Abstract
Thiazolidinedione-8 (S-8) has recently been identified as a potential anti-quorum-sensing/antibiofilm agent against bacteria and fungi. Based on these results, we investigated the possibility of incorporating S-8 in a sustained-release membrane (SRM) to increase its pharmaceutical potential against Candida albicans biofilm. We demonstrated that SRM containing S-8 inhibits fungal biofilm formation in a time-dependent manner for 72 h, due to prolonged release of S-8. Moreover, the SRM effectively delivered the agent in its active form to locations outside the membrane reservoir. In addition, eradication of mature biofilm by the SRM containing S-8 was also significant. Of note, S-8-containing SRM affected the characteristics of mature C. albicans biofilm, such as thickness, exopolysaccharide (EPS) production, and morphogenesis of fungal cells. The concept of using an antibiofilm agent with no antifungal activity incorporated into a sustained-release delivery system is new in medicine and dentistry. This concept of an SRM containing a quorum-sensing quencher with an antibiofilm effect could pave the way for combating oral fungal infectious diseases.
INTRODUCTION
The yeast Candida albicans is found as a commensal microorganism in the digestive tract of mammals (1), as well as in the oral cavity of humans (2). It is also the most common fungal pathogen in humans, causing both mucosal and systemic infections, particularly in immunocompromised individuals (3). Several factors can predispose individuals to candidiasis, including prolonged treatment with antibiotics or corticosteroids, hormone therapy, or disorders like diabetes mellitus, nutritional deficiencies, or immunosuppressive diseases (4). The oral cavity provides an optimal environment for a sessile microbial lifestyle, and Candida strains play a key role in influencing oral diseases. Hyphae and extrapolymeric material are fundamental to the fungal biofilm, due to their pivotal roles in structural integrity and adhesive capacity and their contributions to antifungal resistance. Biofilm formation is an important virulence factor in C. albicans pathogenesis (5); it involves attachment to the host cells and to abiotic surfaces, colonization, and the development of a mature biofilm structure composed of yeast cells, pseudo- and true hyphae, and extracellular matrix (3, 6–8). In comparison to the susceptibility to fungicides of the planktonic form, the fungicide susceptibility of fungi immobilized in a biofilm is very low. This resistance is multifactorial and complex, involving (i) limited drug penetration into the biofilm due to the high density of the extracellular matrix, (ii) drug absorption or binding by the biofilm extracellular matrix, (iii) decreased growth rate, (iv) overexpression of genes involved in drug resistance, particularly those encoding efflux pumps, (v) and multidrug tolerance due to persistent cells (5, 9–11).
Microorganisms have been found to exchange information among themselves. This cross talk is termed quorum sensing (QS). QS is associated with biofilm formation and the increased pathogenicity of fungi in biofilms (12). Thiazolidinediones (TZDs) have been proposed as potential QS inhibitors in Vibrio harveyi (13). Several TZD derivatives have also been tested for their ability to affect C. albicans pathogenicity (9).
The currently accepted treatment regimens for oral candidiasis are clotrimazole (lozenges or troches), nystatin (tablets, lozenges, or pessaries), or miconazole (oral gel or buccal tablets) (14), as well as amphotericin B, fluconazole, flucytosine, and ketoconazole (15, 16). The main disadvantage of these common antifungals for candidiasis is their low substantivity and their potential side effects, especially on the oral ecosystem. Local sustained-release varnishes for prolonged release of drugs in the oral cavity have been developed with active agents such as chlorhexidine, cetylpyridinium chloride, triclosan, and fluorides (17–21). Several sustained-release varnishes have been developed, and some are available for dental applications, mainly for the treatment and prevention of bacterial infections. However, only a few studies have described a sustained-release varnish containing a drug against oral candidiasis (22, 23).
In previous studies, we demonstrated a specific antibiofilm effect and molecular mechanism of action of the TZD derivative thiazolidinedione-8 (S-8) in solution against C. albicans (7, 9). In addition, S-8 was tested for mammalian toxicity in female ICR mice. The maximum tolerated dose (MTD) of S-8 following acute oral administration to ICR female mice was between 1,000 and 2,000 mg/kg of body weight (HBI study no. HUJ/020/AOT, Harlan Biotech, Israel). Based on these results, the aim of the present study was to investigate the possibility of incorporating S-8 into a sustained-release membrane (SRM–S-8) in order to increase its clinical potential. Integrating a drug into a polymeric matrix may affect its biological properties as an antifungal drug. We therefore examined the time-dependent effect, as well as the release mode of the sustained-release delivery system loaded with S-8, toward C. albicans biofilm. In addition, fungal biofilm architecture, thickness, and exopolysaccharide (EPS) production after exposure to SRM-S-8 treatment were analyzed.
MATERIALS AND METHODS
Synthesis of S-8.
The compound S-8 was synthesized in our laboratory and was characterized by nuclear magnetic resonance (NMR) analysis, boiling point, and elemental analysis using the same procedure as described previously (7).
SRM–S-8.
The SRM was prepared by dissolving ethyl cellulose (Dow Chemical Company, Midland, MI) and polyethylene glycol 400 (PEG-400; Dow Chemical Company) in absolute ethanol. The mixture was stirred overnight at room temperature. S-8 was added, and the mixture was stirred to homogeneity. To obtain a membrane, the varnish solution was poured into Teflon molds and dried overnight at 37°C. The membrane was then removed from the mold. The content of active agent in the SRM was calculated from the weight ratio of the drug and polymer used.
Release kinetics of S-8.
Films of SRM–S-8 were cut into 1.7- by 1.7-cm squares. The films were immersed in 50 ml of distilled water containing 1% (wt/vol) sodium lauryl sulfate. The experiments were carried out at 37°C with shaking at 50 rpm. At the designated time points, samples were taken and the amount of drug released from the film was determined. The extraction mixture was maintained at sink conditions (the volume of the liquid the drug is released into is at least 10 times greater than the maximal solubility of the free drug). The experiments were performed in triplicate.
