Abstract
Quorum sensing (QS) regulates group behaviors of Candida albicans such as biofilm, hyphal growth, and virulence factors. The sesquiterpene alcohol farnesol, a QS molecule produced by C. albicans, is known to regulate the expression of virulence weapons of this fungus. Fluconazole (FCZ) is a broad-spectrum antifungal drug that is used for the treatment of C. albicans infections. While FCZ can be cytotoxic at high concentrations, our results show that at much lower concentrations, quercetin (QC), a dietary flavonoid isolated from an edible lichen (Usnea longissima), can be implemented as a sensitizing agent for FCZ-resistant C. albicans NBC099, enhancing the efficacy of FCZ. QC enhanced FCZ-mediated cell killing of NBC099 and also induced cell death. These experiments indicated that the combined application of both drugs was FCZ dose dependent rather than QC dose dependent. In addition, we found that QC strongly suppressed the production of virulence weapons—biofilm formation, hyphal development, phospholipase, proteinase, esterase, and hemolytic activity. Treatment with QC also increased FCZ-mediated cell death in NBC099 biofilms. Interestingly, we also found that QC enhances the anticandidal activity of FCZ by inducing apoptotic cell death. We have also established that this sensitization is reliant on the farnesol response generated by QC. Molecular docking studies also support this conclusion and suggest that QC can form hydrogen bonds with Gln969, Thr1105, Ser1108, Arg1109, Asn1110, and Gly1061 in the ATP binding pocket of adenylate cyclase. Thus, this QS-mediated combined sensitizer (QC)-anticandidal agent (FCZ) strategy may be a novel way to enhance the efficacy of FCZ-based therapy of C. albicans infections.
INTRODUCTION
Candidiasis is a prevalent fungal infection caused by species of the yeast genus Candida. There are more than Candida 20 species, the most common of which is Candida albicans. Candidiasis is the fourth most common cause of health care-associated bloodstream infections in India and the United States (1). This yeast normally lives on the skin and mucous membranes without causing infection. However, overgrowth of these microbes can cause superficial mycoses, invasive mucosal infections, and disseminated systemic disease, particularly in AIDS patients, transplant recipients, and other immunocompromised people (2, 3). It is well documented that the emergence of drug-resistant C. albicans is increasing at an alarming pace (4–6). Therefore, the need for effective anticandidal therapy is increasing, as the available drugs are still very restricted.
Currently available therapies for candidiasis are based on antifungal drugs including azoles, echinocandins, and inhibitors of calcineurin, Hos2 deacetylase, and Hsp90 (7–10). Fluconazole (FCZ) is most widely used to treat candidiasis infections because of its high bioavailability and low toxicity (11–13). However, excessive and indiscriminate clinical use of FCZ has led to the emergence of multiple-drug-resistant (MDR) strains of C. albicans (5, 14, 15). Importantly, these MDR strains occur at frequencies higher than mutation rates and, consistent with this, seem to be genetically identical to the drug-sensitive microbe. Hence, there is an urgent need to develop new effective and safe anticandidal therapeutics to reduce the high mortality rate due to invasive infections and to combat these fungal diseases.
The concept of selectively sensitizing human cancer cells to death induced by a variety of anticancer drugs has been fully justified. However, in the case of pathogenic microorganisms such as C. albicans, it has still not been studied. It is less well known that C. albicans uses cell-to-cell chatting or quorum sensing (QS) signaling for biofilm formation or to produce a wide array of virulence factors, including biofilm and hypha formation (16). These virulence factors are primary sources of MDR development and invasive infections because they are difficult or impossible to eradicate with conventional anticandidal agents (17). Most infectious diseases are caused by C. albicans cells that proliferate within QS-mediated biofilms (16, 18, 19).
The sesquiterpene alcohol farnesol, a QS molecule, is capable of blocking the yeast-to-hyphal/pseudohyphal (filament) switch, biofilm formation, and other virulence factors that are the focus of intense study because of their role in C. albicans pathogenesis (18, 20). There is therefore a pressing need to develop novel antifungal therapy based on QS that can possibly sensitize C. albicans to conventional drugs, particularly FCZ. Efforts to regulate QS have enabled the identification of bioactive molecules produced by plants (21). Recently, several studies have shown that dietary phytochemicals inhibit QS-dependent biofilm formation in various human-pathogenic bacteria (22–27). Hence, it seems logical to target QS signaling to overcome therapy resistance, which can be a promising target for C. albicans cell sensitization to drugs by using dietary phytochemicals for combined chemotherapy.
Thus, the goal of the present study was to determine whether QS regulation by the dietary flavonoid quercetin (QC), isolated from an edible lichen (Usnea longissima), could sensitize C. albicans to FCZ. We used FCZ-resistant C. albicans strain NBC099 to determine the effects of FCZ and QC on its functionality. Increased farnesol production was the key mechanism by which QC enhanced FCZ-mediated cell death in C. albicans. Such novel findings can generate great enthusiasm in the therapeutic microbiology community for the development of next-generation anticandidal therapies.
MATERIALS AND METHODS
Strains, cultures, reagents, and plant sample.
C. albicans strain NBC099 and the ΔCzf1 mutant were routinely cultured in modified Sabouraud dextrose (SD) medium (1% yeast extract, 2% peptone, 2% dextrose, and for solid medium 2% agar) at pH 6.5 and incubated at 30°C. FCZ (MP Biomedicals, India) and QC (Sigma-Aldrich, St. Louis, MO) were dissolved in dimethyl sulfoxide (DMSO; Merck, Darmstadt, Germany). The edible lichen U. longissima was collected from the Govind Wildlife Sanctuary, Uttaranchal, India, in May 2013.
Preparation of NBC099 SCS.
A small amount of stock culture was inoculated onto SD agar containing chloramphenicol with a sterile loop and incubated at 30°C for 24 to 48 h. The cells were then harvested and suspended in sterile phosphate-buffered saline (PBS; Gibco) at an optical density (600 nm) of 0.5. The final suspension was adjusted to contain 1 × 107 cells/ml; this was called the standard cell suspension (SCS).
Anticandidal activity assay.
A disc diffusion method was used to determine anticandidal activity. Briefly, 5 ml of mid-log- or exponential-phase growth of NBC099 was centrifuged at 6,000 rpm for 10 min at 4°C. The pellets were then washed with sterile 1× PBS and resuspended in 500 μl of normal saline solution (NSS). A 100-μl volume of the suspended cells was spread uniformly on agar culture medium, and a presterilized disc (6 mm) was placed on it. Ethanolic U. longissima extract (eUSE), QC, or FCZ was then loaded onto the discs and incubated for 24 h at 30°C.
Time-kill curve assay.
A growth curve assay was performed according to a protocol of Li et al. (3). Cells were grown to exponential phase in liquid medium and harvested, and an SCS was made by suspending the cells in fresh SD broth medium. Different concentrations of eUSE, QC, or FCZ were added. Cells were cultured at 30°C with constant shaking (180 rpm) and counted at different time intervals. No drug supplementation was used in the control group.
Cell viability assay.
SYTO 9 and propidium iodide (PI) staining was used to examine NBC099 cell death. Briefly, cells (2 × 105/ml) were exposed to eUSE, QC, or FCZ and washed in PBS. The cells were then fixed in appropriate buffer, PBS for live cells and 70% isopropanol alcohol for dead cells, at room temperature for 30 min. Cells were washed again twice in PBS. Cell viability was assessed with the LIVE/DEAD cell viability staining kit (Life Technologies). The SYTO 9 (20 mM) and PI (20 mM) stain stock solutions were added, and staining was allowed to progress for 15 min. Live SYTO 9-stained cells and dead PI-stained cells were visualized by laser scanning confocal microscopy (LSCM) with an LSM-510 META (Zeiss, Munich, Germany) with fluorescein isothiocyanate (FITC; excitation and emission wavelengths of 480 and 500 nm, respectively) and tetramethyl rhodamine isothiocyanate (excitation and emission wavelengths of 490 and 635 nm, respectively) optical filters, respectively. In addition, the tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich, St. Louis, MO) was further used to assess cell viability. One hundred microliters of exponentially grown NBC099 SCS was seeded onto a commercially available, presterilized, flat-bottom 96-well polystyrene microtiter plate (Axiva, New Delhi, India) and grown in the presence of QC or FCZ at 30°C for the times required. A 100-μl volume of MTT was added to the cells (0.5 mg/ml, dissolved in SD broth), and they were incubated for 4 h at 30°C. The culture medium was then aspirated, and the cells were further incubated for 15 min with 100 μl of acidic isopropanol (0.09 N HCl) to dissolve the formazan crystals. The A570 of the MTT formazan produced was measured. All data were normalized to the MTT conversion activity of medium-treated control cells.
Assessment of biofilm formation.
