Abstract
Isavuconazole is a novel broad-spectrum triazole antifungal agent. This open-label dose escalation study assessed the safety and pharmacokinetics of intravenous isavuconazole prophylaxis in patients with acute myeloid leukemia who had undergone chemotherapy and had preexisting/expected neutropenia. Twenty-four patients were enrolled, and 20 patients completed the study. The patients in the low-dose cohort (n = 11) received isavuconazole loading doses on day 1 (400/200/200 mg, 6 h apart) and day 2 (200/200 mg, 12 h apart), followed by once-daily maintenance dosing (200 mg) on days 3 to 28. The loading and maintenance doses were doubled in the high-dose cohort (n = 12). The mean ± standard deviation plasma isavuconazole areas under the concentration-time curves for the dosing period on day 7 were 60.1 ± 22.3 μg · h/ml and 113.1 ± 19.6 μg · h/ml for the patients in the low-dose and high-dose cohorts, respectively. The adverse events in five patients in the low-dose cohort and in eight patients in the high-dose cohort were considered to be drug related. Most were mild to moderate in severity, and the most common adverse events were headache and rash (n = 3 each). One patient in the high-dose cohort experienced a serious adverse event (unrelated to isavuconazole treatment), and two patients each in the low-dose and high-dose cohorts discontinued the study due to adverse events. Of the 20 patients who completed the study, 18 were classified as a treatment success. In summary, the results of this analysis support the safety and tolerability of isavuconazole administered at 200 mg and 400 mg once-daily as prophylaxis in immunosuppressed patients at high risk of fungal infections. (This study is registered at ClinicalTrials.gov under registration number NCT00413439.)
INTRODUCTION
Invasive fungal infections (IFIs) are associated with significant morbidity, mortality, and health care costs in patients with hematologic malignancies (1, 2). Allogeneic hematopoietic stem cell transplantation and chemotherapy-associated neutropenia place patients with acute myeloid leukemia (AML) at risk of developing an IFI (3). Aspergillus spp. and Candida spp. are the predominant IFI pathogens in patients with acute leukemia (1, 3, 4). However, other molds, such as the Mucorales, Scedosporium spp., and Fusarium spp., are emerging as pathogens in this setting and are associated with high mortality rates (1).
Because IFIs are often difficult to diagnose, and delays in treatment can significantly increase the risk of mortality (5, 6), antifungal prophylaxis has become a commonly used strategy in patients at high risk of IFIs (7, 8). Fluconazole, itraconazole, posaconazole, voriconazole, and micafungin are recommended for prophylactic use in patients with hematologic malignancies (9, 10). Although the results of randomized clinical trials support the prophylactic benefits of these agents (7, 8, 11), each may be limited in their use: fluconazole has no activity against molds (12); posaconazole demonstrates broad-spectrum activity against both yeasts and molds (13), but optimal absorption of the oral suspension of posaconazole is dependent on administration with a high-fat meal (however, the delayed-release tablets have improved bioavailability) (14); voriconazole is as effective as fluconazole in preventing IFIs (15) but has been associated with breakthrough mucormycosis and a high incidence of side effects (16, 17); itraconazole tablets have variable bioavailability, and its oral suspension has poor tolerability (18); and micafungin is available only as an intravenous (i.v.) formulation and has no activity against the Mucorales or Fusarium species (19).
Isavuconazole is a novel triazole antifungal being developed for the treatment of invasive candidiasis, aspergillosis, and emerging mold infections. Available in both i.v. and oral formulations, isavuconazole is the active antifungal component of isavuconazonium sulfate, a water-soluble prodrug that is rapidly and almost completely (>99%) converted to isavuconazole via plasma esterases (20). The results of in vitro and animal model studies have shown that isavuconazole is active against Candida spp. (both fluconazole-sensitive and fluconazole-resistant strains), Aspergillus spp., Cryptococcus spp., and other molds, including the Mucorales and Scedosporium species (21–28).
This open-label, sequential-cohort, phase 2 study was designed to assess the safety and tolerability of isavuconazole as a prophylactic agent in neutropenic patients who had undergone chemotherapy for AML. The pharmacokinetics (PK) and efficacy of isavuconazole in prophylaxis were also assessed.
MATERIALS AND METHODS
Patients.
Patients ≥18 years of age who were diagnosed with AML, entering first induction treatment or subsequent chemotherapy, had not experienced a prior IFI (29), and were expected to have preexisting or chemotherapy-induced neutropenia (absolute neutrophil count [ANC], <500/mm3) for ≥10 days and <28 days after enrollment were eligible for inclusion in the study. The exclusion criteria comprised the use of any systemic antifungal therapy for >72 h during the 5-day screening period, receipt of any systemic antifungal therapy for proven or probable fungal infection in the previous 12 months, fever (central body temperature, >38°C), pregnancy or breastfeeding, known hypersensitivity to azoles or any component of the study medication, or concomitant use of rifampin, rifabutin, ergot alkaloids, terfenadine, astemizole, cisapride, pimozide, quinidine, long-acting barbiturates, neostigmine, or carbamazepine. Patients were also excluded if they had a concomitant medical condition that in the opinion of the investigator posed an unacceptable risk to the patient, had received any investigational treatment within the 30 days preceding the administration of the study medication, had other or additional medical reasons for neutropenia or immunosuppression, or demonstrated hepatic or renal dysfunction (i.e., total bilirubin, >3 times the upper limit of normal [ULN]; alanine aminotransferase or aspartate aminotransferase level, >5 times the ULN; calculated creatinine clearance, <50 ml/min; or history of oliguria [<20 ml/h] that was unresponsive to fluid challenge).