The amount of drug released was determined using an HP 1050 high-pressure liquid chromatography (HPLC) system. The separation was performed on a Hypersil Gold C18 column (4.6 by 250 mm, 5-μm particle size; Thermo Scientific, Waltham, MA) at room temperature. The mobile phase consisted of acetonitrile/water (70:30, vol/vol), and the flow was set to 1 ml/min. S-8 was detected with a UV detector at 280 nm, and the amount released was calculated from a calibration curve that was linear between 1.5 and 100 μg/ml.
Fungal strains and growth conditions.
C. albicans SC5314 and C. albicans SC5314 carrying the green fluorescent protein (GFP) reporter gene (24), kindly provided by J. Berman (Tel Aviv University, Israel), were grown for 24 to 48 h at 37°C on Sabouraud dextrose agar (SDA; Novamed, Jerusalem, Israel) plates. To prepare a standard cell suspension, a single colony was inoculated in YNB medium (0.67% [wt/vol] yeast nitrogen base without amino acids, 2% [wt/vol] dextrose; Difco, Sparks, MD) and incubated for 18 h at 30°C with agitation. The fungal cells were harvested by centrifugation, washed twice in phosphate-buffered saline (PBS) (pH 7.4), and resuspended at 5 × 106 cells/ml. RPMI medium (Biological Industries, Beit Haemek, Israel) was used for biofilm assays at 37°C.
The effect of SRM–S-8 on biofilm formation.
Amounts of 250 μl of varnish solution with various concentrations of S-8 and a control without S-8 (placebo) were poured into a 48-well microplate and dried overnight at 37°C to form membranes under sterile conditions. SRMs were transferred into a new six-well microplate (Thermo Scientific). An overnight-grown culture of C. albicans was diluted in RPMI medium and added to the SRMs at 5 × 106 cell/ml. Biofilms were grown for 24, 48, and 72 h at 37°C in RPMI medium. The SRMs contained 4, 16, 64, and 256 μg (dry weight) of S-8 per milliliter of fungal inoculum (μg/ml). For the 48- and 72-h incubation periods, the medium was changed every 24 h.
To evaluate long-term effects of S-8, SRMs were preincubated in RPMI medium at 37°C for 72 h, washed, and transferred to a new six-well plate. The SRMs were then incubated with C. albicans diluted as described above for 24 h at 37°C in RPMI medium. Placebo SRM or SRM–S-8 without fungi served as negative controls. The viability of planktonic candida in the supernatant fluid of the biofilms formed was examined by the 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-5-tetrazolium-carboxanilide (XTT) reduction method (7).
The amounts of candida immobilized on the different SRMs were assayed as follows: after washing the biofilms three times with PBS to remove loosely adhering cells, the metabolic activity of the candida cells immobilized in the biofilms was checked by XTT reduction assay at each incubation time point. The results are presented as the percentages of biofilm formation in samples treated with SRM–S-8 compared to the biofilm formation in controls (placebo, taken as 100%). Assays were performed in triplicate and repeated three times.
The effect of SRM–S-8 on preformed biofilms.
To investigate the effect of SRMs on preformed biofilms, C. albicans biofilms were allowed to mature for 24 h at 37°C as described above. The biofilms were washed twice with PBS. SRMs containing the same concentrations of S-8 as described above were then introduced into the wells. Fresh RPMI medium was added, and the plate was further incubated for 24 h at 37°C. The amounts of C. albicans biofilms, as well as of planktonic fungi, were determined quantitatively using a standard XTT reduction assay (Biological Industries) (7). The biofilm structure was visualized by using an aCOLade manual colony counter (Synbiosis, MD). The assays were performed in triplicate and repeated three times.
XTT reduction assay.
Prior to each assay, XTT solution (Biological Industries) was thawed and mixed with N-methyl dibenzopyrazine methyl sulfate (PMS) (Biological Industries) solution at a ratio of 50 to 1 by volume. Wells with biofilms formed on membranes as described above were incubated with 60 μl of XTT-PMS solution in 2 ml of PBS. To test planktonic cell viability, 60 μl of the XTT-PMS solution was added to the supernatants, which were separated from the membranes and placed in a 96-well plate. Plates containing biofilms on membranes, and biofilms around the membranes, and supernatant fluids were then incubated in the dark for 12 h at 37°C according to the manufacturer's protocol. Following incubation, the color change in the solution was measured spectrophotometrically at 492 nm in a GENios plate reader (Tecan, Salzburg, Austria).
Morphology of biofilms around the SRM.
SRMs with the concentrations of S-8 described above were placed in a 12-well polystyrene microplate (Thermo Scientific) and incubated with C. albicans in RPMI medium for 48 h at 37°C. Then, the SRMs were removed from the wells, and the biofilm formed next to the membrane was washed with PBS, followed by staining with 0.02% crystal violet for 45 min (25). The wells were washed with PBS to remove residual crystal violet and dried overnight at room temperature. The fungal morphology in each biofilm was visualized under an Olympus CKX41 inverted microscope (Olympus, Tokyo, Japan) at ×400 magnification and photographed with an Olympus DP72 microscope camera. At least four random fields were observed and analyzed. Three independent experiments were performed.
CLSM of biofilms.
SRMs with the same concentrations of S-8 as described above or placebo were placed in a black glass-bottom 24-well microplate (Ibidi, Martinsried, Germany) and inoculated with GFP-expressing C. albicans at 5 × 106 cell/ml. Biofilms were allowed to form in RPMI medium for 48 h at 37°C. The SRMs were then removed and the biofilms next to them were washed with PBS and incubated for 45 min in PBS containing the fluorescent stain concanavalin A-Alexa Fluor 647 conjugate (ConA; 25 mg/ml) (Invitrogen, Carlsbad, CA). ConA (excitation wavelength of 650 nm and emission at 668 nm) binds to the glucose and mannose residues of cell wall exopolysaccharides (26) and fluoresces red. Stained biofilms (green for fungal cells and red for EPS) were observed with a Zeiss LSM510 confocal laser scanning microscope (CLSM) (Carl Zeiss, Oberkochen, Germany). Three-dimensional images of the biofilms and EPS distribution were constructed using Zen 2009 software (Carl Zeiss). At least three random fields were observed and analyzed. Three independent experiments were performed.