Biofilms of NBC099 were grown in the presence or absence of QC or FCZ on presterilized glass coverslips in sterile SD broth medium for 48 h under aseptic and humid conditions. Biofilm growth and development were examined by LSCM with an LSM-510 META (Zeiss, Munich, Germany) equipped with an argon laser and a helium-neon laser for excitation of fluorophores. After incubation for 48 h, cells were fixed with 70% isopropanol for 10 min at 30°C and then washed twice with PBS. Cell viability in biofilm cultures was assessed by staining with the LIVE/DEAD cell viability staining kit (Life Technologies) as recommended by the manufacturer. The staining of cells and their subsequent visualization were done as described above in the cell viability section. Biofilm formation on a microtiter dish was further assayed. Briefly, NBC099 SCS (150 μl) was pipetted into a 96-well flat-bottom microplate. A 50-μl volume of QC or FCZ was then added, and the plate was incubated at 30°C for 48 h. The medium was discarded, and cells were sterilized by adding 70% ethanol to each well for 1 min. The wells were subsequently washed twice by immersion in distilled water, and cells attached to the wells were stained by adding 125 μl of a 0.1% crystal violet (CV) solution for 15 min. After drying, 150 μl of isopropanol containing 0.04 N HCl and 50 μl of 0.25% sodium dodecyl sulfate (SDS) were added to each well of the microplate. In order to extract CV from the cells, the solution was mixed with a plate mixer for 30 s and the A590 was measured.
GC-MS analysis.
Before gas chromatography-mass spectrometry (GC-MS) analysis, the compound was silylated as described by Proestos and Komaitis (28). The silylated sample was applied to a model DSQ II GC-MS system (Thermo Scientific) equipped with a TR 50-MS capillary column (30 m by 0.32 mm [inside diameter]) coated with material at a film thickness of 0.25 μm. The injector was set at 250°C, and the detector was set at 290°C. GC was performed at a split ratio of 10:1 for 10 min. The temperature program was as follows: 50°C for 1 min and then increase at 20°C/min to 310°C and hold for 15 min. A postrun temperature of 70°C for 10 min was sufficient for the next injection, and the flow rate of the carrier gas (helium) was maintained at 1.0 ml/min. Compound identification was achieved by comparing retention times with those of reference compounds. The spectral data were obtained from National Institute of Standards and Technology library. The experiment was carried out in duplicate.
Hyphal development.
Mid-log-phase growth of NBC099 was diluted to obtain an SCS in culture medium containing 10% heat-inactivated fetal bovine serum (FBS), and various concentrations of QC were added. Cells were grown at 30°C for 72 h. Aliquots of cells were visualized by differential interference contrast microscopy and photographed.
Quantification of extracellular virulence factors of NBC099.
To determine phospholipase activity, the egg yolk agar method described by Samaranayake et al. (29) was used. Briefly, 1 M NaCl, 0.005 M CaCl2, and 10% sterile egg yolk were autoclaved separately and then used to supplement SD agar medium. Ten microliters of NBC099 SCS was placed on a prepoured SD agar plate and incubated at 30°C for 5 days. Enzyme activity was determined by the formation of a precipitated dark brown zone around the inoculum. To determine proteinase activity, we used bovine serum albumin agar medium (0.1% KH2PO4, 0.05% MgSO4, 2% agar, 1% bovine serum albumin) and adjusted the final pH of the medium to 4.5 ± 0.2. Ten microliters of NBC099 SCS was inoculated and incubated at 30°C for 5 days. The presence of proteinase activity was determined by the formation of a transparent halo zone around the inoculum. Tween 80 opacity test medium (1% peptone, 0.5% NaCl, 0.01% CaCl2, 1.8% agar) at pH 6.8 ± 0.2 was used to determine esterase activity. Tween 80 (0.5%) was added to the medium. Ten microliters of NBC099 SCS was carefully inoculated onto the Tween 80 opacity test medium and incubated at 30°C for 5 days. The formation of a halo zone around the inoculation site was considered positive proof of esterase activity. To determine hemolytic activity, SD broth was supplemented with 7% sheep blood and 3% glucose and the final pH was adjusted to 5.6 ± 0.2. Fifty microliters of NBC099 SCS was added to 5 ml of culture broth and incubated at 30°C for 48 h. Decolorization of the red medium was considered positive confirmation of hemolytic activity.
Extraction and quantitation of farnesol.
Briefly, 5 ml of SD broth containing different concentrations of QC was inoculated with NBC099 SCS and incubated at 30°C for 48 h on a New Brunswick Scientific G52 shaker at 150 rpm. The supernatant was separated by centrifugation at 10,000 rpm for 20 min. Filtrates were sterilized by vacuum filtration (Pall Corporation) through 0.45-μm cellulose nitrate filters and then extracted thrice with a one-fifth volume of ethyl acetate (Merck, Darmstadt, Germany). Ethyl acetate was removed under reduced pressure on a rotary evaporator (Tokyo Rikakikai Inc., Tokyo, Japan) and then lyophilized with a freeze dryer (Labconco). The residue was resuspended in absolute ethanol and stored at 4°C until use. Separation of farnesol was obtained by thin-layer chromatography (TLC) on silica gel-precoated 60G F254 TLC plates (Merck, India) with a mobile phase of toluene-ethyl acetate-formic acid (7.5:2:0.5). Farnesol was simultaneously quantified with a CAMAG model 3 TLC scanner equipped with CAMAG winCATS IV software.
SEM.
An exponentially growing NBC099 culture (2 × 105 cells) was treated with QC or FCZ. Cells were harvested after incubation for 48 h, washed twice with PBS, and fixed in 2.0% glutaraldehyde (prepared in 0.1 M phosphate buffer, pH 7.0). The cells were then postfixed in 1% osmium tetroxide buffer. After dehydration in a graded ethanol series, cells were embedded in spur resin and cut into thin sections with an ultramicrotome. The section grids were stained with saturated solutions of uranyl acetate and lead citrate. Scanning electron microscopy (SEM) was performed at a magnification of ×10,000 (JEOL, Tokyo, Japan).
Apoptosis assay.
Fluorescence microscopy was used to analyze apoptotic cell death. Briefly, exponentially growing C. albicans cultures (1 × 109 cells) were grown on glass coverslips (Nunc-LabTek; Nunc, Naperville, IL) in the absence or presence of QC or FCZ for 48 h at 30°C. Following treatment, cells were washed twice with PBS and incubated with monoclonal antibody CK18 (1:100) for detection of apoptosis by LSCM.
In silico docking studies.
Docking studies were performed with Discovery Studio v2.5 software. Adenylate cyclases from Trypanosoma brucei (Protein Data Bank code 1FX2) as the receptor and QC (PubChem CID 5280343) as the ligand were considered. Discovery Studio v2.5 included scoring functions such as LigScore1 & 2 (polar surface in receptor-ligand interactions), PLP1 & 2 (hydrogen bond formation), Jain (hydrophobic interactions), PMF (protein-ligand binding free energy), Ludi (degree of freedom), and Dock score. QC was docked into the active site of the receptor by using the Ligand Fit option. The docked pose with lower energy was recorded.
Statistical analyses.
All statistical analyses were performed with the Student t test, and P < 0.01 was considered the significance level.
RESULTS
Ethanolic extract of U. longissima sensitizes FCZ-resistant NBC099 to cell death.
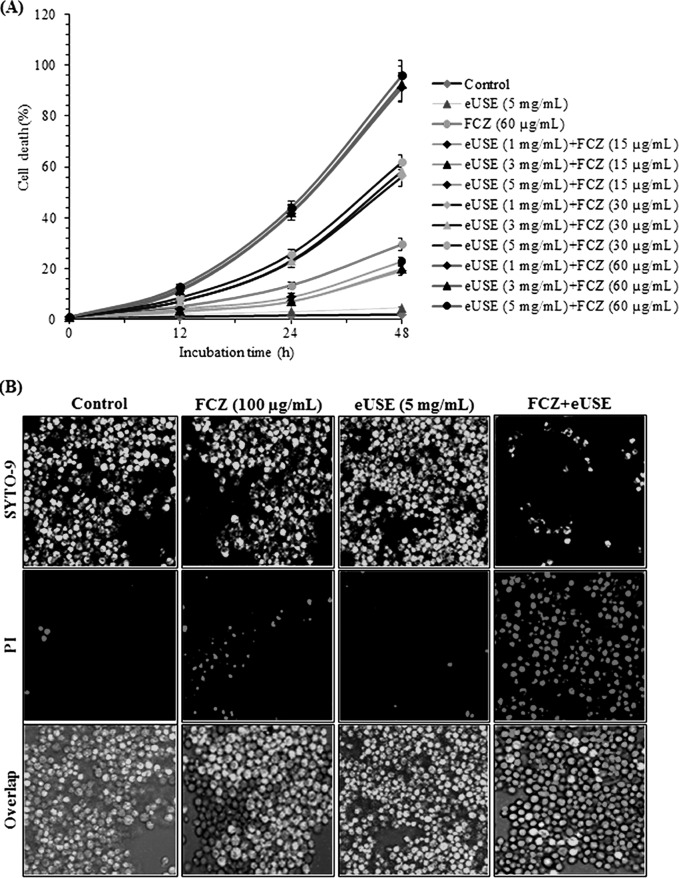
Air-dried and finely ground plant matter (50 g) was extracted with methanol (750 ml for 12 h) at room temperature; this process was repeated three times, and combined extracts were evaporated under reduced pressure in a rotary evaporator (Tokyo Rikakikai Inc., Tokyo, Japan) and then lyophilized (Labconco) to obtain dry residue (eUSE; 5.6 g). We examined whether eUSE could sensitize FCZ-resistant C. albicans NBC099 to cell death in the presence of the antifungal drug FCZ. In orthogonal experiments, we incubated NBC099 with various concentrations of eUSE (1, 3, and 5 mg/ml dissolved in DMSO) and FCZ (15, 30, and 60 μg/ml) for 48 h and measured cell killing and viability. Our results specified that the synergistic effect of both depended on the concentration of FCZ but not that of eUSE. More specifically, 5 mg/ml eUSE alone had almost no cell killing effect and 60 μg/ml FCZ alone had a weak effect, but this effect was enhanced significantly when eUSE and FCZ were mixed together (Fig. 1A and B). Interestingly, the cell killing effect of eUSE plus FCZ was enhanced when the concentration of FCZ was increased even though the concentration of eUSE remained unchanged. In contrast, increasing the concentration of eUSE did not enhance the synergistic cell killing potential when the concentration of FCZ remained unchanged (see Fig. S1 in the supplemental material at https://docs.google.com/document/d/18UTEqWtPbnI9YCrE19HU4k653vkgNPHSbw-xCBMr854/edit). Similarly, the combination of eUSE and FCZ was found to inhibit NBC099 cell viability, which was further confirmed by the MTT assay (Fig. 2A) and LSCM (Fig. 2B). Overall, these results suggested that FCZ along with eUSE had great anticandidal activity; however, eUSE acts as a sensitizer of FCZ-resistant C. albicans NBC099 to cell death.
