Abstract
The development of a topical agent that would strengthen the nail, improve the natural barrier, and provide better drug penetration to the nail bed is needed. In this study, we examined the effects of a hydroxypropyl chitosan (HPCH)-based nail solution using a bovine hoof model. Following application of the nail solution, changes in the hardness of the hoof samples were measured using the Vickers method. Tensile and flexural strengths were tested by stretching or punching the samples, respectively. The ultrastructure was examined using scanning electron microscopy (SEM), and samples stained with periodic acid-Schiff (PAS) stain were used to determine the fungal penetration depth. The comparators included 40% urea and 70% isopropyl alcohol solutions. The HPCH nail solution increased hoof sample hardness in comparison to the untreated control sample (mean, 22.3 versus 19.4 Vickers pyramid number [HV]). Similarly, the HPCH solution increased the tensile strength (mean, 33.07 versus 28.42 MPa) and flexural strength (mean, 183.79 versus 181.20 MPa) compared to the untreated control. In contrast, the comparators had adverse effects on hardness and strength. SEM showed that the HPCH solution reduced the area of sample crumbling following abrasion compared to the untreated control (7,418 versus 17,843 pixels), and the PAS-stained images showed that the HPCH solution reduced penetration of the dermatophyte hyphae (e.g., penetration by Trichophyton mentagrophytes was <25 μm at day 9 versus 275 μm in the untreated control). Unlike chemicals normally used in cosmetic treatments, repeated application of the HPCH nail solution may help prevent the establishment of new or recurring fungal nail infection.
INTRODUCTION
The treatment of onychomycosis has improved recently with the addition of novel topical agents used either alone or in combination with systemic oral drugs. However, recurrent or persistent infection occurs in up to 25% of patients (1). Several predisposing factors may contribute to a patient's susceptibility to fungal nail infection, including age, impaired blood circulation, diabetes, physical trauma, occupation, and lifestyle (1–6). The health of the nail itself may have an important bearing on the ability of the dermatophyte fungi to establish an infection, as most cases of onychomycosis are secondary to a previously established skin infection and/or nail trauma, derived either from mechanical insults or from exposure to chemical agents which can alter the natural nail barrier function. In this regard, the use of urea and/or isopropyl alcohol, chemicals often used in nail care to dissolve the nail keratin or to remove solutions from the nail surface, can undermine the nail structure and make it more prone to fungal infections.
A topical agent that strengthens the nail, improving the natural barrier to infection, might represent a valuable option for preventing new or recurrent fungal infections. The hydroxypropyl chitosan (HPCH)-based nail solution (Genadur) developed by Polichem is based on aminosaccharide chitin extracted from the crab carapace. The other two components, methyl sulfonyl methane and Equisetum arvense (an herbaceous perennial plant commonly known as horsetail), provide sulfur and silica, which contribute to the strengthening of the nail and which provide minerals to form the collagen that cements the nail cells. This water-based HPCH solution forms a protective film that remineralizes and restructures nails, protects keratin, and maintains hydration. In clinical studies, it has been shown to reduce the signs of fragility and roughness in the nails of patients with psoriatic nail dystrophy (7–9). The HPCH-based nail solution is not intended as a cure for onychomycosis, as it does not exhibit antifungal activity; instead, it is able to form a film on the nail surface that may prevent fungal invasion. Moreover, the solution, being devoid of any pharmacological or toxicological effect, can be used indefinitely.
In this paper, we describe the effects of an HPCH-based nail solution compared to the effects of urea and isopropyl alcohol on hardness and on ultrastructure and tensile and flexural strength using bovine hooves, which have been validated as equivalent in structure to human nails (10). We used bovine hooves in this study because they are less expensive and more easily obtained than human cadaver nails. In addition, although the bovine keratin is chemically slightly different from that of humans, these differences were not believed to affect the results of the investigation. On the other hand, the great interindividual variability of human nails, e.g., due to sex, age, thickness, and length, may have affected the results. In our investigation, the bovine samples were cut to a standardized size and thickness for the various assays.
MATERIALS AND METHODS
Test items.