Study design.
This was an open-label, multicenter, sequential-cohort, phase 2, dose escalation study conducted at four sites in Germany between June 2006 and April 2007 (registered at ClinicalTrials.gov under registration number NCT00413439). Each patient was screened during a 5-day period before the initiation of study treatment (days −5 to −1) to ensure that the eligibility criteria were met and to collect baseline demographic and clinical data. Intravenous dosing of the prodrug was initiated, at the earliest, 24 h after the last chemotherapy dose, and it was continued until 48 h after neutropenia resolved (ANC, >500/mm3), with a treatment limit of 28 days. The patients attended follow-up visits at 14 and 35 days after the last dose of isavuconazole. The prodrug isavuconazonium sulfate was administered to all patients; the doses of the prodrug are expressed in mg equivalents of the active drug isavuconazole.
The data from healthy volunteers were the basis for dose selection. Isavuconazole has potent activity in vitro against Candida spp. (MIC50, <0.008 μg/ml) and Aspergillus spp. (MIC, <2 μg/ml). The doses were selected to achieve near-steady-state plasma isavuconazole levels within 3 to 4 days of treatment initiation that were higher than these concentrations. The anticipated mean maximum isavuconazole concentration in plasma (Cmax) was <10 μg/ml, and the anticipated trough levels were 1.5 to 4 μg/ml.
The patients in the low-dose cohort received three i.v. loading doses of isavuconazole at 400 mg, 200 mg, and 200 mg on day 1 (each infusion administered over 4 h, at equal intervals), followed by further loading doses of i.v. isavuconazole at 200 mg twice daily on day 2 (each infusion administered over 2 h, with 10 h between infusions), and then a once-daily maintenance dose of i.v. isavuconazole at 200 mg (administered over 2 h) from day 3 to the end of treatment.
The patients in the high-dose cohort received doses that were 2-fold higher than those used in the low-dose cohort, i.e., 800 mg/400 mg/400 mg on day 1, 400 mg twice daily on day 2, and 400 mg once daily from day 3 to the end of treatment. The infusion rates were adjusted in the high-dose cohort to ensure that isavuconazole was administered according to the same time schedule used in the patients in the low-dose cohort. An option for oral dosing was also available (8/4/4 capsules [100 mg each] on day 1, 4 capsules twice daily on day 2, and 4 capsules once daily from day 3 to the end of treatment).
Cohort testing was conducted sequentially: the study began with the low-dose cohort, followed by the high-dose cohort. Initiation of the study in the high-dose cohort was permitted only if all of the following conditions were met in the low-dose cohort during study treatment: at least nine patients were enrolled, at least six of whom received ≥9 days of therapy, and no dose-limiting toxicity was observed. Dose-limiting toxicity was defined as at least three patients at the same dose level reporting the same drug-related adverse event (AE) of grade 3 (severe) or higher, severe QT prolongation in at least one patient (QTc, >500 ms; or change from baseline of >60 ms), unexplained electrocardiogram (ECG) abnormality in at least two patients, and liver function test increases of >3 times the ULN, with a plausible temporal association in at least two patients.
Follow-up visits were scheduled for patients in both cohorts at 14 and 35 days after the end of isavuconazole treatment. The patients who prematurely discontinued the study treatment were allowed to continue to participate in the study, i.e., attend the remaining scheduled visits and undergo prespecified testing procedures.
The study protocol was approved by the independent ethics committee at each participating study site, and each patient provided written informed consent before participating in the study. The study was conducted in compliance with the International Conference on Harmonisation, good clinical practice guidelines, the Declaration of Helsinki, and local regulations.
Pharmacokinetic analyses.
In both cohorts, serial blood samples were collected to determine isavuconazole plasma concentrations at 12 h after the start of treatment (C12), the maximum plasma concentration (Cmax), and the area under the plasma concentration-time curve from time zero to 24 h after the initiation of isavuconazole administration (AUC0–24) on days 1 (predose and at 4, 6, 12, and 24 h after the start of the first infusion) and 7 (predose and at 2, 3, 4, 6, 12, and 24 h after the start of infusion). Isavuconazole plasma trough levels (Cmin) were determined using blood samples collected predose on days 2, 3, 5, 7, 8, 14, and 21 and at the end of treatment.
Isavuconazole plasma concentrations were determined using a validated liquid chromatography-mass spectroscopy assay, with C12, Cmax, and AUC0–24 derived via noncompartmental analysis using WinNonlin Professional (version 5.1b; Pharsight Corporation, Mountain View, CA) and the actual blood sampling time.
Safety and tolerability analyses.
Safety and tolerability were assessed in both patient cohorts via AE monitoring, laboratory testing, 12-lead ECG testing, clinical symptoms, vital sign measurements, and physical examinations.
An AE was defined as any untoward medical occurrence that did not necessarily have a causal relationship with the treatment in a patient administered a study drug. All AEs were recorded and graded on a 4-point scale (1, mild; 2, moderate; 3, severe; 4, life-threatening), as per the Common Terminology Criteria for Adverse Events guidelines (30). Infections and related microbiology findings were described as AEs.
Treatment-emergent AEs (TEAEs) were defined as all events occurring after the first treatment administration and up to 28 days posttreatment. Serious AEs (SAEs) were defined as AEs that resulted in death, were life-threatening, required inpatient hospitalization or prolongation of existing hospitalization, or resulted in persistent or significant disability/incapacity. AEs were monitored throughout the study.
Routine clinical laboratory assessments of hematology, biochemistry, and urinalysis were performed at screening, on days 7, 14, and 21, at the end of treatment, and at the follow-up visits if abnormalities were observed at the end of treatment. Additional standard biochemistry assessments for safety were also performed on days 3 and 5. Serum pregnancy testing was performed at screening, at the end of treatment, and at the final follow-up visit.