FIG 1.
Effects of eUSE and FCZ on NBC099 survival. (A) Time-kill curves determined by colony counting. Cells were exposed to various concentrations of eUSE and FCZ. Data at the respective time points are mean cell counts (log10 cells/ml) of the starting inoculum (CFU/ml) ± the standard deviations of three independent experiments. (B) Growth inhibition as examined by the disc diffusion method. Pellets harvested from the mid-log phase were resuspended in NSS. A 100-μl volume of suspended cells was spread uniformly on an SD agar plate, and a presterilized disc (6 mm) was placed on it. Subsequently, QC and FCZ were loaded for 24 h of incubation at 30°C. For each treatment, six experiments were performed.
FIG 2.
Impact of eUSE and FCZ on NBC099 cell death. (A) Cells were treated with various amounts of eUSE and FCZ for the times indicated. Cell death was examined by using MTT dye. All data were normalized to the MTT conversion activity of medium-treated control cells. Each value is the mean ± the standard deviation of three independent experiments. (B) Treated cells were harvested and processed with the LIVE/DEAD cell viability kit (Life Technologies) as described in the manufacturer's protocol. Live SYTO 9-stained green cells and dead PI-stained red cells were visualized by LSCM with an LSM-510 META (Zeiss, Munich, Germany) with FITC (excitation and emission wavelengths of 480 and 500 nm) and tetramethyl rhodamine isothiocyanate (excitation and emission wavelengths of 490 and 635 nm) optical filters, respectively.
eUSE contains QC as a sensitizer of NBC099 cells.
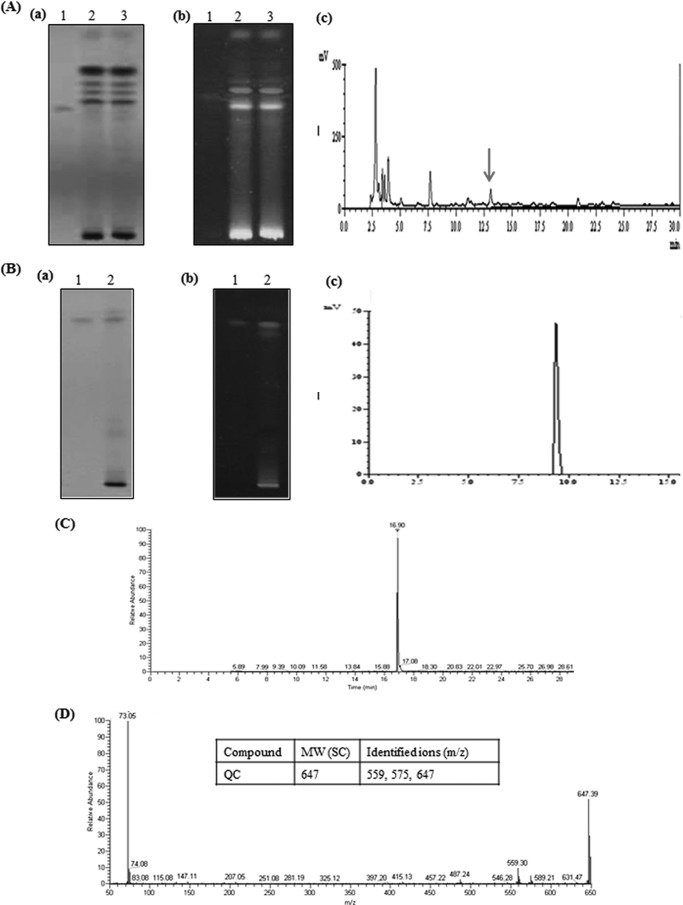
Next, we sought to identify the phytochemical in eUSE that could be responsible for its sensitizing action. For this, eUSE was dissolved in distilled water and then partitioned successively with chloroform (which produced chloroform extract [CE]), dichloromethane (which produced dichloromethane extract [DCME]), ethyl acetate (which produced ethyl acetate extract [EAE]), and butanol (which produced butanol extract [BE]). The chloroform- and dichloromethane-partitioned fractions were concentrated to give residues of 0.11 g (CE) and 0.074 g (DCME), respectively. Similarly, the ethyl acetate and butanol fractions were also concentrated to give residues of 2.12 g (EAE) and 2.37 g (BE), respectively. All of the fractions were tested for anticandidal activity against NBC099, and only EAE showed anticandidal activity when combined with FCZ (see Fig. S2 in the supplemental material at https://docs.google.com/document/d/18UTEqWtPbnI9YCrE19HU4k653vkgNPHSbw-xCBMr854/edit). EAE was applied to silica gel-coated 60G F254 TLC plates (Merck; 20 by 10 cm, 0.25 mm) and separated by elution with toluene-ethyl acetate-formic acid (7.5:2:0.5). The developed plates were overlaid with NBC099-seeded SD agar medium along with FCZ and incubated for 24 h at 30°C (data not shown). The spot corresponding to a growth inhibition zone was identified as a flavonoid, QC, which was confirmed by high-performance TLC (HPTLC; CAMAG, Muttenz, Switzerland) and C18 reversed-phase high-performance liquid chromatography (RP-HPLC; Shimadzu, Kyoto, Japan) with toluene-ethyl acetate-formic acid (7.5:2:0.5) (Fig. 3A, a and b; see Fig. S3A in the supplemental material at https://docs.google.com/document/d/18UTEqWtPbnI9YCrE19HU4k653vkgNPHSbw-xCBMr854/edit) and methanol-H2O-acetic acid (60:39:1.0; 1.0 ml/min) (Fig. 3A, c), respectively, as the mobile phase. The spot was recovered and again subjected to HPTLC and RP-HPLC, which confirmed the presence of 0.21% QC (Fig. 3B, a to c; see Fig. S3B in the supplemental material at https://docs.google.com/document/d/18UTEqWtPbnI9YCrE19HU4k653vkgNPHSbw-xCBMr854/edit). In addition to HPTLC and HPLC analyses, we further performed GC-MS analysis through silylation of the purified compound on GC-MS (Thermo Scientific model DSQ II) equipped with a TR 50-MS column (30 m by 0.32 mm [inside diameter]) coated with material at a film thickness of 0.25 μm. The results confirmed the presence of QC (Fig. 3C and D), and the major ions identified were at 559, 575, and 647 m/z (Fig. 3D, inset), which was consistent with previously reported data (28).
FIG 3.
Identification of QC in eUSE. (A) Methanolic extract of eUSE was dissolved in water and then partitioned successively with chloroform, dichloromethane, ethyl acetate, and butanol. Only EAE was found to be effective against NBC099 when applied with FCZ. Then EAE and the reference compound were subjected to TLC with silica gel 60G F254 plates (20 by 10 cm; 0.25 mm) by elution with toluene-ethyl acetate-formic acid (7.5:2:0.5) and visualized under UV at 254 (a) and 366 (b) nm. Quantification of the compound was done with a model 3 CAMAG TLC scanner with CAMAG winCATS IV software and QC (lanes 1) and EAE (lanes 2 and 3) as reference compounds. (c) RP-HPLC with a C18 column was also used for EAE. Compound separation was achieved with methanol-H2O-acetic acid (60:39:1) as the mobile phase at a flow rate of 1.0 ml/min. The arrow shows the position of QC. (B) Identification of purified fraction by HPTLC under UV light at 254 (a) and 366 (b) nm and RP-HPLC (c). Lanes: 1, reference compound QC; 2, purified fraction. GC-MS analysis. (C) The purified fraction was subjected to silylation, and its molecular weight of 637 was measured at 16.90 min. (D) Production of fragmented ions. The inset shows the molecular weight (MW) of purified compound and its important ions identified during GC-MS analysis.