The HPCH-based nail solution, containing hydroxypropyl chitosan, Equisetum arvense glycolic extract, methylsulfonyl methane, diethylene glycol monoethylether, water, and ethanol, was the primary test article in this study. The comparators were 70% isopropyl alcohol and 40% urea water solutions.
Fungal strains.
Two strains obtained from the American Type Culture Collection (ATCC), Trichophyton mentagrophytes ATCC 24953 and Trichophyton rubrum MYA 4438, were used in the penetration studies.
Preparation of hoof samples.
In order to ensure uniform test product application, hoof samples were polished with abrasive paper, wiped with a wet cloth to remove excess material, and cut into slices of various thicknesses depending on the test assay. Each sample for each assay was processed in the same manner and was subsequently stored at 20°C ± 2°C at 50% ± 2% relative humidity throughout the testing procedure. Each test product was applied as a thin layer to one surface of the sample using a special brush and was allowed to air dry. After 24 h, the sample was rinsed with water, air dried, and re-treated with the product. This procedure was repeated daily for a total of 6 days to mimic a repeat-dose regimen. The test groups included the HPCH-based nail solution, the 40% urea solution, the 70% isopropyl alcohol solution, and an untreated control.
Hardness test.
Hoof slices (3 mm thick; n = 20 per test article) were tested for hardness using the Vickers method, which measures the footprint left by an indenter on the surface of the sample. The unit of hardness given by the test is known as the Vickers pyramid number (HV) and represents the test material's ability to resist deformation from a standard source, which in this case is a diamond in the form of a square-based pyramid (11).
Tensile strength.
To determine the relative tensile strength, uniaxial samples (n = 20 per test article) were prepared at a thickness of 0.5 to 0.6 mm with widened shoulders to facilitate testing with an RS 232 Sauter FH 500 instrument. The sample was pulled with a controlled, gradually increasing force until the sample changed shape or was ruptured. Tensile strength was measured as the force per unit area and was expressed in megapascals (MPa) (12).
Flexural strength.
The RS 232 Sauter FH 500 instrument was also used to test the flexural strength of the hoof samples. The samples (n = 20 per test article) were prepared at a thickness of 0.5 to 0.6 mm and were processed to achieve a constant rectangular section throughout the length of the sample. The load was gradually increased to obtain the rupture of the tested specimens. The flexural or bend strength, defined as a material's ability to resist deformation under load, was also measured in megapascals (MPa) (13).
Ultrastructure.
Ultrastructure images of the samples were obtained by scanning electron microscopy (SEM) (Hitachi S-2400 scanning electron microscope) of 0.5-cm2 samples that were 2 to 5 mm thick (n = 20 per test article). Images were taken before and after abrasion of the hoof surface with abrasive paper applied in 2 directions for 15 s each. The index of crumbling, defined as the difference between the percent area of shades of gray before and after the abrasion, is an indicator of the protective effect of the applied test product.
Dermatophyte penetration.
In order to study the effects of test articles on the ability of dermatophytes to penetrate the hoof samples, disposable biopsy specimen punches were used to cut 4-mm disks from autoclaved samples measuring 0.5 to 1 mm thick (n = 5 per test article). Treatments were applied to the disks once daily for 6 days prior to inoculation with dermatophytes.
Mature colonies of Trichophyton mentagrophytes ATCC 24953 and T. rubrum MYA 4438 were harvested in phosphate-buffered saline (PBS) to a concentration of 2 to 5 × 105 CFU/ml. This inoculum was used to seed potato dextrose agar (PDA) plates upon which the disks were placed. The disks were evaluated after 1, 3, 5, 7, 9, 11, 13, and 15 days of incubation at 30°C; these days were chosen as optimal time points for demonstrating the various rates of penetration.
The ability of the fungi to invade the hoof disk was evaluated using two methodologies: determination of the number of fungal CFU infecting the hoof samples and histopathological examination. For determining the number of CFU, additional hoof disks were wiped to remove any residual inoculum from the surface, weighed, and then ground to powder. Ground hooves were cultured on PDA, incubated for 4 days at 30°C, and then analyzed for a colony count. Since determination of the number of CFU by this method is only semiquantitative and is not standardized, the depth of penetration by fungal hyphae was also determined by histological evaluation. The disks were stained with periodic acid-Schiff (PAS) stain for visualization of fungal elements in order to monitor the penetration of the fungal infection.