The amount of total EPS production in each sample was calculated as the intensity of the red fluorescence using Image J, version 3.91 (http://rsb.info.nih.gov/ij). The data are presented as total EPS production by C. albicans cells in each layer of biofilm (5 μm). The percentages of total EPS production by biofilms treated with SRMs containing S-8 at 4 μg/ml and 16 μg/ml are presented as area under the curve (AUC) values and compared to the EPS production by biofilms treated with SRMs without S-8.
Statistical analysis.
The means ± standard deviations (SD) of the results of three independent experiments were calculated. The statistical analysis was performed using Student's t test with a significance level of P < 0.05 compared to the results for controls.
RESULTS
Kinetics of S-8 release from the SRM.
The results shown in Fig. 1 demonstrate the sustained release of S-8 from the SRM over 96 h. The release was time dependent, with 80% of the drug released within 60 h, after which the release rate decreased. Ninety percent of the drug loaded into the SRMs was released within 96 h.
FIG 1.
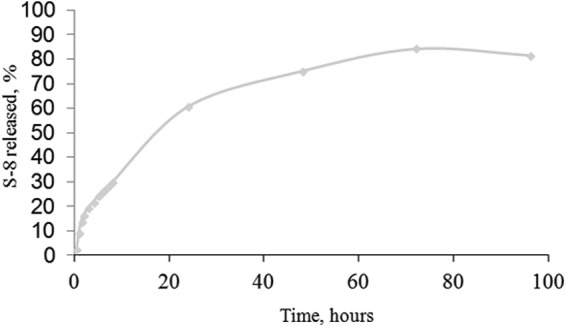
Kinetics of the release of S-8 from the SRM.
Effect of SRM on biofilm formation.
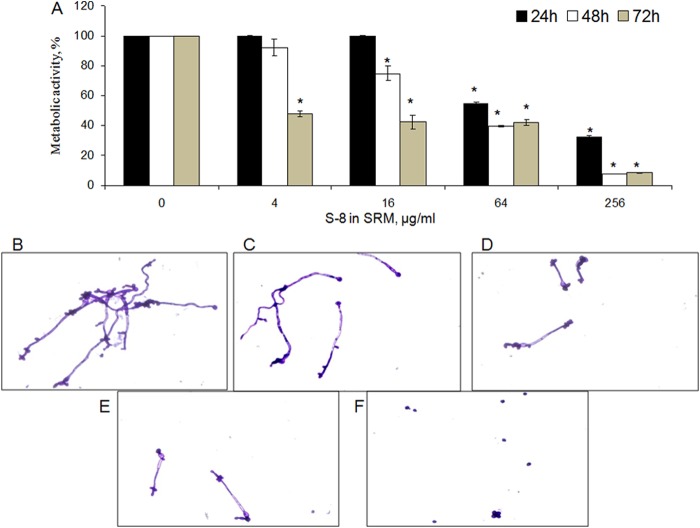
Candida biofilm accumulation on and around the SRM was quantified by XTT assay. Membrane-associated biofilms were inhibited by S-8 in a dose- and time-dependent manner (Fig. 2). After 24 h of incubation, the half-minimal biofilm inhibitory concentration (MBIC50) (the concentration of S-8 in the SRM that inhibited biofilm formation by 50% compared to that on control SRMs with no S-8) was recorded only at the highest tested dose of S-8 in the SRM (256 μg/ml). Increasing the exposure time to 48 h resulted in a 4-fold reduction of the MBIC50 dose, to 64 μg/ml. A dramatic inhibitory effect of SRM–S-8 was observed after 72 h of incubation. At 4 and 16 μg/ml S-8, the SRMs reduced biofilm formation by more than 50%. Increasing the SRM–S-8 concentration to 64 and 256 μg/ml caused further pronounced inhibition (by 95%) compared to the results for the control membrane with no S-8 (Fig. 2).
FIG 2.
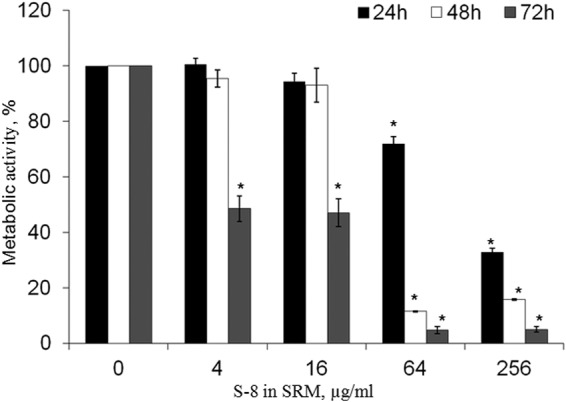
Effect of SRM–S-8 on C. albicans membrane-associated biofilm formation. C. albicans cells were incubated with SRM containing 4 to 256 μg/ml S-8 for 24, 48, and 72 h. The metabolic activity of membrane-associated biofilm cells was measured by XTT reduction assay. The XTT values of the untreated control were set as 100%. The results are presented as means ± SD from three independent experiments. *, significantly lower than the metabolic activity for the untreated control (P < 0.05).