Enhanced production of farnesol by QC inhibited the production of virulence factors.
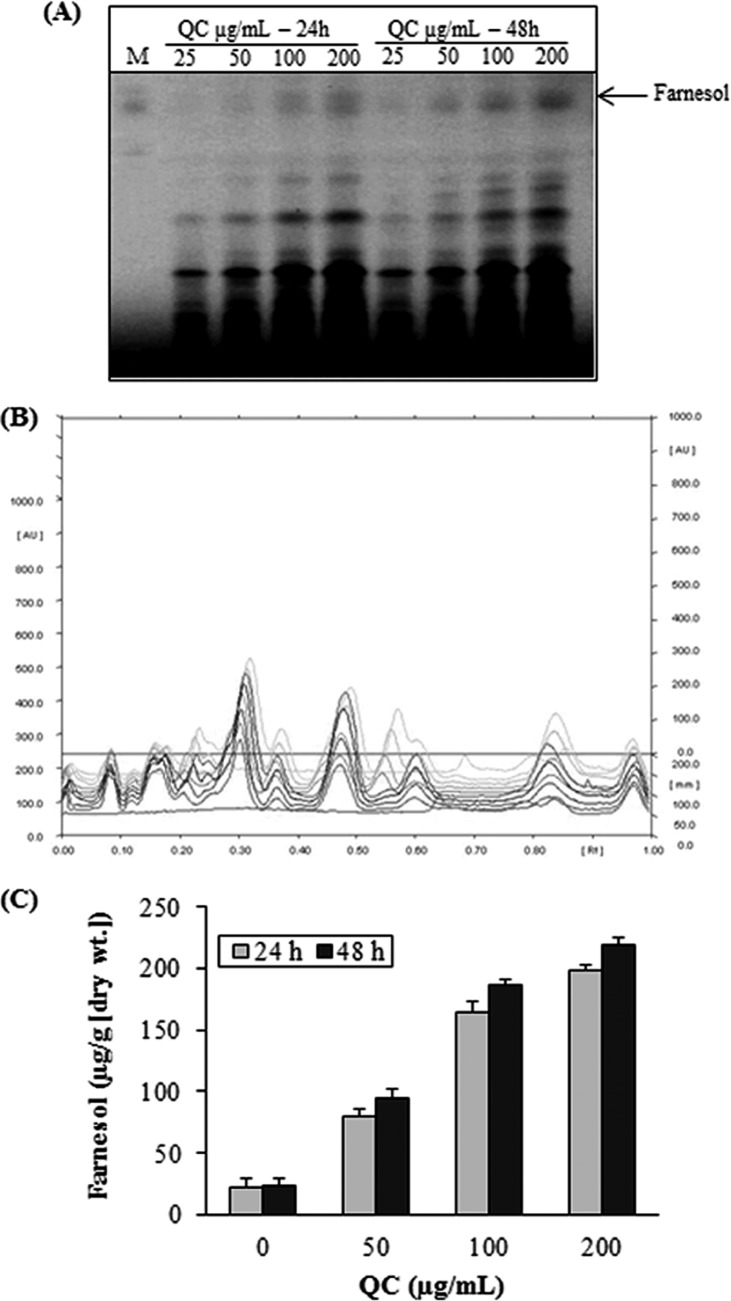
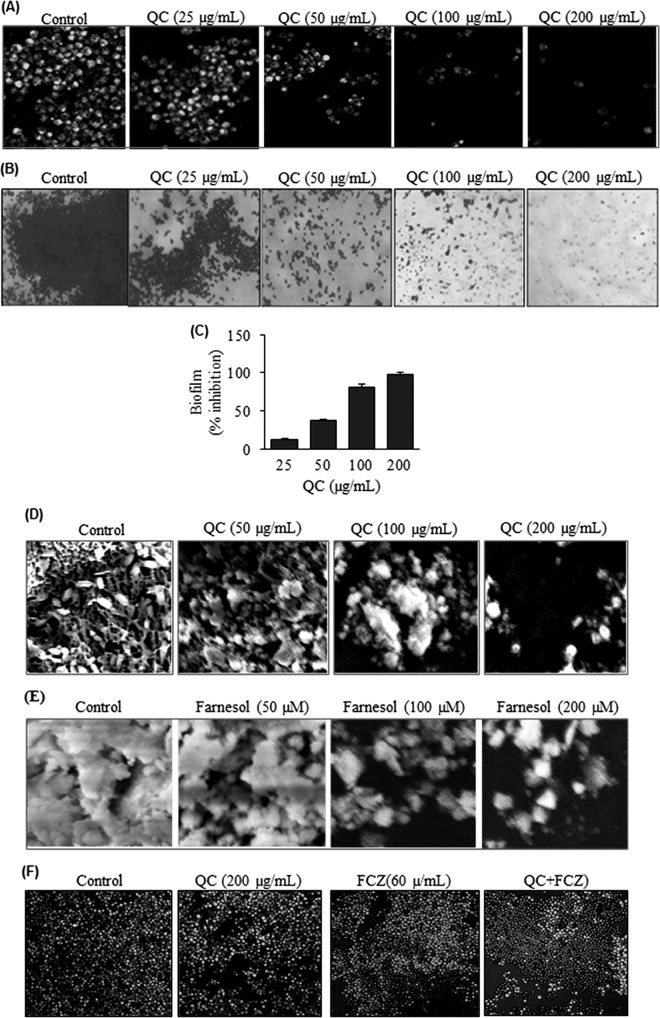
Farnesol has been described as a QS molecule secreted by C. albicans that is able to regulate group behaviors, like virulence, competence, conjugation, antibiotic production, and biofilm formation (30). We were interested in determining if farnesol production would be enhanced in NBC099 culture medium after QC treatment. HPTLC analysis data revealed that exposure of QC to NBC099 induced extracellular farnesol production in a concentration-dependent manner, as indicated by increasing band density (Fig. 4A to C). The highest concentration of farnesol (209 μM) was detected in 200 μg/ml QC-treated NBC099 filtrate after 48 h of incubation. It was ∼10 times that of the untreated control (20.1 μM). Separation of farnesol on silica gel 60 F254 TLC plates (Merck, India) was achieved by using a mobile phase of toluene-ethyl acetate-formic acid (7.5:2:0.5).
FIG 4.
Effects of QC on farnesol production. Supernatant was collected from QC-treated or untreated broth culture of NBC099 cells and extracted thrice with a one-fifth volume of ethyl acetate. The solvent was removed, and the residue was resuspended in absolute ethanol and applied to silica gel 60G F254 plates. Separation of farnesol was obtained with a solvent system consisting of toluene-ethyl acetate-formic acid (7.5:2:0.5), and the results were quantified by HPTLC with CAMAG winCATS IV software. Panels: A, UV at 254 nm; B, overlapping spectra; C, quantified contents of farnesol versus concentrations of QC (inset, incubation times). Each value is the mean result ± the standard deviation of two independent experiments.
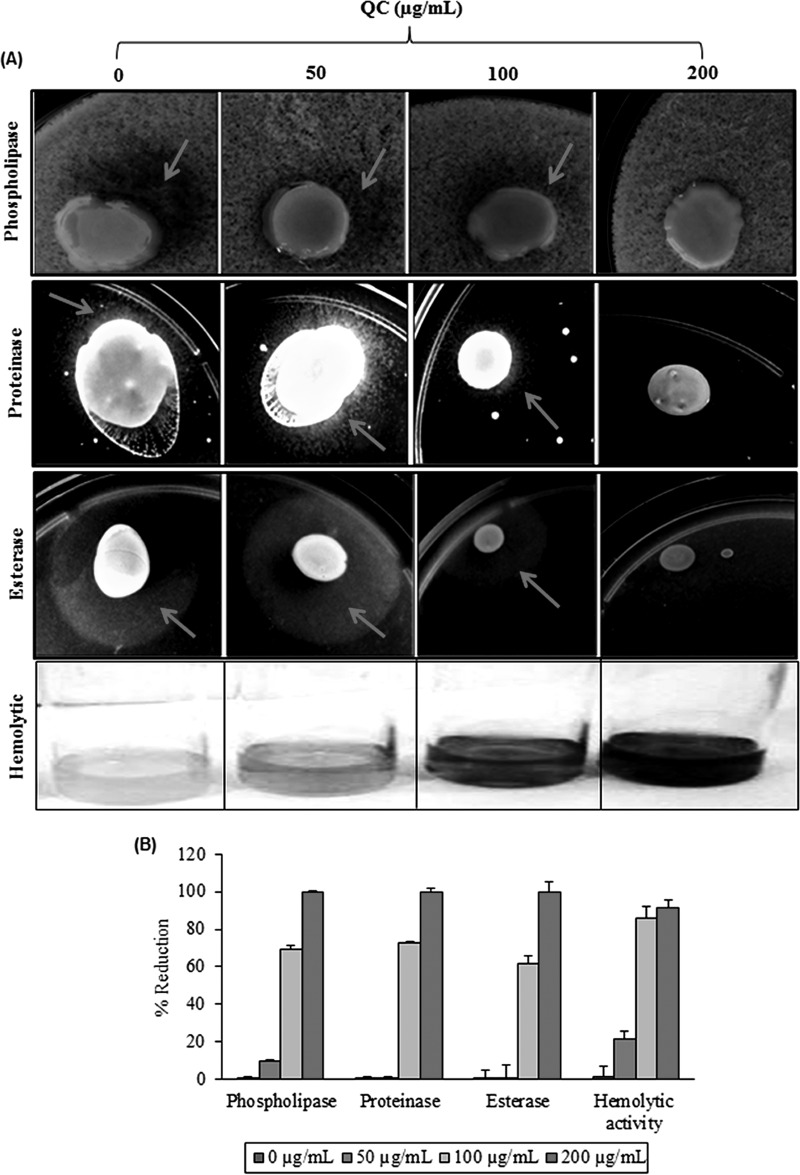
Next we examined whether QC could inhibit hypha formation by NBC099 cells, for which we incubated them with different QC doses and examined the samples by phase-contrast microscopy (Leica) to detect a switch between yeast morphology and hyphal morphology, a key virulence factor. QC significantly suppressed hypha formation in a concentration-dependent manner (Fig. 5A). The CV staining experiment further confirmed the 100% hypha formation inhibition by QC, compared to the untreated control (Fig. 5B and C). Farnesol can also affect the expression of virulence factors such as phospholipase, proteinase, esterase, and hemolysin, which are reported to facilitate the proliferation and pathogenicity of C. albicans (14). Further, SD agar medium supplemented with different amounts of QC and incubated for 5 days showed inhibition of phospholipase, proteinase, and esterase activities (Fig. 6A). Similarly, inhibition of hemolytic activity was also observed in QC-treated cells (Fig. 6A). Hypha formation and expression of virulence factors were completely blocked by QC at 200 μg/ml (Fig. 6B), indicating the reduction of C. albicans pathogenicity by QC.