Statistical analyses.
The results for hardness and ultrastructure were analyzed using the Kruskal-Wallis test, followed by the Wilcoxon-Mann-Whitney test. For tensile strength and flexural strength, an analysis of variance (ANOVA) was performed, followed by the t test.
RESULTS
Hardness test.
The results of the hardness test are summarized in Fig. 1. As can be seen, application of the HPCH-based nail solution to the hooves significantly increased their hardness in comparison to that of the untreated control sample (mean [± standard deviation], 22.27 ± 2.01 HV versus 19.36 ± 1.23 HV, respectively; P < 0.001). In contrast, urea and isopropyl alcohol had significant adverse effects on the hardness of the bovine hoof (mean, 17.98 ± 1.39 HV and 17.59 ± 1.22 HV, respectively; P < 0.001). The effect of the HPCH-based test product was also significant compared to that of urea or isopropyl alcohol (P < 0.001 for each).
FIG 1.
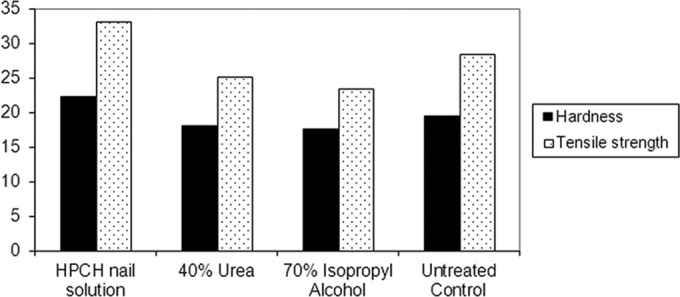
Comparison of the effects of HPCH-based nail solution and comparators on the hardness and tensile strength of hoof samples (in Vickers pyramid number [HV] and megapascals [MPa], respectively). *, P < 0.001 for HPCH-based nail solution versus comparators for hardness and tensile strength.
Tensile strength.
As reported in Fig. 2, the application of the HPCH-based nail solution increased the tensile strength of the hoof samples over that of the untreated control (mean, 33.07 ± 5.46 versus 28.42 ± 5.39 MPa, respectively; P < 0.05). In contrast, the application of urea or isopropyl alcohol rendered the samples more fragile (mean, 25.03 ± 4.02 and 23.41 ± 7.48 MPa, respectively). The effect of the HPCH-based test product also was significant versus that of urea and isopropyl alcohol (P < 0.001 each).
FIG 2.
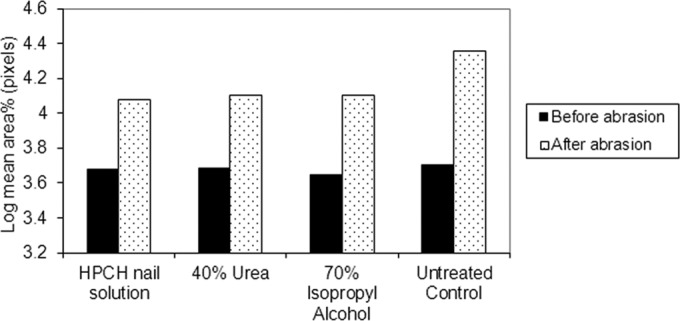
Comparison of the effects of HPCH-based nail solution and comparators on the crumbling index following sample abrasion in pixels.
Flexural strength.
The results obtained on the flexural strength suggest that the application of the HPCH-based nail solution appeared to increase the resistance of the hoof samples to flexing or bending over that of the untreated control (mean, 183.79 ± 44.75 versus 181.20 ± 49.71 MPa, respectively). In contrast, urea and isopropyl alcohol made the samples more easily flexed (178.31 ± 61.89 and 178.97 ± 35.71 MPa, respectively), although there were no significant differences between the treatments.
Ultrastructure.