Twelve-lead ECG testing was performed predose on day 1 and within 15 min of completing each infusion on days 1, 2, and 3, at the end of treatment, and at follow-up. The 24-h Holter ECG test was conducted at screening and on days 1, 2, and 7.
Vital sign measurements and physical examinations were conducted at screening, on days 1, 2, 3, 5, 7, 14, and 21, and at the end of treatment. Vital signs were measured predose and at the end of study drug infusion during each of these scheduled visits.
Efficacy analyses.
Efficacy was assessed in each patient cohort via the rates of treatment success versus failure. Treatment success was defined as the absence of breakthrough fungal infections and the lack of need for other systemic antifungal therapy during the study. Treatment failure was defined as the occurrence of a probable or proven breakthrough fungal infection or as the need for another systemic antifungal therapy before the end of isavuconazole treatment due to possible fungal infection. Proven and probable infections were defined according to the consensus criteria of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (29). A possible fungal infection was based on evidence of lung infiltrates via a high-resolution computerized tomography scan, persistent fever (>72 h), or any other condition requiring additional systemic antifungal therapy. Patients who were classified as treatment failures were treated with alternative antifungal therapy, according to local standards.
Statistical analyses.
This was an exploratory study that was not statistically powered. The study protocol required that at least nine patients be enrolled in each cohort. The patients who received ≥1 dose of isavuconazole were eligible for inclusion in the safety, tolerability, and efficacy analyses. The patients who received ≥1 dose of isavuconazole and whose PK data were adequate for the calculation of at least one PK parameter were included in the PK analyses.
Patient demographics and baseline characteristics were summarized using descriptive statistics, as were safety, tolerability, and efficacy outcomes and isavuconazole plasma concentrations at prespecified time points.
The dose proportionality of the isavuconazole Cmax and AUC0–24 parameters was evaluated using a one-way analysis of variance model in which the factor dose was applied to logarithmically transformed and dose-normalized values of Cmax and AUC0–24. The ratios of Cmax and AUC0–24 geometric means (calculated as the ratio of the high-dose cohort to the low-dose cohort) and the corresponding 95% confidence intervals (CIs) were obtained from this model; dose proportionality was concluded if the 95% CIs for Cmax and AUC0–24 geometric mean ratios fell entirely within the 80% to 125% CI acceptance range.
RESULTS
Patients.
A total of 24 inpatients (12 in each cohort) were enrolled in the study. Eleven of the 12 patients in the low-dose cohort and all 12 patients in the high-dose cohort received ≥1 dose of the study drug; these patients comprised the safety population. Baseline demographic and clinical characteristics were generally similar in the two cohorts (Table 1).
TABLE 1.
Baseline demographics and clinical characteristicsa
| Characteristic | Low-dose cohort (n = 11)b | High-dose cohort (n = 12)c |
|---|---|---|
| Age (mean [range]) (yr) | 47.8 (32–67) | 48.4 (24–62) |
| Sex (no. [%]) | ||
| Men | 7 (63.6) | 10 (83.3) |
| Women | 4 (36.4) | 2 (16.7) |
| Caucasian (no. [%]) | 11 (100.0) | 12 (100.0) |
| Wt (mean [range]) (kg) | 74.2 (54.9–101.4) | 86.6 (56.2–117.0) |
| Ht (mean [range]) (cm) | 174.6 (160–186) | 178.9 (164–189) |
| BMI (mean [range]) (kg/m2)d | 24.2 (18.3–31.3) | 26.9 (20.4–36.1) |
For the safety population (received ≥1 dose of study medication).
Isavuconazole administered as a 200-mg once-daily maintenance regimen.
Isavuconazole administered as a 400-mg once-daily maintenance regimen.
BMI, body mass index.
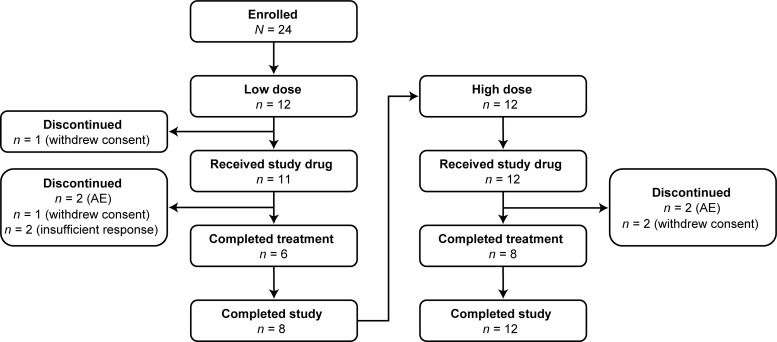
Six (50%) patients in the low-dose cohort and eight (66.7%) patients in the high-dose cohort safety population completed the study treatment (Fig. 1). The reasons for treatment and study discontinuation included the withdrawal of consent, hypersensitivity, and infusion-related reactions (Table 2).
FIG 1.
Patient flow diagram. Two patients in the low-dose cohort and four patients in the high-dose cohort prematurely discontinued study treatment but completed the study (i.e., attended remaining scheduled visits and underwent prespecified testing procedures). The study began with 12 patients (of the 24 enrolled) in the low-dose cohort, followed by a second set of 12 patients in the high-dose cohort; none of the 12 patients in the high-dose cohort had participated in the low-dose phase of the study.
TABLE 2.