FIG 5.
Inhibition of hypha formation by QC. (A) A mid-log-phase culture of NBC099 was diluted to 1 × 106 cells/ml with SD broth medium containing 10% FBS and exposed to various concentrations of QC. Cells were grown at 30°C for 72 h. Aliquots were placed on slides, visualized by differential interference contrast microscopy, and photographed at a magnification of ×40. (B) Aliquots of NBC099 (1 × 106 cells/ml) were placed on glass slides and grown for 72 h at 30°C under aseptic and moist conditions. The cells were washed with PBS and stained with 0.2% CV solution for 5 min at room temperature. The cells then were dried, visualized by differential interference contrast microscopy, and photographed at a magnification of ×20. (C) Graph of percent inhibition of hypha formation versus various concentrations of QC. Each value is the mean result ± the standard deviation of three independent experiments.
FIG 6.
Inhibitory effect of QC on the production of virulence factors. (A) To determine phospholipase activity, SD agar medium containing 10% sterile egg yolk was inoculated with 10 μl of a mid-log-phase NBC099 cell culture and incubated for 5 days. The presence of enzyme activity was determined by the formation of a precipitated dark brown zone around the inoculum. Proteinase activity was determined with bovine serum albumin agar. Ten microliters of an active culture of NBC099 was inoculated onto the plates and incubated for 5 days. The presence of proteinase activity was determined by the formation of a transparent halo zone around the inoculum. Tween 80 opacity test agar medium was used to examine esterase activity. NBC099-inoculated medium was incubated for 5 days, and the formation of a halo zone around the inoculation site was considered positive proof of esterase activity. To determine hemolytic activity, SD broth containing 7% sheep blood and 3% glucose was inoculated with 5 ml of active culture of NBC099. After 48 h of incubation, decolorization of the red medium was considered positive proof of hemolytic activity. (B) Percent reduction of virulence factor activity in the absence or presence of QC. Each value is the mean result ± the standard deviation of six independent experiments.
QC inhibited farnesol-dependent biofilm formation in NBC099.
Biofilm formation is an important factor in C. albicans pathogenesis and involves attachment, colonization, and the development of resistance to therapeutic drugs (31). Therefore, we were quite interested in examining whether QC could inhibit biofilm formation by NBC099. Cells were treated with QC and examined for inhibition of biofilm formation by LSCM. Almost complete inhibition was observed when cells were exposed to 200 μg/ml QC (Fig. 7A). Moreover, inhibition of biofilm formation was also confirmed by CV staining under phase-contrast microscopy (Fig. 7B) and quantified spectrophotometrically (Fig. 7C). A thick biofilm coating was observed in untreated controls, whereas a visible reduction in numbers of microcolonies was observed in the biofilms of QC-treated NBC099. In addition, QC deteriorated the architecture of the biofilm, which was evident from SEM analysis (Fig. 7D). The antibiofilm activity of QC was concentration dependent. A number of studies revealed that farnesol disrupts biofilm formation by C. albicans (20, 30). Consistent with this notion, we detected about 95% inhibition of the biofilm formation in culture medium treated with farnesol compared to the untreated control (Fig. 7E). Next, we sought to determine if the antibiofilm activity of QC was related to farnesol induction. To do this, we used a farnesol-deficient ΔCzf1 mutant strain of C. albicans. The results revealed that QC does not exhibit antibiofilm potential (see Fig. S4 in the supplemental material at https://docs.google.com/document/d/18UTEqWtPbnI9YCrE19HU4k653vkgNPHSbw-xCBMr854/edit). This clearly suggests that biofilm formation is inhibited as a result of farnesol production upon exposure to QC.
FIG 7.
Inhibition of biofilm formation by QC. (A) NBC099 cells were grown on glass coverslips under aseptic and humid conditions in the presence or absence of QC. Degrees of biofilm formation were assessed in terms of cell viability in biofilm cultures with the LIVE/DEAD cell viability kit (Life Technologies). Live SYTO 9-stained cells were visualized by LSCM with an FITC (excitation and emission wavelengths of 480 and 500 nm) filter. (B) Cells grown on glass slides were washed with PBS and stained with 0.2% CV solution for 5 min at room temperature. After the cells were dried, biofilm formation was visualized by differential interference contrast microscopy and photographed. (C) NBC099 cells were grown in a 96-well flat-bottom microplate in the presence or absence of QC for 48 h. The medium was then discarded, and the cells were sterilized by adding 70% ethanol to each well for 1 min and washed twice with distilled water. Cells were stained by adding 0.2% CV solution for 15 min. After the microplate was dried, isopropanol containing 0.04 N HCl and 0.25% sodium dodecyl sulfate was added and the A590 was measured. Results are expressed as percentages of inhibition relative to the control. Each value is the mean result ± the standard deviation of three independent experiments. (D and E) Exponentially growing NBC099 cells that had been exposed to QC (D) and farnesol (E) for 48 h were harvested, washed, fixed in 2.0% glutaraldehyde, and then postfixed in 1% osmium tetroxide buffer. After dehydration in a graded ethanol series, the cells were embedded in spur resin and sections were cut on an Ultramicrotome. The sectioned grids were stained with saturated solutions of uranyl acetate and lead citrate and examined by SEM. (F) Sensitivity of QC-treated NBC099 biofilms to FCZ. NBC099 biofilms were grown in the absence or presence of QC. After 72 h, the biofilms were exposed to FCZ for 36 h. Cell viability was assayed by staining with the LIVE/DEAD Cell Viability kit. PI-stained areas are dead cells, and SYTO 9-stained areas are live cells, as analyzed by LSCM.
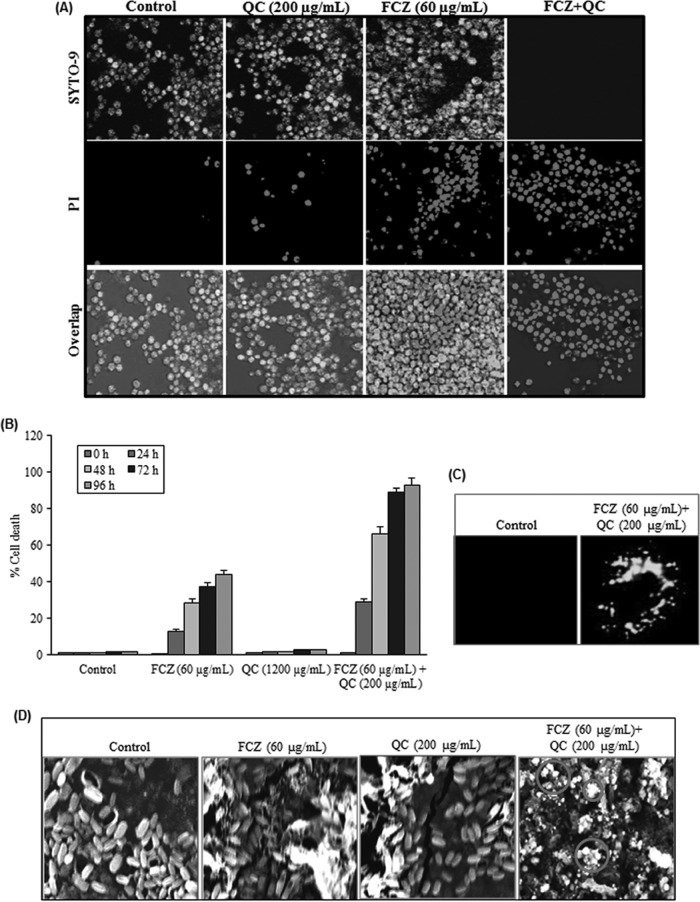
Sensitivity to FCZ, a standard drug routinely used to treat C. albicans infections, was also assessed. The cell viability staining results showed that biofilms grown in the presence of QC were significantly more susceptible to FCZ (Fig. 7F). FCZ efficiently penetrated and killed the cells in QC-treated biofilms, leaving 8% ± 10% of the cells alive., while in the non-QC-treated control, only 10% ± 15% of the cells were killed by FCZ. QC-treated planktonic NBC099 cells were 2 to 3 orders of magnitude more sensitive to FCZ (data not shown).