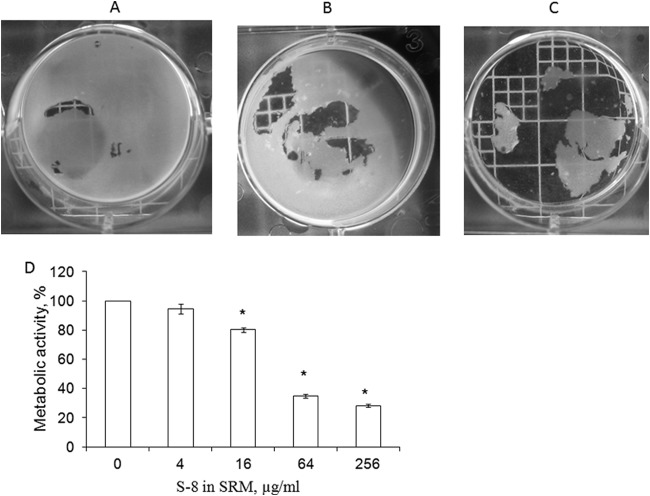
A similar inhibitory effect of SRM–S-8 on C. albicans biofilm formation was observed around the membrane. A decrease in the metabolic activity of the fungal biofilms was strongly associated with the incubation period (Fig. 3A). Indeed, the MBIC50 after 24 h of incubation was 256 μg/ml S-8, while after 48 h, it was reduced to 64 μg/ml S-8, and finally, after 72 h, it was 4 μg/ml S-8 (Fig. 3A).
FIG 3.
Effect of SRM–S-8 on C. albicans biofilm formed around the membrane. (A) C. albicans cells were incubated with SRM containing 4 to 256 μg/ml S-8 for 24, 48, and 72 h. The metabolic activity of cells in the biofilm around the membrane was measured by XTT reduction assay. The XTT values of the untreated control were set as 100%. The results are presented as means ± SD of three independent experiments. *, significantly lower than the metabolic activity for the untreated control (P < 0.05). (B to F). Morphologies of 48-h biofilms around the SRM. (B) Untreated control. (C to F) Biofilms that developed with 4, 16, 64, and 256 μg/ml S-8 incorporated into the SRM, respectively. Magnification, ×400. At least four random fields were observed. Three independent experiments were performed.
We performed a temporal evaluation to determine whether the prolonged effect of SRMs was still maintained after 72 h. In this set of experiments, prior to 24 h of incubation with the fungi, SRMs (with S-8 and placebo) were immersed in fungal growth medium for 72 h. The antibiofilm effect of the SRMs on the membrane-associated biofilm was still maintained after the 72-h period of preincubation in growth medium. The MBIC50 was recorded at a concentration of 64 μg/ml S-8 in the SRM (Fig. 4). Elevating the S-8 concentration to 256 μg/ml further reduced biofilm formation, by 80% compared to the results for the placebo (Fig. 4).
FIG 4.
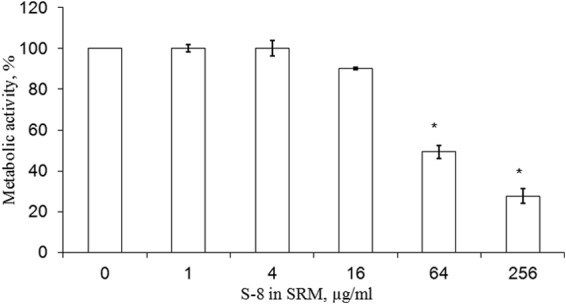
Long-term effect of SRM–S-8 on C. albicans membrane-associated biofilm formation. Prior to 24-h inoculation with C. albicans cells, the SRMs were preincubated with fungal growth medium for 72 h. The metabolic activity of cells in the membrane-associated biofilms was measured by XTT reduction assay. The XTT values of the untreated control were set as 100%. The results are presented as means ± SD of three independent experiments. *, significantly lower than the values for the untreated control (P < 0.05).
We further examined the effect of SRM–S-8 on mature biofilm. Preformed C. albicans biofilm was significantly attenuated in the presence of SRM–S-8 (Fig. 5). At concentrations of 4 μg/ml (Fig. 5B) and 64 μg/ml (Fig. 5C) S-8, the SRMs caused dose-dependent detachment of the preformed biofilms compared to the results for controls (Fig. 5A). In accordance with the microscope observations, quantitative analysis using the XTT assay revealed that SRM–S-8 at the doses described above reduced the metabolic activity in developed biofilms by 16% (P < 0.05) and 66% (P < 0.05), respectively, compared to the results for the untreated control (Fig. 5D). Thus, S-8 at 64 μg/ml in SRM was recorded as the half-minimal biofilm eradication concentration 50 (MBEC50) (the concentration of S-8 in the SRM that eradicates preformed biofilm by 50% compared to its eradication by control SRM with no S-8). The highest tested dose of S-8 in the SRM (256 μg/ml) reduced mature biofilm by 72% (P < 0.05) compared to the reduction of biofilm by the untreated control (Fig. 5D). None of the concentrations of SRM tested had any observable effect on fungal viability (data not shown).
FIG 5.
Effect of treatment with SRM–S-8 on maintenance of preformed biofilms. C. albicans biofilms were allowed to form for 24 h and then treated for 24 h with SRM containing 4 to 256 μg/ml S-8. The structures of preformed biofilms exposed to SRM–S-8 and untreated controls were visualized using a colony-count plate and photographed. (A) Untreated control. (B, C) Preformed biofilms exposed to 4 and 64 μg/ml S-8 in the SRM, respectively. Three independent experiments were performed. (D) Biofilm metabolic activity was estimated by XTT reduction assay. The XTT values of the untreated control were set as 100%. The results are presented as means ± SD of three independent experiments. *, significantly lower than the value for the untreated control (P < 0.05).
SRM–S-8 alters fungal morphology.
The effect of the SRM on biofilm morphology was monitored using light microscopy. SRM–S-8 caused a dose-dependent decrease in hyphal length (Fig. 3B to F). Increasing the concentration of S-8 to 256 μg/ml (Fig. 3F) resulted in dramatic inhibition of the yeast-to-hypha transition. Moreover, the fungal mycelium density decreased with increasing S-8 concentrations compared to the mycelium density of fungi treated with the placebo (Fig. 3B to F).