Study withdrawals and treatment discontinuation of patients who received at least one dose of isavuconazole
| Patient | M/Fa | Age (yr) | Duration of study treatment (days) | Treatment discontinuation or study withdrawalb | Reason for treatment discontinuation or study withdrawal |
|---|---|---|---|---|---|
| Low-dose cohort (n = 11)c | |||||
| 1 | F | 33 | 1 | Study withdrawal | AE (hypersensitivity reaction)d |
| 2 | F | 32 | 2 | Study withdrawal | AE (infusion-related reaction) |
| 3 | M | 39 | 2 | Study withdrawal | Patient withdrew consent |
| 4 | F | 54 | 12 | Treatment discontinuation | Protocol-defined treatment failure due to predefined indicator of possible fungal infection (lung infiltrates)e |
| 5 | F | 50 | 7 | Treatment discontinuation | Protocol-defined treatment failure due to predefined indicator of possible fungal infection (treatment-refractory fever)f |
| High-dose cohort (n = 12)g | |||||
| 1 | F | 58 | 11 | Treatment discontinuation | Patient withdrew consent |
| 2 | F | 48 | 1 | Treatment discontinuation | AE (dizziness, hypersensitivity, and nausea) |
| 3 | M | 53 | 5 | Treatment discontinuation | AE (skin infection/petechiae) |
| 4 | M | 57 | 12 | Treatment discontinuation | Patient withdrew consent |
M/F, male/female.
Patients prematurely discontinued study treatment but completed the study (i.e., attended remaining scheduled visits and underwent prespecified testing procedures).
Isavuconazole administered as a 200-mg once-daily maintenance regimen.
AE, adverse event.
No mycologic evidence of fungal infection based on galactomannan antigen testing conducted before, during, or following detection of lung infiltrates.
No mycologic evidence of fungal infection based on blood culture results from blood samples collected during the period of persistent fever.
Isavuconazole administered as a 400-mg once-daily maintenance regimen.
Pharmacokinetics.
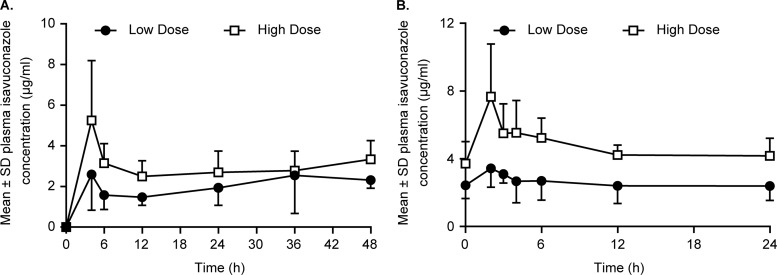
Twenty-one and 18 patients were evaluable for PK analyses on days 1 and 7, respectively. The mean isavuconazole plasma concentration-versus-time profiles for both isavuconazole dose cohorts on days 1 and 7 are shown in Fig. 2. Mean isavuconazole C12 values of 1.5 and 2.5 μg/ml were obtained in the low-dose and high-dose cohorts, respectively, following the initial loading dose on day 1 (Table 3).
FIG 2.
Mean ± standard deviation (SD) isavuconazole plasma concentration-time profiles after intravenous administration of isavuconazole on days 1 (A) and 7 (B) in the low-dose and high-dose cohorts.
TABLE 3.
Mean isavuconazole plasma pharmacokinetic parametersa
| Parameterd | Low-dose cohortb |
High-dose cohortc |
||
|---|---|---|---|---|
| Day 1 (n = 10) | Day 7 (n = 8)e | Day 1 (n = 11) | Day 7 (n = 10) | |
| C12 (μg/ml) | 1.5 (0.4) | 2.5 (0.8) | ||
| Cmax (μg/ml) | 3.6 (1.0) | 8.0 (2.8) | ||
| AUC0–24 (μg · h/ml) | 60.1 (22.3) | 113.1 (19.6) | ||
PK analysis set; values reflect mean (standard deviation [SD]).
Isavuconazole administered as a 200-mg once-daily maintenance regimen.
Isavuconazole administered as a 400-mg once-daily maintenance regimen.
C12, plasma concentration at 12 h after the start of treatment; Cmax, maximum plasma concentration of drug; AUC0–24, area under the plasma concentration-time curve from time 0 to 24 h after the initiation of isavuconazole intake.
For AUC0–24, n = 7.
The mean isavuconazole Cmax and AUC0–24 values on day 7 are listed in Table 3. Interpatient variability was relatively low on day 7 (steady state) for Cmax (27.9% for the 200-mg dose and 35.6% for the 400-mg dose) and AUC0–24 (37.2% for the 200-mg dose and 17.3% for the 400-mg dose).
On day 7, the geometric least-squares mean ratios for Cmax and AUC0–24 were 106.6% and 97.7%, respectively. The 95% CI of the ratio of isavuconazole dose-normalized geometric mean Cmax (81.7, 139.2) and AUC0–24 (78.5, 121.8) values were slightly outside the predefined acceptance range of 80% to 125%.
Safety and tolerability. (i) Adverse events.
A total of 77 TEAEs were reported for 10 (90.9%) patients in the low-dose cohort compared with 120 TEAEs in 12 (100%) patients in the high-dose cohort. The most commonly reported TEAEs were fever (n = 6 [54.5%]), diarrhea (n = 4 [36.4%]), and rash (n = 4 [36.4%]) in the low-dose cohort, compared with fever (n = 12 [100%]) and nausea (n = 8 [66.7%]) in the high-dose cohort. The majority of the TEAEs reported in both cohorts were of mild-to-moderate intensity (Table 4). One SAE was reported, a case of life-threatening respiratory distress in a patient in the high-dose cohort, which was not considered to be attributable to the study medication.