QC sensitizes FCZ-resistant NBC099 by inducing apoptotic cell death.
It is apparent that fungus cells, like cancer cells, also undergo sensitization to cell death and to apoptosis by dietary phytochemicals. Considering the extent to which dietary phytochemicals trigger important processes in many human cancer cell lines, it is not be surprising that sensitization to cell death also appears to be prevalent in diverse fungal species. We therefore next checked whether QC-controlled, QS-dependent production of virulence factors and biofilm formation sensitized NBC099 cells to FCZ-mediated cell death and apoptosis. Cells were treated with QC and FCZ alone, as well as together. LSCM results showed almost no cell death with QC alone, and FCZ alone had a weak anticandidal effect; however, the percentage of PI-stained cells was enhanced significantly when cells were treated with a combination of QC and FCZ (Fig. 8A and B). In addition, we investigated whether QC-FZC combination-mediated cell death could be a result of apoptosis. Treated cells were labeled with M30 CytoDEATH, an antibody that binds to a caspase-cleaved epitope of the cytokeratin 18 cytoskeletal protein as a marker of apoptosis, and examined by LSCM with a green filter. As shown in Fig. 8C, exposure of cells to a combination of QC and FCZ resulted in induction of apoptosis, in contrast to QC and FCZ alone and the untreated control. SEM was also used to confirm the induction of apoptotic cell death (Fig. 8D), and the results were generally consistent with M30 CytoDEATH labeling. Interestingly, the anticandidal effect of the combination of QC and FCZ was enhanced when the concentration of FCZ was increased, while an increase in the concentration of QC caused no enhancement when the concentration of FCZ remained unchanged (data not shown). The dietary flavonoid QC therefore can be said to increase the susceptibility of C. albicans cells to apoptotic cell death caused by FCZ at the recommended dose of 60 μg/ml.
FIG 8.
Enhancement of FCZ-mediated apoptotic cell death by QC. (A) Exponentially growing NBC099 cells were treated with QC and FCZ, harvested, and processed with the LIVE/DEAD cell viability kit (Life Technologies) as described in the manufacturer's protocol. Live SYTO 9-stained green cells and dead PI-stained red cells were visualized by LSCM with FITC (excitation and emission wavelengths of 480 and 500 nm) and tetramethyl rhodamine isothiocyanate (excitation and emission wavelengths of 490 and 635 nm) optical filters, respectively. (B) Cells were treated with QC and FCZ for 48 h. Cell death was examined with MTT dye. All data were normalized to the MTT conversion activity of medium-treated control cells. Each value is the mean ± the standard deviation of three independent experiments. (C) Exponentially growing NBC099 cells were grown on glass coverslips in the absence or presence of QC plus FCZ for 48 h at 30°C, washed, and then incubated with monoclonal antibody CK18 (1:100) for detection of apoptosis by LSCM. (D) Cells were grown in the presence or absence of QC and FCZ for 48 h and processed for SEM analysis as described in the legend to Fig. 7D. Cell death and apoptosis induced by QC plus FCZ, as indicated by the circles, were examined by SEM.
QC inhibition of adenylate cyclase activity confirmed by molecular docking.
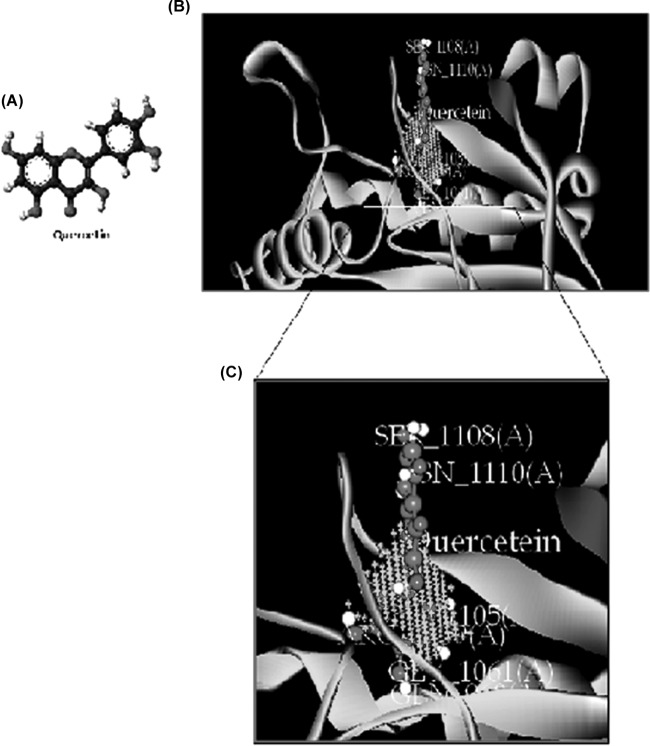
Silencing of the adenylate cyclase-encoding gene cacdc35 in C. albicans, which is essential for hyphal growth, virulence, and biofilm formation, has been found to establish vaginal infection in a mucosal membrane mouse model and produced avirulence in a mouse model of systemic infection. Therefore, adenylyl cyclase, a regulator of the cyclic AMP (cAMP) signaling pathway, may be a useful target for anticandidal drugs (32). To investigate the possible inhibitory action of QC on adenylate cyclase, we used information available from the X-ray crystal structure of T. brucei to model the three-dimensional (3D) structure of the adenylate cyclase catalytic domain. We used the Discovery Studio v2.5 docking program to see the interaction between the protein and its ligand. Docking of QC in the putative ATP binding pocket indicated that QC formed potential hydrogen bonds with some catalytically important residues (Gln969, Thr1105, Ser1108, Arg1109, Asn1110, and Gly1061), which were the same residues that appear to stabilize the flipped ATP through hydrogen bonding and hydrophobic interactions (Fig. 9B and C). This structural model revealed a substantial region of interaction with the ATP active pocket for subsequent production of cAMP, which helps to regulate developmental programs of C. albicans, including invasive hyphal growth, phenotypic switching to a mating-competent cell type, and biofilm formation (33). The calculated Ebinding value of QC was found to be −20.808 kcal/mol. These predicted results are generally consistent with the experimental data.
FIG 9.
Molecular docking of the interaction between QC and adenylate cyclase. (A) 3D structure of QC. (B) Binding orientation of QC in adenylate cyclase. The protein is depicted as a ribbon, and secondary structures (i.e., helix, strand, and loop) are shown. (C) Both QC and ligand contact residues are represented in stick form.
DISCUSSION
In this study, we examined the effect of QC isolated from U. longissima on FCZ-mediated apoptotic cell death in resistant C. albicans strain NBC099. FCZ is undergoing clinical testing and has been shown to have minimal toxicity (3, 34). Many strains of C. albicans are resistant to FCZ therapy, while FCZ can be cytotoxic at high concentrations (15, 35). We therefore aimed to sensitize FCZ-resistant C. albicans strain NBC099 with QC. In this study, we demonstrated that a combination of QC and FCZ strongly induced apoptotic cell death in NBC099 cells by modulating QS signaling.
First, we demonstrated that the viability of NBC099 was not affected at 48 h after treatment with 200 μg/ml QC alone. We then examined the viability of NBC099 cells after treatment with FCZ alone and detected a weak effect up to 60 μg/ml. Our results were similar to the previously published data that demonstrated the resistance of C. albicans to 60 μg/ml FCZ (3). The sensitization of human cancer cells by QC has been studied well in various carcinoma cell lines (36–38), but the sensitization of human fungal pathogens by QC has not been investigated. Upon combined treatment with QC and FCZ, NBC099 cells showed strongly enhanced cell death. A combination of QC and FCZ demonstrated a positive synergistic action. Our results are very similar to those of previous reports on combined treatment with tumor necrosis factors and QC, which has been successfully tested in various cancer cell lines (39, 40).