SRM–S-8 modifies biofilm architecture.
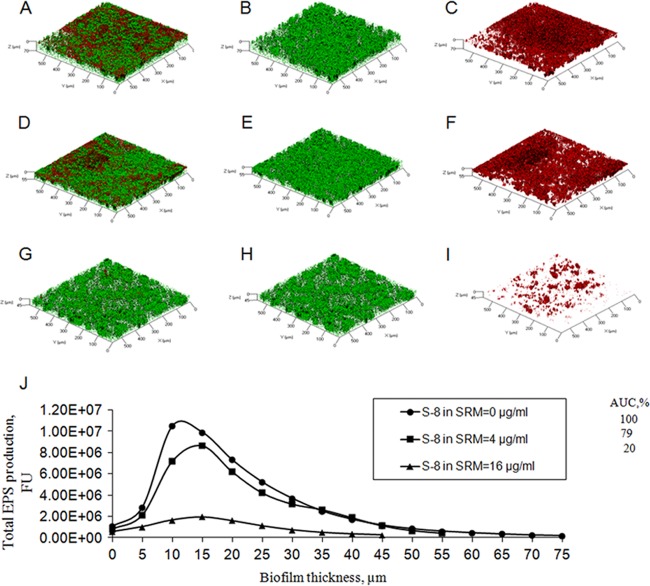
Three-dimensional images constructed using CLSM showed notable alterations in C. albicans biofilm structure due to SRM–S-8 treatment. The thickness of the fungal biofilm was reduced dose dependently, by 15 μm (22%) at 4 μg/ml S-8 (Fig. 6D to F) and by 25 μm (36%) at 16 μg/ml S-8 (Fig. 6G, H, and I) compared to the thickness of biofilm treated with the control SRM (Fig. 6A to C). Moreover, 16 μg/ml S-8 in the SRM resulted in fungal biofilm with reduced biomass and density and a nonintact structure (Fig. 6H) compared to the results for the control (Fig. 6B). EPS formation and distribution within the biofilm were already moderately modified by 4 μg/ml S-8 (Fig. 6F), while a further increase to 16 μg/ml S-8 resulted in a dramatic reduction of EPS (Fig. 6I) compared to the results for the control (Fig. 6C). In addition, total EPS, calculated as the AUC, was reduced by 21% by the lowest concentration of S-8 tested (4 μg/ml) and was decreased 80% further at a concentration of 16 μg/ml S-8 in the SRM compared to the total EPS with the control treatment (Fig. 6J).
FIG 6.
CLSM images of biofilms around the SRM–S-8. The z axes of three-dimensional constructed images indicate biofilm thickness. (A, D, G) Merged images of metabolically active GFP-expressing fungal cells embedded in EPS. (B, E, H) Images of fungal cells alone. (C, F, I) Images of EPS alone. (A, B, C) Untreated control. Biofilms were developed with 4 μg/ml (D, E, F) and 16 μg/ml (G, H, I) S-8 incorporated into the SRM. Magnification, ×10. At least three random fields were observed and analyzed. Three independent experiments were performed. (J) Quantitative measurements of total EPS production. Data are presented as total EPS production by C. albicans cells in each layer of biofilm (5 μm). Percentages of total EPS production by biofilms treated with SRM containing 4 μg/ml and 16 μg/ml S-8 are presented as area under the curve (AUC) values and compared to AUC values for biofilms treated with control SRM without S-8. FU, fluorescence unit.
DISCUSSION
It is clear today that alternatives to the customary use of antifungal agents are greatly needed. Attempts to kill the fungi, as frequently performed today in cases of fungal infections, have numerous limitations. As much of the fungi's virulence is due to its accumulation on surfaces, a novel approach should involve a drug that interferes with the microorganism's biofilm-forming ability (27–29). This effect can be maximized by sustained release of an effective agent.
Traditional treatments for oral candidiasis include topical and systemic routes. Topical antifungals are usually the drug of choice for uncomplicated, localized candidiasis (30, 31); these include oral gels, lozenges, or varnishes applied to treat denture stomatitis (32, 33). The drugs amphotericin B, clotrimazole, flucytosine, miconazole, nistatin, itraconazole, and ketoconazole have been the mainstream antifungal regime therapies for many years. However, drug toxicity, the emergence of resistant strains, and low efficacy have limited their clinical use.
Several sustained-release formulations have been proposed for the treatment of oral diseases like tooth decay and periodontal diseases (17, 19, 34). However, very few attempts to use this technology for potential antifungal treatments have been documented (35). Studies have demonstrated an anticandidal effect of the fungistatic drug clotrimazole incorporated into sustained-release varnishes in vivo and in vitro (22, 23).
Our study showed that S-8 incorporated into an SRM did not affect fungal viability; however, it did inhibit biofilm formation. This effect, at concentrations of up to 256 μg/ml, is unique, as it influences the candida yeast in its ecological niche without the potential for the emergence of resistant strains. Similarly, finasteride, a human 5-α-reductase inhibitor, was highly effective in the prevention of C. albicans urinary biofilm formation at doses of 16 μg/ml and the treatment of preformed biofilms at doses of 128 μg/ml, while it had no activity against fungal planktonic growth at up to 256 μg/ml (36). The effect of S-8 incorporated into the SRM was dose and time dependent. After 24 h of incubation with the fungi, the MBIC50 was recorded at only the highest tested dose of S-8 (256 μg/ml) incorporated into the membrane; however, prolonged fungal exposure to the SRM (up to 72 h) dramatically enhanced biofilm inhibition, by 64-fold (MBIC50 = 4 μg/ml). These findings could be explained by the gradual release of S-8 from the SRM, leading to a cumulative inhibitory effect and supporting the prolonged activity of the formulation.