TABLE 4.
Treatment-emergent AEs and drug-related TEAEs by severity gradea
| TEAE characteristics by type | No. (%) by severity in: |
|||||
|---|---|---|---|---|---|---|
| Low-dose cohort (n = 11)b |
High-dose cohort (n = 12)c |
|||||
| Mild | Moderate | Severe | Mild | Moderate | Severe | |
| All TEAEs | ||||||
| Total no. | 45 | 28 | 4 | 45 | 49 | 25 |
| Most commonly reportedd | ||||||
| Fever | 3 (27.3) | 1 (9.1) | 2 (18.2) | 2 (16.7) | 6 (50.0) | 4 (33.3) |
| Diarrhea | 3 (27.3) | 1 (9.1) | 0 (0) | 4 (33.3) | 2 (16.7) | 0 (0) |
| Rash | 1 (9.1) | 3 (27.3) | 0 (0) | 2 (16.7) | 1 (8.3) | 1 (8.3) |
| Cough | 1 (9.1) | 2 (18.2) | 0 (0) | 3 (25.0) | 1 (8.3) | 1 (8.3) |
| Nausea | 0 (0) | 1 (9.1) | 0 (0) | 4 (33.3) | 2 (16.7) | 2 (16.7) |
| Thrombocytopenia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (8.3) | 3 (25.0) |
| Drug-related | ||||||
| Total no. | 6 | 3 | 2 | 9 | 8 | 4 |
| Most commonly reportede | ||||||
| Hypotension | 2 (18.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vertigo | 2 (18.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Headache | 0 (0) | 1 (9.1) | 0 (0) | 1 (8.3) | 1 (8.3) | 0 (0) |
| Rash | 1 (9.1) | 0 (0) | 0 (0) | 1 (8.3) | 1 (8.3) | 0 (0) |
| Cough | 0 (0) | 0 (0) | 0 (0) | 2 (16.7) | 0 (0) | 0 (0) |
Safety population (received ≥1 dose of study medication); AE severity defined by the Common Terminology Criteria for Adverse Events guidelines (30). TEAE, treatment-emergent adverse event.
Isavuconazole administered as a 200-mg once-daily maintenance regimen; no life-threatening TEAEs were reported in this cohort.
Isavuconazole administered as a 400-mg once-daily maintenance regimen; a single life-threatening TEAE of respiratory distress that was not drug related was reported in this cohort.
TEAEs were reported by >30% of patients in either cohort; each AE was counted only once at its most extreme severity in each patient.
TEAEs were reported by ≥2 patients in either cohort.
A total of 11 TEAEs that occurred in five (45.5%) patients in the low-dose cohort and 21 TEAEs that occurred in eight (66.7%) patients in the high-dose cohort were considered by the investigators to be drug related (Table 4). The most commonly experienced drug-related TEAEs were headache (n = 3 [13.0%]) and rash (n = 3 [13.0%]). The majority of the drug-related TEAEs in both cohorts were of mild-to-moderate intensity; no drug-related TEAE was considered life-threatening. More patients experienced drug-related TEAEs during the maintenance phase in the high-dose cohort (n = 6 [54.5%]) than in the low-dose cohort (n = 2 [25.0%]; Table 5). Comparatively few patients in each cohort experienced drug-related TEAEs during the loading-dose phase. Two patients in the low-dose cohort and two patients in the high-dose cohort discontinued the study due to TEAEs (Table 2).
TABLE 5.
Drug-related TEAEs by dosing phasea
| TEAE data | Low-dose cohortb |
High-dose cohortc |
||||
|---|---|---|---|---|---|---|
| 800 mg, day 1 | 400 mg, day 2 | 200 mg, day 2 to EOTd | 1,600 mg, day 1 | 800 mg, day 2 | 400 mg, day 3 to EOT | |
| n | 11 | 10 | 8 | 12 | 11 | 11 |
| Patients with drug-related TEAEs (no. [%]) | 3 (27.3) | 2 (20.0) | 2 (25.0) | 3 (25.0) | 1 (9.1) | 6 (54.5) |
| Total no. of TEAEs | 4 | 3 | 5 | 5 | 1 | 15 |
| Most commonly reported AEs (no. [%])e | ||||||
| Cough | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (18.2) |
| Headache | 1 (9.1) | 1 (10.0) | 0 (0) | 0 (0) | 0 (0) | 2 (18.2) |
| Hypotension | 0 (0) | 1 (10.0) | 1 (12.5) | 0 (0) | 0 (0) | 0 (0) |
| Rash | 0 (0) | 0 (0) | 1 (12.5) | 1 (8.3) | 0 (0) | 1 (9.1) |
| Vertigo | 1 (9.1) | 1 (10.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
TEAE, treatment-emergent adverse event.
Isavuconazole administered as a 200-mg once-daily maintenance regimen.
Isavuconazole administered as a 400-mg once-daily maintenance regimen.
EOT, end of treatment.
TEAEs were reported by ≥2 patients in either cohort.
(ii) Other safety outcomes.
Postbaseline abnormalities in the liver function tests were experienced by patients in the high-dose cohort: one patient had alanine aminotransferase levels at >5 times the ULN, one patient had aspartate aminotransferase levels at >3 times the ULN, and one patient had alkaline phosphatase levels at >2 times the ULN. The study records did not capture whether these abnormalities were all experienced by one patient or by up to three different patients. No patients in the low-dose cohort experienced abnormal liver function tests. The changes from baseline in the laboratory and ECG (12-lead and Holter) parameters, vital signs, and physical examination findings were not clinically relevant and were consistent with the underlying disease of AML and associated neutropenia. QTc prolongation was not observed during isavuconazole therapy.