A very high index of crumbling, measured for the HPCH-based nail solution as the difference in the areas of gray shading on SEM images taken before and after sample abrasion, was recorded in the untreated hooves. In contrast, the application of the HPCH-based nail solution resulted in significantly smaller shaded areas in the images captured before and after abrasion than in those of the control (P < 0.05 and P < 0.001, respectively), indicating that the HPCH-based nail solution rendered the samples less brittle. Similarly, urea and isopropyl alcohol significantly reduced the index of crumbling compared to that of the untreated control after abrasion (P < 0.001).
Dermatophyte penetration.
Figure 3 shows the fungal burden (CFU) per gram of hooves pretreated with either the HPCH-based nail solution or isopropyl alcohol or urea and infected with either T. mentagrophytes or T. rubrum. Also, the fungal burden of dermatophytes was consistently lower in the hooves treated with the HPCH-based nail solution than in the untreated control. Additionally, the fungal burden of the hooves treated with the HPCH-based nail solution was generally lower than that of the hooves pretreated with urea or isopropyl alcohol at every time point.
FIG 3.
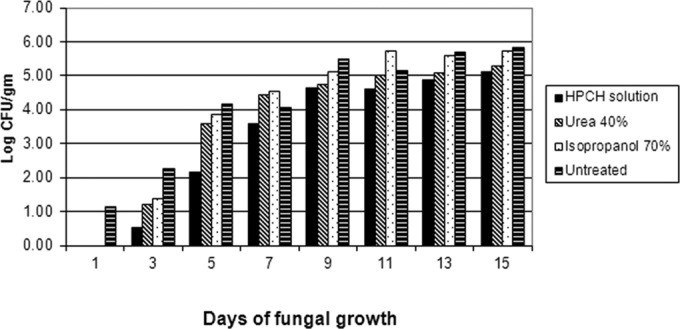
Fungal burdens of hooves pretreated with HPCH-based nail solution, urea, or isopropanol and the untreated control after exposure to dermatophytes.
Further, there was a notable delay in the proliferation of T. rubrum growth on the HPCH-based nail solution-treated hooves. There were no colonies of T. rubrum isolated from the HPCH-based nail solution-treated hooves evaluated at days 3 and 5, but there was a 3.30-log CFU/g colony count in the untreated hooves at day 5.
As shown in Table 1, the depth of fungal penetration by the two dermatophyte species was much lower in the HPCH-based nail solution-treated hooves than that achieved in the untreated control or in the hooves treated with urea or isopropyl alcohol. In fact, the penetration depth of the HPCH-based nail solution-treated hooves by T. mentagrophytes was <25 μm at day 9 compared to 275 μm in the untreated control. The penetration depth in the HPCH-based nail solution-treated hooves by T. rubrum at day 5 was <25 μm compared to 125 μm in the untreated control.
TABLE 1.
Tissue penetration of hooves inoculated with dermatophytes
| Days of fungal growth | Tissue penetration (μm) ofa: |
|||||||
|---|---|---|---|---|---|---|---|---|
| HPCH-based nail solution |
40% urea |
70% isopropyl alcohol |
Untreated control |
|||||
| TM | TR | TM | TR | TM | TR | TM | TR | |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | <25 | 0 |
| 3 | 25 | 0 | 100 | 0 | 50 | 0 | 50 | 0 |
| 5 | <25 | <25 | 175 | 150 | 175 | 150 | 150 | 125 |
| 7 | <25 | 75 | 225 | 175 | 250 | 200 | 100 | 250 |
| 9 | <25 | 100 | 175 | 200 | NSb | 200 | 275 | 225 |
| 11 | 150 | 100 | 150 | 200 | 200 | 200 | NS | 150 |
| 13 | 175 | 125 | 250 | NS | 275 | 200 | 300 | NS |
| 15 | 225 | 100 | 225 | 225 | 300 | 225 | 300 | 150 |
TM, T. mentagrophytes; TR, T. rubrum.
NS, no sample; damaged during processing.