In addition, biofilm formation both on and near the membrane was similarly affected in a time-dependent manner. This indicates that the SRM is also effective at delivering the agent in its active form to locations outside the reservoir of the membrane. Since fungal infection is capable of spreading within the oral cavity, dispersal of the orally administered drug throughout the oral cavity is desirable.
The light microscopy images of immobilized C. albicans cells after exposure to different doses of S-8 in the SRM showed morphological changes in the candida cells exposed to the released S-8. The length of the fungal cells in the biofilms around the membranes was already shorter after exposure to the SRM with 16 μg/ml S-8, whereas 256 μg/ml S-8 caused drastic inhibition of the yeast-to-hypha transition. This observation is of clinical importance, as C. albicans is a dimorphic fungal pathogen that is capable of reversible transitions between yeast and hyphal forms. Morphogenesis is an important factor in C. albicans' lifestyle and pathogenicity (7). Strains that are unable to perform the morphologic switch are hypovirulent in models of systemic candidiasis (37, 38). For instance, it has been found that hyphal formation is necessary for C. albicans to accumulate in formed biofilms on medical devices (39, 40). Various small molecules have been reported to alter the yeast-to-hypha transition in C. albicans (41), making them potential candidates for the sustained-release delivery of antifungal drugs.
Although prevention of fungal biofilm is of the utmost importance, disruption of an existing biofilm is also of great clinical relevance. Our results showed that S-8 released from the SRM disrupted mature biofilm in a dose-dependent manner. This observation is in accordance with our previous study, in which S-8 in solution at a sub-MIC level caused significant detachment of C. albicans biofilm (7). Mature fungal biofilms are difficult to eradicate, and they are much less sensitive to fungicides (42, 43). The MBEC50 for SRM–S-8 was recorded as 64 μg/ml, while the formulation did not affect fungal viability and growth at that concentration. In contrast, commonly used antifungals can only affect mature biofilms at doses that are much greater than the MICs for planktonic cells. Biofilms from C. albicans strains were intrinsically resistant to fluconazole, with MICs of 0.25 to 16 μg/ml versus MBEC50s of >1,024 μg/ml (10). Other well-known antifungal agents showed much less activity against biofilms than against planktonic cells. The MICs for amphotericin B, flucytosine, itraconazole, and ketoconazole were determined to be 1.3, 0.2, <0.025, and <0.025 μg/ml, respectively, while the MBEC50s were found to be 48.5 to 54.2, 20.7 to 42, 35.5 to 56, and 23.5 to 46.5 μg/ml, respectively (44). Furthermore, elimination of mature biofilms is of great clinical significance, since they are strongly related to device-associated Candida infections, including denture stomatitis (45). Due to the slow release of S-8 by the membrane, the half-minimal biofilm eradication concentration (MBEC50) was higher than the MBEC50 of S-8 in solution detected in our previous study (7).
The C. albicans biofilm architecture was dramatically impaired after exposure to the SRM containing S-8. Our data clearly showed a dose-dependent decrease in biofilm thickness, which could be explained by the differences in cell morphology observed by light microscopy, as well as differences in the amounts of EPS in samples treated with SRM–S-8 compared to controls. C. albicans biofilms secrete a thick layer of EPS, which is associated with immobilization and inhibition of antifungal drug penetration into the biofilm (46, 47). A notable reduction in EPS production in biofilms exposed to SRM–S-8 can be related to the reduced biomass and modified morphology of the cells. A previous report on the ability of S-8 in solution to modify C. albicans biofilm-associated gene transcription (7) supports this notion, but further research is required.
Although still far from clinical implementation, S-8, especially in the SRM pharmaceutical form, is a potential novel antibiofilm drug. Even the highest concentration of S-8 loaded into the SRM is still far below the maximum tolerated dose (MTD) tested in mice. Moreover, the drug is released from the SRM in a gradual sustained-release mode, and therefore, the amounts released are much lower than the maximal load of S-8 in the SRM.
A previous study (7) has demonstrated that S-8 in solution is able to inhibit the adhesion of C. albicans to mammalian cells. We have shown that S-8 is active after 72 h of release, therefore allowing a prolonged inhibitory effect on the invasion process of candida. Furthermore, the local sustained release allows better penetration of the agent to the epithelium.
Similar to other SRMs (34), the antibiofilm/anti-quorum-sensing delivery system, in the form of a varnish, can be applied on oral surfaces, including teeth, implants, restorations, orthodontic appliances, and soft tissues, as alternatives to conventional fungicidal regimes. The varnish is simple for a subject to apply at home and does not interfere with normal daily oral hygiene. In addition, further studies on the use of SRMs in other infection sites (bacterial and/or fungal), such as in catheters, implants, and feeding tubes, can be projected.
Our previous study showed that S-8 and the C. albicans QS molecule farnesol exert antibiofilm activity and inhibit the yeast-to-hyphal transition by similar mechanisms, i.e., repressing mitogen-activated protein kinase and cyclic AMP-protein kinase A signaling pathways and stimulating the expression of hyphal suppressor genes like TUP1 (7). In addition, thiazolidinediones (TZDs) have been proposed as potential QS inhibitors for the bacterium Vibrio harveyi (13). Given all of the findings described above, we propose that one of the possible modes of the antibiofilm action of S-8 toward fungi could be interference in the fungal QS cascade, as it may directly or indirectly interact with the synthesis and/or activity of C. albicans QS molecules. Further research is needed in order to evaluate the precise mechanism of the antibiofilm action of S-8 as an anti-QS molecule.
The concept of using an antibiofilm agent incorporated into a sustained-release delivery system is novel in medicine and dentistry. Our formulation of an SRM containing a QS quencher with an antibiofilm effect presents a promising avenue for combating infectious fungal diseases in the oral cavity.
