540. doi: 10.1016/0020-7519(86)90089-5. [DOI] [PubMed] [Google Scholar]
- Le T.H., Pearson M.S., Blair D., Dai N., Zhang L.H., McManus D.P. Complete mitochondrial genomes confirm the distinctiveness of the horse-dog and sheep-dog strains of Echinococcus granulosus. Parasitology. 2002;124:97–112. doi: 10.1017/s0031182001008976. [DOI] [PubMed] [Google Scholar]
- Loos-Frank B. An up-date of Verster's (1969) ‘Taxonomic revision of the genus Taenia Linnaeus’ (Cestoda) in table format. Syst. Parasitol. 2000;45:155–183. doi: 10.1023/a:1006219625792. [DOI] [PubMed] [Google Scholar]
- Macpherson C.N.L., Wachira T.M. Cystic echinococcosis in Africa south of the Sahara. In: Andersen F.L., Ouhelli H., Kachani M., editors. Compendium of Cystic Echinococcosis in Africa and in Middle Eastern Countries with Special Reference to Morocco. Brigham Young University; Provo, Utah: 1997. pp. 245–277. [Google Scholar]
- Macpherson C.N.L., Else J.E., Suleman M. Experimental infection of the baboon (Papio cynocephalus) with Echinococcus granulosus of camel, cattle, sheep and goat origin from Kenya. J. Helminthol. 1986;60:213–217. doi: 10.1017/s0022149x00026122. [DOI] [PubMed] [Google Scholar]
- Mathis A., Deplazes P., Eckert J. An improved test system for PCR-based specific detection of Echinococcus multilocularis eggs. J. Helminthol. 1996;70:219–222. doi: 10.1017/s0022149x00015443. [DOI] [PubMed] [Google Scholar]
- McCully R.M., van Niekerk J.W., Kruger S.P. Observations on the pathology of bilharziasis and other parasitic infestations of Hippopotamus amphibius Linnaeus, 1758, from the Kruger National Park. Onderstepoort J. Vet. Res. 1967;34:563–617. [PubMed] [Google Scholar]
- Mogoye B.K., Menezes C.N., Wong M.L., Stacey S., von Delft D., Wahlers K. First insights into species and genotypes of Echinococcus in South Africa. Vet. Parasitol. 2013;196:427–432. doi: 10.1016/j.vetpar.2013.03.033. [DOI] [PubMed] [Google Scholar]
- Mwambete D.K., Ponce-Gordo F., Cuesta-Bandera C. Genetic identification and host range of the Spanish strains of Echinococcus granulosus. Acta Trop. 2004;91:87–93. doi: 10.1016/j.actatropica.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Nakao M., Yokoyama N., Sako Y., Fukunaga M., Ito A. The complete mitochondrial DNA sequence of the cestode Echinococcus multilocularis (Cyclophyllidea: Taeniidae) Mitochondrion. 2002;1:497–509. doi: 10.1016/s1567-7249(02)00040-5. [DOI] [PubMed] [Google Scholar]
- Nakao M., Sako Y., Ito A. Isolation of polymorphic microsatellite loci from the tapeworm Echinococcus multilocularis. Infect. Genet. Evol. 2003;3:159–163. doi: 10.1016/s1567-1348(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Nakao M., Sako Y., Ito A. The mitochondrial genome of the tapeworm Taenia solium: a finding of the abbreviated stop codon U. J. Parasitol. 2003;89:633–635. doi: 10.1645/0022-3395(2003)089[0633:TMGOTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Nakao M., McManus D.P., Schantz P.M., Craig P.S., Ito A. A molecular phylogeny of the genus Echinococcus inferred from complete mitochondrial genomes. Parasitology. 2007;134:713–722. doi: 10.1017/S0031182006001934. [DOI] [PubMed] [Google Scholar]