Efficacy.
Overall, 18 of the 20 patients (90.0%) who completed the study were classified as a treatment success, six (75%) of whom were in the low-dose cohort and 12 (100%) of whom were in the high-dose cohort (P = 0.147). Two patients in the low-dose cohort who completed the study had protocol-defined treatment failures due to a possible fungal infection (Table 2). Both patients discontinued isavuconazole prophylaxis and were treated with empirical caspofungin therapy. One patient was still considered a treatment failure at the final follow-up visit, while the second patient demonstrated no evidence of fungal infection based on further mycological investigation. There was no instance of probable or proven breakthrough fungal infection in either cohort during isavuconazole prophylaxis.
DISCUSSION
This is the first study to assess the PK, safety, and tolerability of isavuconazole for the prevention of fungal infections in patients with severe and prolonged neutropenia. In studies of healthy volunteers, isavuconazole has been shown to be well tolerated and to have a low clearance, large volume of distribution, high bioavailability, and long elimination half-life (20, 31). The present study extends these data to provide PK parameters for two high- and low-dose regimens of i.v. isavuconazole. The interpatient variabilities for both Cmax and AUC0–24 on day 7 were low. In addition, there was no evidence to suggest that concomitant medication use influenced isavuconazole PK; however, a number of key medications, such as rifampin, were specifically excluded from this study, limiting conclusions on the possible interactions with these medications (data not shown).
Previously reported PK outcomes in healthy volunteers suggest that i.v. isavuconazole has predictable linear PK (31). In the current study, the 95% CIs of the ratios of isavuconazole dose-normalized geometric mean Cmax and AUC0–24 values were slightly outside the normal acceptance range. As a result, dose proportionality could not be conclusively determined in this study. This was possibly a consequence of the small sample size in this trial, and future studies with larger numbers of patients may demonstrate dose proportionality.
None of the predefined safety criteria for preventing escalation to the higher-dose isavuconazole regimen were met. Moreover, there were no unexpected safety concerns during the higher-dose treatment. The number of patients who experienced drug-related TEAEs during maintenance treatment was higher in the high-dose cohort (55%) than in the low-dose cohort (25%). However, comparatively few patients experienced drug-related TEAEs during loading with isavuconazole at 1,600 mg (25%, high-dose cohort) or 800 mg (27%, low-dose cohort; 9%, high-dose cohort).
The monitoring of AEs showed that the majority of the drug-related TEAEs were of mild-to-moderate severity, and rash and headache were the most commonly reported drug-related TEAEs. One patient in the high-dose group and two patients in the low-dose group discontinued treatment due to TEAEs of hypersensitivity, nausea, and dizziness (Fig. 1) during administration of the loading doses; however, these reactions resolved after the termination of the infusions and administration of antihistamine treatment. In addition, there were no deaths, and the SAE reported was not related to isavuconazole treatment. Abnormal liver function tests were reported in up to three patients in the high-dose group. No QTc prolongation was observed during isavuconazole treatment.
In conclusion, the results of this exploratory study support the safety and tolerability of isavuconazole prophylaxis (in 200-mg and 400-mg once-daily maintenance regimens) in neutropenic patients with AML. Pharmacokinetic parameters were determined at both dose levels in this patient population. In addition, 18 of the 20 patients who completed the study were classified as a treatment success. In the completed and ongoing phase 3 trials (ClinicalTrials.gov registration numbers) in the primary treatment of invasive candidiasis (NCT00413218), invasive aspergillosis (NCT00412893 and NCT00634049), and emerging fungal pathogens (NCT00634049), the patients assigned to isavuconazole treatment received 200 mg of i.v. drug three times a day on days 1 and 2, followed by either 200 mg of i.v. or oral drug once daily from day 3 onwards. The results of these trials will provide more definitive information on the efficacy, safety, and tolerability of isavuconazole therapy in this patient population.
ACKNOWLEDGMENTS
Angelika Böhme was an employee of Medizinische Klinik III, J. W. Goethe-Universität, Frankfurt, Germany, at the time of the study. Andrew J. Ullmann was an employee of Medical Center of the Johannes Gutenberg University, Mainz, Germany, at the time of the study.
Isavuconazole is in codevelopment by Astellas and Basilea Pharmaceutica International, Ltd. Funding for this study was provided by Basilea Pharmaceutica International, Ltd. Editorial assistance was provided by Tracy Wetter and Rick Davis from Complete Healthcare Communications, Inc., and Radhika Bhatia and Neil Thomas of Envision Scientific Solutions. Editorial assistance was funded by Astellas and Basilea Pharmaceutica International, Ltd.
Oliver A. Cornely is supported by the German Federal Ministry of Research and Education (BMBF grant 01KN1106); has received research grants from 3M, Actelion, Astellas, Basilea Pharmaceutica International, Ltd., Bayer, Celgene, Cubist, F2G, Genzyme, Gilead, GSK, Merck/MSD, Miltenyi, Optimer, Pfizer, Quintiles, and ViroPharma; has consulted for 3M, Astellas, Basilea Pharmaceutica International, Ltd., Cubist, F2G, Gilead, GSK, Merck/MSD, Optimer, Pfizer, Sanofi Pasteur, and Summit/Vifor; and has received lecture honoraria and travel support from Astellas, Gilead, Merck/MSD, and Pfizer. Angelika Böhme has been a board member of Gilead and MSD. She has been on the speakers' bureau of MSD and has received support for travel and accommodation from Bristol-Myers Squibb, Celgene, Hexal, MSD, Novartis, and Teva. Her institution has received grants from Hexal, MSD, and Teva. Anne Schmitt-Hoffmann is an employee of Basilea Pharmaceutica International, Ltd. Andrew J. Ullmann has received support for travel to meetings for the current study from Astellas and Basilea Pharmaceutica International, Ltd. He is a consultant for and on the speakers' bureaus of Astellas, Gilead, MSD, and Pfizer. He has also received support for travel and accommodation from Astellas, Boehringer Ingelheim, Gilead, MSD, and Pfizer for activities unrelated to the current study. His institution has received grants from Astellas, Gilead, MSD, and Pfizer.