ACKNOWLEDGMENTS
This work was partially supported by The International Association for Dental Research (IADR)-GlaxoSmithKline (GSK) Innovation in Oral Care Award no. 0304914.
We thank Mark Tarshish and Vitaly Gutkin for assistance with the CLSM and ESEM work, respectively.
This work is dedicated to our colleague the late Prof. Morris Srebnik.
REFERENCES
- 1.Nadas GC, Taulescu MA, Ciobanu L, Fit NI, Flore C, Rapuntean S, Bouari CM, Catoi C. 2013. The interplay between NSAIDs and Candida albicans on the gastrointestinal tract of guinea pigs. Mycopathologia 175:221–230. doi: 10.1007/s11046-013-9613-8. [DOI] [PubMed] [Google Scholar]
- 2.Pereira CA, Toledo BC, Santos CT, Pereira Costa AC, Back-Brito GN, Kaminagakura E, Jorge AO. 2013. Opportunistic microorganisms in individuals with lesions of denture stomatitis. Diagn Microbiol Infect Dis 76:419–424. doi: 10.1016/j.diagmicrobio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayer FL, Wilson D, Hube B. 2013. Candida albicans pathogenicity mechanisms. Virulence 4:119–128. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finkel JS, Mitchell AP. 2011. Genetic control of Candida albicans biofilm development. Nat Rev Microbiol 9:109–118. doi: 10.1038/nrmicro2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blankenship JR, Mitchell AP. 2006. How to build a biofilm: a fungal perspective. Curr Opin Microbiol 9:588–594. doi: 10.1016/j.mib.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Feldman M, Al-Quntar A, Polacheck I, Friedman M, Steinberg D. 2014. Therapeutic potential of thiazolidinedione-8 as an antibiofilm agent against Candida albicans. PLoS One 9:e93225. doi: 10.1371/journal.pone.0093225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srinivasan A, Uppuluri P, Lopez-Ribot J, Ramasubramanian AK. 2011. Development of a high-throughput Candida albicans biofilm chip. PLoS One 6:e19036. doi: 10.1371/journal.pone.0019036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kagan S, Jabbour A, Sionov E, Alquntar AA, Steinberg D, Srebnik M, Nir-Paz R, Weiss A, Polacheck I. 2014. Anti-Candida albicans biofilm effect of novel heterocyclic compounds. J Antimicrob Chemother 69:416–427. doi: 10.1093/jac/dkt365. [DOI] [PubMed] [Google Scholar]
- 10.Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother 45:2475–2479. doi: 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramage G, Saville SP, Thomas DP, Lopez-Ribot JL. 2005. Candida biofilms: an update. Eukaryot Cell 4:633–638. doi: 10.1128/EC.4.4.633-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albuquerque P, Casadevall A. 2012. Quorum sensing in fungi—a review. Med Mycol 50:337–345. doi: 10.3109/13693786.2011.652201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brackman G, Al Quntar AA, Enk CD, Karalic I, Nelis HJ, Van Calenbergh S, Srebnik M, Coenye T. 2013. Synthesis and evaluation of thiazolidinedione and dioxazaborocane analogues as inhibitors of AI-2 quorum sensing in Vibrio harveyi. Bioorg Med Chem 21:660–667. doi: 10.1016/j.bmc.2012.11.055. [DOI] [PubMed] [Google Scholar]
- 14.Van Roey J, Haxaire M, Kamya M, Lwanga I, Katabira E. 2004. Comparative efficacy of topical therapy with a slow-release mucoadhesive buccal tablet containing miconazole nitrate versus systemic therapy with ketoconazole in HIV-positive patients with oropharyngeal candidiasis. J Acquir Immune Defic Syndr 35:144–150. doi: 10.1097/00126334-200402010-00007. [DOI] [PubMed] [Google Scholar]
- 15.Rao NG, Han G, Greene JN, Tanvetyanon T, Kish JA, De Conti RC, Chuong MD, Shridhar R, Biagioli MC, Caudell JJ, Trotti AM III. 2013. Effect of prophylactic fluconazole on oral mucositis and candidiasis during radiation therapy for head-and-neck cancer. Pract Radiat Oncol 3:229–233. doi: 10.1016/j.prro.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Brito GN, Inocêncio AC, Querido SM, Jorge AO, Koga-Ito CY. 2011. In vitro antifungal susceptibility of Candida spp. oral isolates from HIV-positive patients and control individuals. Braz Oral Res 25:28–33. doi: 10.1590/S1806-83242011005000001. [DOI] [PubMed] [Google Scholar]
- 17.George AM, Kalangi SK, Vasudevan M, Krishnaswamy NR. 2010. Chlorhexidine varnishes effectively inhibit Porphyromonas gingivalis and Streptococcus mutans—an in vivo study. J Indian Soc Periodontol 14:178–180. doi: 10.4103/0972-124X.75913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinberg D, Moldovan M, Molukandov D. 2001. Testing a degradable topical varnish of cetylpyridinium chloride in an experimental dental biofilm model. J Antimicrob Chemother 48:241–243. doi: 10.1093/jac/48.2.241. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg D, Rozen R, Klausner EA, Zachs B, Friedman M. 2002. Formulation, development and characterization of sustained release varnishes containing amine and stannous fluorides. Caries Res 36:411–416. doi: 10.1159/000066539. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg D, Tal T, Friedman M. 2006. Sustained-release delivery systems of triclosan for treatment of Streptococcus mutans biofilm. J Biomed Mater Res B Appl Biomater 77B:282–286. doi: 10.1002/jbm.b.30266. [DOI] [PubMed] [Google Scholar]
- 21.Zyskind D, Steinberg D, Friedman M, Bernimoulin JP. 1992. Inhibition of plaque accumulation under periodontal dressing by sustained-release varnish of chlorhexidine. Clin Prev Dent 14:29–33. [PubMed] [Google Scholar]