QS is a regulatory process of gene expression and group behavior in response to changes in cell population density. C. albicans is one of the most prevalent human fungal pathogens that reside in the commensal microbiota of the gastrointestinal tract and mucosal membranes (30). Its capacity to switch between budding and hyphal growth is a key virulence factor (30). Production of virulence factors regulated by QS signaling is a key weapon that causes pathogenesis and developing resistance in C. albicans, with hypha and biofilm formation as prominent members. Because hypha and biofilm formation prevents cell death and apoptosis, a point where QS converges, strategies targeting these virulence factors to remove its inhibitory effect seem to be useful in overcoming the drug resistance of C. albicans. Suppression of hypha and biofilm formation and related genes has been demonstrated upon QC-based nanomaterial treatment in C. albicans (41). We therefore analyzed the effect of QC in FCZ-resistant NBC099 on the production of virulence factors. Hypha and biofilm formation was strongly suppressed in QC-treated NBC099 cells. Moreover, QC was also able to inhibit the production of other key C. albicans virulence factors, such as phospholipase, proteinase, esterase, and hemolysin, which promote proliferation and invasion of host tissue, degrade immunoglobulins, inhibit phagocytosis, and induce inflammatory reactions (42, 43). These findings indicate that the inhibition of hyphal growth, biofilm formation, and virulence enzymes and hemolytic activities can be a key mechanism of action by which QC enhances FCZ-mediated apoptotic cell death in resistant C. albicans.
We further identified a potential mechanism leading to farnesol-dependent apoptotic cell death and changes in virulence factor expression by QC. Farnesol-treated NBC099 cells significantly inhibited yeast-to-mycelium conversion and biofilm formation. Farnesol, a QS molecule, is released by C. albicans, which inhibits hypha and biofilm formation as a mechanism of regulation (20, 44). Ramage and colleagues found that C. albicans biofilm density and morphology were drastically altered by high concentrations of farnesol, most likely as a direct consequence of the inhibitory effect of farnesol on the morphogenetic process (20). In addition, farnesol has also been reported to induce apoptotic cell death and cytotoxicity at certain concentrations in C. albicans via caspase activation (45). In line with previous reports, the present study demonstrated that the inhibitory effect of QC on virulence factor production appears to result from induction of the QS molecule farnesol, since the ΔCzf1 mutation inhibited QC-mediated suppression of biofilm formation in C. albicans. Other research groups have also reported that Czf1 is required for filament and biofilm inhibition by farnesol (18), which is in line with our results because QC regulates morphology conversion and virulence behaviors and, along with FCZ, induces apoptotic cell death due to increasing concentrations of farnesol. These results clearly suggest that QC enhances farnesol-dependent inhibition of virulence factors, biofilm formation, and hypha development and eventually induces apoptotic cell death.
To the best of our knowledge, no study exploring the interaction between QC and adenylate cyclase has previously been published. Adenylate cyclase is an enzyme that has key regulatory roles in C. albicans, affecting hyphal growth and drug resistance (32, 33). In addition to the effect of QC on the inhibition of virulence factors and induction of apoptotic cell death, we have also studied the possible interaction between QC and adenylate cyclase by using computational docking. The docking results showed the strong molecular interactions between QC and the ATP binding pocket of adenylate cyclase through the formation of hydrogen bonds and hydrophobic and ionic interactions with the important residues of the ATP binding pocket of adenylate cyclase, thus inhibiting the pathogenicity of C. albicans.
Conclusion.
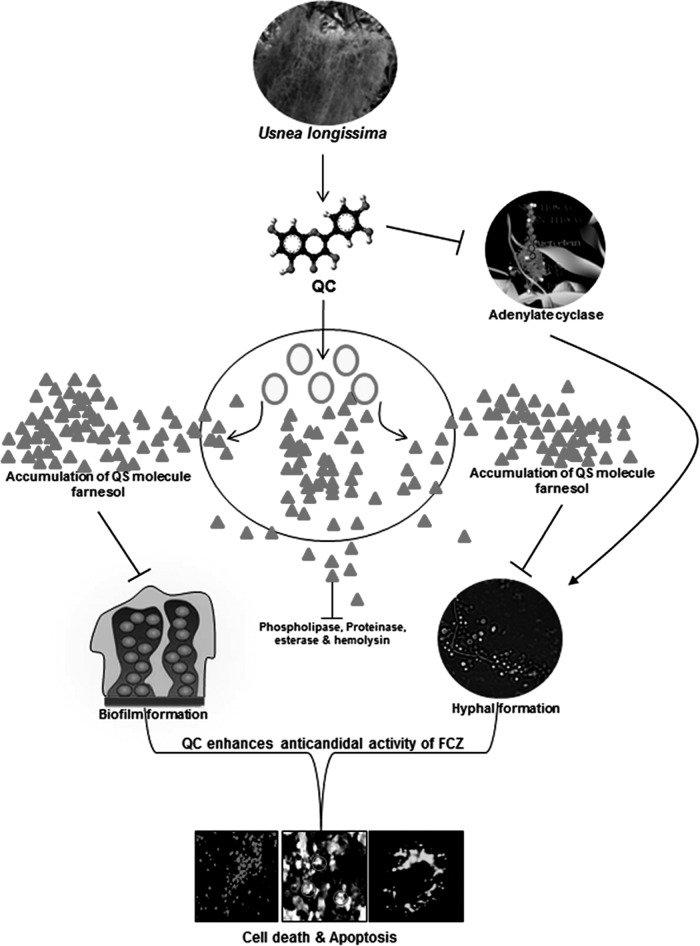
In this investigation, we demonstrated that QC isolated from the edible lichen U. longissima potently enhances FCZ-mediated apoptotic cell death by modulating QS signaling by increasing the production of farnesol, which is known to coregulate hyphal development, biofilm formation, and virulence factor production (Fig. 10).
FIG 10.
Possible mechanism by which QC induces FCZ-mediated apoptotic cell death in FCZ-resistant C. albicans NBC099. To summarize our research, QC inhibited farnesol-mediated hyphal development, biofilm formation, and production of virulence factors in C. albicans NBC099 cells. Moreover, QC was found to inhibit adenylate cyclase activity in NBC099, a key enzyme responsible for suppression of farnesol production. Later, QC increased the sensitization of NBC099 cells to FCZ, thereby inducing cell death and eventually apoptotic cell death.
ACKNOWLEDGMENTS
This work was financially supported by the Council of Scientific and Industrial Research, New Delhi, India (BSC-0106, OLP-0089). We are thankful to C. S. Nautiyal, director of the CSIR-National Botanical Research Institute, for providing all of the research facilities.
REFERENCES
- 1.Chander J, Singla N, Sidhu SK, Gombar S. 2013. Epidemiology of Candida blood stream infections: experience of a tertiary care centre in North India. J Infect Dev Ctries 7:670–675. doi: 10.3855/jidc.2623. [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li DD, Xu Y, Zhang DZ, Quan H, Mylonakis E, Hu DD, Li MB, Zhao LX, Zhu LH, Wang Y, Jiang YY. 2013. Fluconazole assists berberine to kill fluconazole-resistant Candida albicans. Antimicrob Agents Chemother 57:6016–6027. doi: 10.1128/AAC.00499-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castelo-Branco DS, Brilhante RS, Paiva MA, Teixeira CE, Caetano EP, Ribeiro JF, Cordeiro RA, Sidrim JJ, Monteiro AJ, Rocha MF. 2013. Azole-resistant Candida albicans from a wild Brazilian porcupine (Coendou prehensilis): a sign of an environmental imbalance? Med Mycol 51:555–560. doi: 10.3109/13693786.2012.752878. [DOI] [PubMed] [Google Scholar]
- 5.Youngsaye W, Hartland CL, Morgan BJ, Ting A, Nag PP, Vincent B, Mosher CA, Bittker JA, Dandapani S, Palmer M, Whitesell L, Lindquist S, Schreiber SL, Munoz B. 2013. ML212: A small-molecule probe for investigating fluconazole resistance mechanisms in Candida albicans. Beilstein J Org Chem 9:1501–1507. doi: 10.3762/bjoc.9.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Ami R, Olshtain-Pops K, Krieger M, Oren I, Bishara J, Dan M, Wiener-Well Y, Weinberger M, Zimhony O, Chowers M, Weber G, Potasman I, Chazan B, Kassis I, Shalit I, Block C, Keller N, Kontoyiannis DP, Giladi M. 2012. Antibiotic exposure as a risk factor for fluconazole-resistant Candida bloodstream infection. Antimicrob Agents Chemother 56:2518–2523. doi: 10.1128/AAC.05947-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins N, Leach MD, Cowen LE. 2012. Lysine deacetylases Hda1 and Rpd3 regulate Hsp90 function thereby governing fungal drug resistance. Cell Rep 2:878–888. doi: 10.1016/j.celrep.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowen LE, Lindquist S. 2005. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 309:2185–2189. doi: 10.1126/science.1118370. [DOI] [PubMed] [Google Scholar]