REFERENCES
- 1.Pagano L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, Pastore D, Picardi M, Bonini A, Chierichini A, Fanci R, Caramatti C, Invernizzi R, Mattei D, Mitra ME, Melillo L, Aversa F, Van Lint MT, Falcucci P, Valentini CG, Girmenia C, Nosari A. 2006. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 91:1068–1075. [PubMed] [Google Scholar]
- 2.Menzin J, Meyers JL, Friedman M, Perfect JR, Langston AA, Danna RP, Papadopoulos G. 2009. Mortality, length of hospitalization, and costs associated with invasive fungal infections in high-risk patients. Am J Health Syst Pharm 66:1711–1717. doi: 10.2146/ajhp080325. [DOI] [PubMed] [Google Scholar]
- 3.Caira M, Girmenia C, Fadda RM, Mitra ME, Picardi M, Van Lint MT, Nosari A, Candoni A, Bonini A, Mattei D, de Waure C, Fianchi L, Valentini CG, Aversa F, Leone G, Pagano L. 2008. Invasive fungal infections in patients with acute myeloid leukemia and in those submitted to allogeneic hemopoietic stem cell transplant: who is at highest risk? Eur J Haematol 81:242–243. doi: 10.1111/j.1600-0609.2008.01096.x. [DOI] [PubMed] [Google Scholar]
- 4.Candoni A, Caira M, Cesaro S, Busca A, Giacchino M, Fanci R, Delia M, Nosari A, Bonini A, Cattaneo C, Melillo L, Caramatti C, Milone G, Scime R, Picardi M, Fanin R, Pagano L. 2013. Multicentre surveillance study on feasibility, safety and efficacy of antifungal combination therapy for proven or probable invasive fungal diseases in haematological patients: the SEIFEM real-life combo study. Mycoses 57:342–350. doi: 10.1111/myc.12161. [DOI] [PubMed] [Google Scholar]
- 5.Sinko J, Csomor J, Nikolova R, Lueff S, Krivan G, Remenyi P, Batai A, Masszi T. 2008. Invasive fungal disease in allogeneic hematopoietic stem cell transplant recipients: an autopsy-driven survey. Transpl Infect Dis 10:106–109. doi: 10.1111/j.1399-3062.2007.00264.x. [DOI] [PubMed] [Google Scholar]
- 6.Pagano L, Caira M, Candoni A, Offidani M, Martino B, Specchia G, Pastore D, Stanzani M, Cattaneo C, Fanci R, Caramatti C, Rossini F, Luppi M, Potenza L, Ferrara F, Mitra ME, Fadda RM, Invernizzi R, Aloisi T, Picardi M, Bonini A, Vacca A, Chierichini A, Melillo L, de Waure C, Fianchi L, Riva M, Leone G, Aversa F, Nosari A. 2010. Invasive aspergillosis in patients with acute myeloid leukemia: a SEIFEM-2008 registry study. Haematologica 95:644–650. doi: 10.3324/haematol.2009.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 8.Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H, Durrant S. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 356:335–347. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 9.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF, Infectious Diseases Society of America . 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 10.Ullmann AJ, Akova M, Herbrecht R, Viscoli C, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Calandra T, Castagnola E, Cornely OA, Donnelly JP, Garbino J, Groll AH, Hope WW, Jensen HE, Kullberg BJ, Lass-Florl C, Lortholary O, Meersseman W, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Cuenca-Estrella M, ESCMID Fungal Infection Study Group . 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect 18(Suppl 7):S53–S67. doi: 10.1111/1469-0691.12041. [DOI] [PubMed] [Google Scholar]
- 11.van Burik JA, Ratanatharathorn V, Stepan DE, Miller CB, Lipton JH, Vesole DH, Bunin N, Wall DA, Hiemenz JW, Satoi Y, Lee JM, Walsh TJ, National Institute of Allergy and Infectious Diseases Mycoses Study Group . 2004. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis 39:1407–1416. doi: 10.1086/422312. [DOI] [PubMed] [Google Scholar]
- 12.Tamura K, Drew R. 2008. Antifungal prophylaxis in adult hematopoietic stem cell transplant recipients. Drugs Today (Barc) 44:515–530. doi: 10.1358/dot.2008.44.7.1230943. [DOI] [PubMed] [Google Scholar]
- 13.Rachwalski EJ, Wieczorkiewicz JT, Scheetz MH. 2008. Posaconazole: an oral triazole with an extended spectrum of activity. Ann Pharmacother 42:1429–1438. doi: 10.1345/aph.1L005. [DOI] [PubMed] [Google Scholar]
- 14.Merck & Co, Inc. 2014. Noxafil (posaconazole) highlights of prescribing information. Merck & Co, Inc., Whitehouse Station, NJ: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205596s000lbl.pdf. [Google Scholar]
- 15.Wingard JR, Carter SL, Walsh TJ, Kurtzberg J, Small TN, Baden LR, Gersten ID, Mendizabal AM, Leather HL, Confer DL, Maziarz RT, Stadtmauer EA, Bolanos-Meade J, Brown J, Dipersio JF, Boeckh M, Marr KA, Blood and Marrow Transplant Clinical Trials Network . 2010. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood 116:5111–5118. doi: 10.1182/blood-2010-02-268151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattiuzzi GN, Cortes J, Alvarado G, Verstovsek S, Koller C, Pierce S, Blamble D, Faderl S, Xiao L, Hernandez M, Kantarjian H. 2011. Efficacy and safety of intravenous voriconazole and intravenous itraconazole for antifungal prophylaxis in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome. Support Care Cancer 19:19–26. doi: 10.1007/s00520-009-0783-3. [DOI] [PubMed] [Google Scholar]