- Nakao M., Lavikainen A., Yanagida T., Ito A. Phylogenetic systematics of the genus Echinococcus (Cestoda: Taeniidae) Int. J. Parasitol. 2013;43:1017–1029. doi: 10.1016/j.ijpara.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Nakao M., Yanagida T., Konyaev S., Lavikainen A., Odnokurtsev V.A., Zaikov V.A. Mitochondrial phylogeny of the genus Echinococcus (Cestoda: Taeniidae) with emphasis on relationships among Echinococcus canadensis genotypes. Parasitology. 2013;140:1625–1636. doi: 10.1017/S0031182013000565. [DOI] [PubMed] [Google Scholar]
- Nelson G.S., Rausch R.L. Echinococcus infections in man and animals in Kenya. Ann. Trop. Med. Parasitol. 1963;57:136–149. doi: 10.1080/00034983.1963.11686169. [DOI] [PubMed] [Google Scholar]
- Obwaller A., Schneider R., Walochnik J., Gollackner B., Deutz A., Janitschke K. Echinococcus granulosus strain differentiation based on sequence heterogeneity in mitochondrial genes of cytochrome c oxidase-1 and NADH dehydrogenase-1. Parasitology. 2004;128:569–575. doi: 10.1017/s0031182004004871. [DOI] [PubMed] [Google Scholar]
- Posada D., Crandall K.A. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Rausch R.L. Life cycle patterns and geographic distribution. In: Thompson R.C.A., Lymbery A.J., editors. Echinococcus and Hydatid Disease. CAB International; Wallingford: 1995. pp. 89–134. [Google Scholar]
- Schurer J., Shury T., Leighton F., Jenkins E. Surveillance for Echinococcus canadensis genotypes in Canadian ungulates. Int. J. Parasitol. Parasites Wildl. 2013;2:97–101. doi: 10.1016/j.ijppaw.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, MA: 2002. PAUP*. Phylogenetic Analysis Using Parsimony (and Other Methods). Version 4 Beta. [Google Scholar]
- Terefe Y., Hailemariam Z., Menkir S., Nakao M., Lavikainen A., Haukisalmi V. Phylogenetic characterisation of Taenia tapeworms in spotted hyenas and reconsideration of the “Out of Africa” hypothesis of Taenia in humans. Int. J. Parasitol. 2014;44:533–541. doi: 10.1016/j.ijpara.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Varcasia A., Garippa G., Pipia A.P., Scala A., Brianti E., Giannetto S. Cystic echinococcosis in equids in Italy. Parasitol. Res. 2008;102:815–818. doi: 10.1007/s00436-007-0862-7. [DOI] [PubMed] [Google Scholar]
- Verster A.J. Review of Echinococcus species in South Africa. Onderstepoort J. Vet. Res. 1965;32:7–118. [PubMed] [Google Scholar]
- Verster A.J., Collins M. The incidence of hydatidosis in the Republic of South Africa. Onderstepoort J. Vet. Res. 1966;33:49–72. [PubMed] [Google Scholar]
- Woodford M.H., Sachs R. The incidence of cysticercosis, hydatidosis and sparganosis in wild herbivores of the Queen Elizabeth National Park, Uganda. Bull. Epizoot. Dis. Afr. 1973;21:265–271. [Google Scholar]
- Young E. Echinococcosis (hydatodosis) in wild animals of the Kruger National Park. J. S. Afr. Vet. Assoc. 1975;46:285–286. [PubMed] [Google Scholar]
- Young E. Some important parasitic and other diseases of lion, Panthera leo, in the Kruger National Park. J. S. Afr. Vet. Assoc. 1975;46:181–183. [PubMed] [Google Scholar]
- Ziadinov I., Mathis A., Trachsel D., Rysmukhambetova A., Abdyjaparov T.A., Kuttubaev O.T. Canine echinococcosis in Kyrgyzstan: using prevalence data adjusted for measurement error to develop transmission dynamics models. Int. J. Parasitol. 2008;38:1179–1190. doi: 10.1016/j.ijpara.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