- 22.Czerninski R, Sivan S, Steinberg D, Gati I, Kagan L, Friedman M. 2010. A novel sustained-release clotrimazole varnish for local treatment of oral candidiasis. Clin Oral Investig 14:71–78. doi: 10.1007/s00784-009-0275-3. [DOI] [PubMed] [Google Scholar]
- 23.Czerninski R, Pikovsky A, Gati I, Friedman M, Steinberg D. 28 May 2014. Comparison of the efficacy of a novel sustained release clotrimazole varnish and clotrimazole troches for the treatment of oral candidiasis. Clin Oral Investig doi: 10.1007/s00784-014-1259-5. [DOI] [PubMed] [Google Scholar]
- 24.Gerami-Nejad M, Zacchi LF, McClellan M, Matter K, Berman J. 2013. Shuttle vectors for facile gap repair cloning and integration into a neutral locus in Candida albicans. Microbiology 159:565–579. doi: 10.1099/mic.0.064097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldman M, Tanabe S, Howell A, Grenier D. 2012. Cranberry proanthocyanidins inhibit the adherence properties of Candida albicans and cytokine secretion by oral epithelial cells. BMC Complement Altern Med 12:6. doi: 10.1186/1472-6882-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol 183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomes PN, da Silva WJ, Pousa CC, Narvaes EA, Del Bel Cury AA. 2011. Bioactivity and cellular structure of Candida albicans and Candida glabrata biofilms grown in the presence of fluconazole. Arch Oral Biol 56:1274–1281. doi: 10.1016/j.archoralbio.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Privett BJ, Nutz ST, Schoenfisch MH. 2010. Efficacy of surface-generated nitric oxide against Candida albicans adhesion and biofilm formation. Biofouling 26:973–983. doi: 10.1080/08927014.2010.534552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL. 2001. Biofilm formation by Candida dubliniensis. J Clin Microbiol 39:3234–3240. doi: 10.1128/JCM.39.9.3234-3240.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powderly WG, Gallant JE, Ghannoum MA, Mayer KH, Navarro EE, Perfect JR. 1999. Oropharyngeal candidiasis in patients with HIV: suggested guidelines for therapy. AIDS Res Hum Retroviruses 15:1619–1623. doi: 10.1089/088922299309658. [DOI] [PubMed] [Google Scholar]
- 31.Rex JH, Walsh TJ, Sobel JD, Filler SG, Pappas PG, Dismukes WE, Edwards JE. 2000. Practice guidelines for the treatment of candidiasis. Clin Infect Dis 30:662–678. doi: 10.1086/313749. [DOI] [PubMed] [Google Scholar]
- 32.Dias AP, Samaranayake LP, Lee MT. 1997. Miconazole lacquer in the treatment of denture stomatitis: clinical and microbiological findings in Chinese patients. Clin Oral Investig 1:47–52. doi: 10.1007/s007840050008. [DOI] [PubMed] [Google Scholar]
- 33.Parvinen T, Kokko J, Yli-Urpo A. 1994. Miconazole lacquer compared with gel in treatment of denture stomatitis. Scand J Dent Res 102:361–366. [DOI] [PubMed] [Google Scholar]
- 34.Friedman M, Steinberg D. 1990. Sustained-release delivery systems for treatment of dental diseases. Pharm Res 7:313–317. doi: 10.1023/A:1015898717936. [DOI] [PubMed] [Google Scholar]
- 35.Yang W, Wiederhold NP, Williams RO III. 2008. Drug delivery strategies for improved azole antifungal action. Expert Opin Drug Deliv 5:1199–1216. doi: 10.1517/17425240802457188. [DOI] [PubMed] [Google Scholar]
- 36.Chavez-Dozal AA, Lown L, Jahng M, Walraven CJ, Lee SA. 2014. In vitro analysis of finasteride activity against Candida albicans urinary biofilm formation and filamentation. Antimicrob Agents Chemother 58:5855–5862. doi: 10.1128/AAC.03137-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorenz MC, Bender JA, Fink GR. 2004. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell 3:1076–1087. doi: 10.1128/EC.3.5.1076-1087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. 2003. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell 2:1053–1060. doi: 10.1128/EC.2.5.1053-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nobile CJ, Nett JE, Andes DR, Mitchell AP. 2006. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot Cell 5:1604–1610. doi: 10.1128/EC.00194-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shareck J, Belhumeur P. 2011. Modulation of morphogenesis in Candida albicans by various small molecules. Eukaryot Cell 10:1004–1012. doi: 10.1128/EC.05030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hobson RP, Munro CA, Bates S, MacCallum DM, Cutler JE, Heinsbroek SE, Brown GD, Odds FC, Gow NA. 2004. Loss of cell wall mannosylphosphate in Candida albicans does not influence macrophage recognition. J Biol Chem 279:39628–39635. doi: 10.1074/jbc.M405003200. [DOI] [PubMed] [Google Scholar]
- 42.Baillie GS, Douglas LJ. 1998. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob Agents Chemother 42:1900–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kojic EM, Darouiche RO. 2004. Candida infections of medical devices. Clin Microbiol Rev 17:255–267. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawser SP, Douglas LJ. 1995. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother 39:2128–2131. doi: 10.1128/AAC.39.9.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939–949. doi: 10.1016/S0092-8674(00)80358-X. [DOI] [PubMed] [Google Scholar]
- 46.Lal P, Sharma D, Pruthi P, Pruthi V. 2010. Exopolysaccharide analysis of biofilm-forming Candida albicans. J Appl Microbiol 109:128–136. [DOI] [PubMed] [Google Scholar]
- 47.Nett JE, Sanchez H, Cain MT, Andes DR. 2010. Genetic basis of Candida biofilm resistance due to drug-sequestering matrix glucan. J Infect Dis 202:171–175. doi: 10.1086/651200. [DOI] [PMC free article] [PubMed] [Google Scholar]