- 17.Marty FM, Cosimi LA, Baden LR. 2004. Breakthrough zygomycosis after voriconazole treatment in recipients of hematopoietic stem-cell transplants. N Engl J Med 350:950–952. doi: 10.1056/NEJM200402263500923. [DOI] [PubMed] [Google Scholar]
- 18.Marr KA, Crippa F, Leisenring W, Hoyle M, Boeckh M, Balajee SA, Nichols WG, Musher B, Corey L. 2004. Itraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplants. Blood 103:1527–1533. doi: 10.1182/blood-2003-08-2644. [DOI] [PubMed] [Google Scholar]
- 19.Groll AH, Stergiopoulou T, Roilides E, Walsh TJ. 2005. Micafungin: pharmacology, experimental therapeutics and clinical applications. Expert Opin Invest Drugs 14:489–509. doi: 10.1517/13543784.14.4.489. [DOI] [PubMed] [Google Scholar]
- 20.Schmitt-Hoffmann A, Roos B, Heep M, Schleimer M, Weidekamm E, Brown T, Roehrle M, Beglinger C. 2006. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother 50:279–285. doi: 10.1128/AAC.50.1.279-285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guinea J, Pelaez T, Recio S, Torres-Narbona M, Bouza E. 2008. In vitro antifungal activities of isavuconazole (BAL4815), voriconazole, and fluconazole against 1,007 isolates of zygomycete, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob Agents Chemother 52:1396–1400. doi: 10.1128/AAC.01512-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seifert H, Aurbach U, Stefanik D, Cornely O. 2007. In vitro activities of isavuconazole and other antifungal agents against Candida bloodstream isolates. Antimicrob Agents Chemother 51:1818–1821. doi: 10.1128/AAC.01217-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verweij PE, Gonzalez GM, Wiedrhold NP, Lass-Florl C, Warn P, Heep M, Ghannoum MA, Guinea J. 2009. In vitro antifungal activity of isavuconazole against 345 Mucorales isolates collected at study centers in eight countries. J Chemother 21:272–281. doi: 10.1179/joc.2009.21.3.272. [DOI] [PubMed] [Google Scholar]
- 24.Thompson GR III, Wiederhold NP, Fothergill AW, Vallor AC, Wickes BL, Patterson TF. 2009. Antifungal susceptibilities among different serotypes of Cryptococcus gattii and Cryptococcus neoformans. Antimicrob Agents Chemother 53:309–311. doi: 10.1128/AAC.01216-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohwada J, Tsukazaki M, Hayase T, Oikawa N, Isshiki Y, Fukuda H, Mizuguchi E, Sakaitani M, Shiratori Y, Yamazaki T, Ichihara S, Umeda I, Shimma N. 2003. Design, synthesis and antifungal activity of a novel water soluble prodrug of antifungal triazole. Bioorg Med Chem Lett 13:191–196. doi: 10.1016/S0960-894X(02)00892-2. [DOI] [PubMed] [Google Scholar]
- 26.Majithiya J, Sharp A, Parmar A, Denning DW, Warn PA. 2009. Efficacy of isavuconazole, voriconazole and fluconazole in temporarily neutropenic murine models of disseminated Candida tropicalis and Candida krusei. J Antimicrob Chemother 63:161–166. doi: 10.1093/jac/dkn431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warn PA, Sharp A, Mosquera J, Spickermann J, Schmitt-Hoffmann A, Heep M, Denning DW. 2006. Comparative in vivo activity of BAL4815, the active component of the prodrug BAL8557, in a neutropenic murine model of disseminated Aspergillus flavus. J Antimicrob Chemother 58:1198–1207. doi: 10.1093/jac/dkl396. [DOI] [PubMed] [Google Scholar]
- 28.Luo G, Gebremariam T, Lee H, Edwards JE Jr, Kovanda L, Ibrahim AS. 2014. Isavuconazole therapy protects immunosuppressed mice from mucormycosis. Antimicrob Agents Chemother 58:2450–2453. doi: 10.1128/AAC.02301-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, Denning DW, Donnelly JP, Edwards JE, Erjavec Z, Fiere D, Lortholary O, Maertens J, Meis JF, Patterson TF, Ritter J, Selleslag D, Shah PM, Stevens DA, Walsh TJ, Invasive Fungal Infections Cooperative Group of the European Organization for Research and Treatment of Cancer, Mycoses Study Group of the National Institute of Allergy and Infectious Diseases . 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 30.National Cancer Institute. 2006. Common terminology criteria for adverse events v3.0 (CTCAE). Cancer Therapy Evaluation Program, National Cancer Institute, Bethesda, MD: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. [Google Scholar]
- 31.Schmitt-Hoffmann A, Roos B, Maares J, Heep M, Spickerman J, Weidekamm E, Brown T, Roehrle M. 2006. Multiple-dose pharmacokinetics and safety of the new antifungal triazole BAL4815 after intravenous infusion and oral administration of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother 50:286–293. doi: 10.1128/AAC.50.1.286-293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]