- 9.Cowen LE, Carpenter AE, Matangkasombut O, Fink GR, Lindquist S. 2006. Genetic architecture of Hsp90-dependent drug resistance. Eukaryot Cell 5:2184–2188. doi: 10.1128/EC.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowen LE. 2008. The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype. Nat Rev Microbiol 6:187–198. doi: 10.1038/nrmicro1835. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar S, Uppuluri P, Pierce CG, Lopez-Ribot JL. 2014. In vitro study of sequential fluconazole/caspofungin treatment against Candida albicans biofilms. Antimicrob Agents Chemother 58:1183–1186. doi: 10.1128/AAC.01745-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han S, Kim J, Yim H, Hur J, Song W, Lee J, Jeon S, Hong T, Woo H, Yim DS. 2013. Population pharmacokinetic analysis of fluconazole to predict therapeutic outcome in burn patients with Candida infection. Antimicrob Agents Chemother 57:1006–1011. doi: 10.1128/AAC.01372-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiori A, Van Dijck P. 2012. Potent synergistic effect of doxycycline with fluconazole against Candida albicans is mediated by interference with iron homeostasis. Antimicrob Agents Chemother 56:3785–3796. doi: 10.1128/AAC.06017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angiolella L, Stringaro AR, De Bernardis F, Posteraro B, Bonito M, Toccacieli L, Torosantucci A, Colone M, Sanguinetti M, Cassone A, Palamara AT. 2008. Increase of virulence and its phenotypic traits in drug-resistant strains of Candida albicans. Antimicrob Agents Chemother 52:927–936. doi: 10.1128/AAC.01223-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu LH, Wei X, Ma M, Chen XJ, Xu SB. 2012. Possible inhibitory molecular mechanism of farnesol on the development of fluconazole resistance in Candida albicans biofilm. Antimicrob Agents Chemother 56:770–775. doi: 10.1128/AAC.05290-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogan DA. 2006. Talking to themselves: autoregulation and quorum sensing in fungi. Eukaryot Cell 5:613–619. doi: 10.1128/EC.5.4.613-619.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis K. 2007. Persister cells, dormancy and infectious disease. Nat Rev Microbiol 5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 18.Langford ML, Hargarten JC, Patefield KD, Marta E, Blankenship JR, Fanning S, Nickerson KW, Atkin AL. 2013. Candida albicans Czf1 and Efg1 coordinate the response to farnesol during quorum sensing, white-opaque thermal dimorphism, and cell death. Eukaryot Cell 12:1281–1292. doi: 10.1128/EC.00311-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deveau A, Hogan DA. 2011. Linking quorum sensing regulation and biofilm formation by Candida albicans. Methods Mol Biol 692:219–233. doi: 10.1007/978-1-60761-971-0_16. [DOI] [PubMed] [Google Scholar]
- 20.Ramage G, Saville SP, Wickes BL, Lopez-Ribot JL. 2002. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol 68:5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borges A, Serra S, Cristina Abreu A, Saavedra MJ, Salgado A, Simoes M. 2014. Evaluation of the effects of selected phytochemicals on quorum sensing inhibition and in vitro cytotoxicity. Biofouling 30:183–195. doi: 10.1080/08927014.2013.852542. [DOI] [PubMed] [Google Scholar]
- 22.Kalia VC. 2013. Quorum sensing inhibitors: an overview. Biotechnol Adv 31:224–245. doi: 10.1016/j.biotechadv.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Singh BN, Singh HB, Singh A, Singh BR, Mishra A, Nautiyal CS. 2012. Lagerstroemia speciosa fruit extract modulates quorum sensing-controlled virulence factor production and biofilm formation in Pseudomonas aeruginosa. Microbiology 158:529–538. doi: 10.1099/mic.0.052985-0. [DOI] [PubMed] [Google Scholar]
- 24.Singh BN, Singh BR, Singh RL, Prakash D, Dhakarey R, Upadhyay G, Singh HB. 2009. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem Toxicol 47:1109–1116. doi: 10.1016/j.fct.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 25.Singh BN, Singh BR, Singh RL, Prakash D, Sarma BK, Singh HB. 2009. Antioxidant and anti-quorum sensing activities of green pod of Acacia nilotica L. Food Chem Toxicol 47:778–786. doi: 10.1016/j.fct.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Adonizio A, Kong KF, Mathee K. 2008. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by south Florida plant extracts. Antimicrob Agents Chemother 52:198–203. doi: 10.1128/AAC.00612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adonizio A, Dawlaty J, Ausubel FM, Clardy J, Mathee K. 2008. Ellagitannins from Conocarpus erectus exhibit anti-quorum sensing activity against Pseudomonas aeruginosa. Planta Med 74:1035–1035. doi: 10.1055/s-0028-1084373. [DOI] [Google Scholar]
- 28.Proestos C, Komaitis M. 2013. Analysis of naturally occurring phenolic compounds in aromatic plants by RP-HPLC coupled to diode array detector (DAD) and GC-MS after silylation. Foods 2:90–99. doi: 10.3390/foods2010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samaranayake LP, Raeside JM, MacFarlane TW. 1984. Factors affecting the phospholipase activity of Candida species in vitro. Sabouraudia 22:201–207. doi: 10.1080/00362178485380331. [DOI] [PubMed] [Google Scholar]
- 30.Lu Y, Su C, Unoje O, Liu H. 2014. Quorum sensing controls hyphal initiation in Candida albicans through Ubr1-mediated protein degradation. Proc Natl Acad Sci U S A 111:1975–1980. doi: 10.1073/pnas.1318690111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finkel JS, Mitchell AP. 2011. Genetic control of Candida albicans biofilm development. Nat Rev Microbiol 9:109–118. doi: 10.1038/nrmicro2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rocha CRC, Schröppel K, Harcus D, Marcil A, Dignard D, Taylor BN, Thomas DY, Whiteway M, Leberer E. 2001. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol Biol Cell 12:3631–3643. doi: 10.1091/mbc.12.11.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogan DA, Muhlschlegel FA. 2011. Candida albicans developmental regulation: adenylyl cyclase as a coincidence detector of parallel signals. Curr Opin Microbiol 14:682–686. doi: 10.1016/j.mib.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Ferry DR, Smith A, Malkhandi J, Fyfe DW, deTakats PG, Anderson D, Baker J, Kerr DJ. 1996. Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res 2:659–668. [PubMed] [Google Scholar]
- 35.Zhao J, Xu Y, Li C. 2013. Association of T916C (Y257H) mutation in Candida albicans ERG11 with fluconazole resistance. Mycoses 56:315–320. doi: 10.1111/myc.12027. [DOI] [PubMed] [Google Scholar]
- 36.Jacquemin G, Granci V, Gallouet AS, Lalaoui N, Morle A, Iessi E, Morizot A, Garrido C, Guillaudeux T, Micheau O. 2012. Quercetin-mediated Mcl-1 and survivin downregulation restores TRAIL-induced apoptosis in non-Hodgkin's lymphoma B cells. Haematologica 97:38–46. doi: 10.3324/haematol.2011.046466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Psahoulia FH, Drosopoulos KG, Doubravska L, Andera L, Pintzas A. 2007. Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Mol Cancer Ther 6:2591–2599. doi: 10.1158/1535-7163.MCT-07-0001. [DOI] [PubMed] [Google Scholar]
- 38.Kim YH, Lee DH, Jeong JH, Guo ZS, Lee YJ. 2008. Quercetin augments TRAIL-induced apoptotic death: involvement of the ERK signal transduction pathway. Biochem Pharmacol 75:1946–1958. doi: 10.1016/j.bcp.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen W, Wang X, Zhuang J, Zhang L, Lin Y. 2007. Induction of death receptor 5 and suppression of survivin contribute to sensitization of TRAIL-induced cytotoxicity by quercetin in non-small cell lung cancer cells. Carcinogenesis 28:2114–2121. doi: 10.1093/carcin/bgm133. [DOI] [PubMed] [Google Scholar]
- 40.Russo M, Nigro P, Rosiello R, D'Arienzo R, Russo GL. 2007. Quercetin enhances CD95- and TRAIL-induced apoptosis in leukemia cell lines. Leukemia 21:1130–1133. (Letter.) doi: 10.1038/sj.leu.2404610. [DOI] [PubMed] [Google Scholar]
- 41.Vashisth P, Nikhil K, Pemmaraju SC, Pruthi PA, Mallick V, Singh H, Patel A, Mishra NC, Singh RP, Pruthi V. 2013. Antibiofilm activity of quercetin-encapsulated cytocompatible nanofibers against Candida albicans. J Bioact Compat Polym 28:652–665. doi: 10.1177/0883911513502279. [DOI] [Google Scholar]
- 42.Schaller M, Januschke E, Schackert C, Woerle B, Korting HC. 2001. Different isoforms of secreted aspartyl proteinases (Sap) are expressed by Candida albicans during oral and cutaneous candidosis in vivo. J Med Microbiol 50:743–747. [DOI] [PubMed] [Google Scholar]
- 43.Reynaud AH, Nygaard-Ostby B, Boygard GK, Eribe ER, Olsen I, Gjermo P. 2001. Yeasts in periodontal pockets. J Clin Periodontol 28:860–864. doi: 10.1034/j.1600-051x.2001.028009860.x. [DOI] [PubMed] [Google Scholar]
- 44.Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, Dussault P, Nickerson KW. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol 67:2982–2992. doi: 10.1128/AEM.67.7.2982-2992.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shirtliff ME, Krom BP, Meijering RA, Peters BM, Zhu J, Scheper MA, Harris ML, Jabra-Rizk MA. 2009. Farnesol-induced apoptosis in Candida albicans. Antimicrob Agents Chemother 53:2392–2401. doi: 10.1128/AAC.01551-08. [DOI] [PMC free article] [PubMed] [Google Scholar]