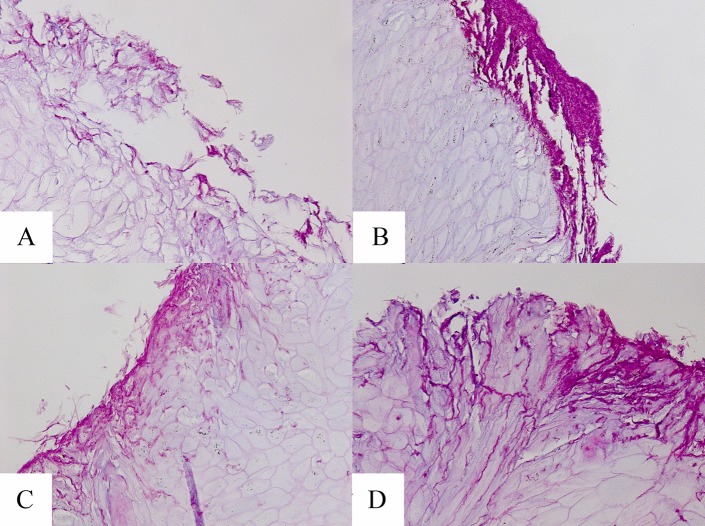
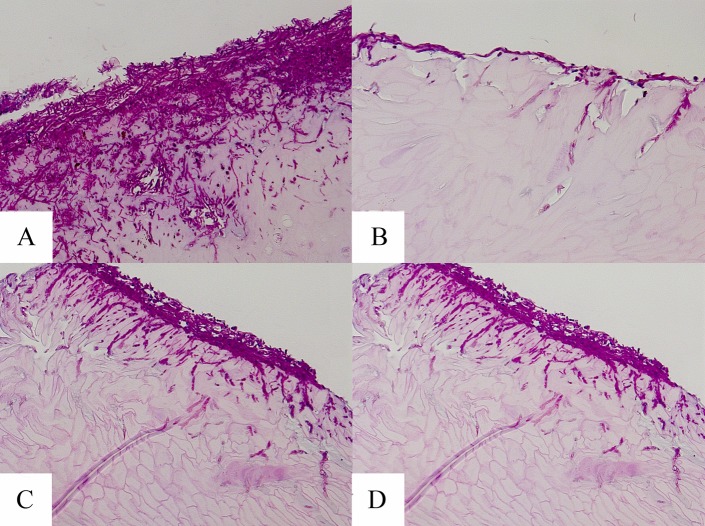
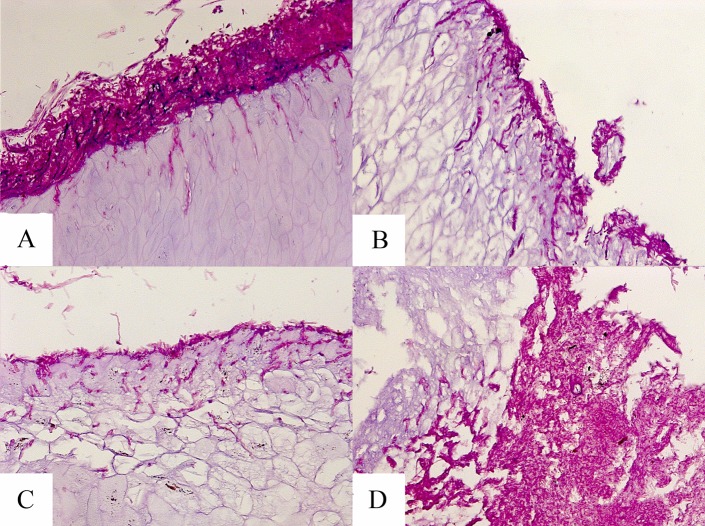
Examples of PAS-stained hoof slices inoculated with T. mentagrophytes and T. rubrum at day 7 are shown in Fig. 4 and 5, respectively. As shown in these figures, the hooves treated with the HPCH-based nail solution demonstrated superficial fungal elements with limited hoof penetration, in contrast to the hooves treated with isopropyl alcohol or urea or the untreated control. Moreover, images taken following inoculation with either dermatophyte strain showed marked degradation of the hooves treated with isopropyl alcohol or urea by day 15, as demonstrated in Fig. 6.
FIG 4.
Microscopy of hooves at day 7 postexposure to T. mentagrophytes (PAS stain, ×20 magnification). (A) Untreated control; (B) HPCH-based nail solution; (C) isopropyl alcohol; (D) and urea.
FIG 5.
Microscopy of hooves at day 7 postexposure to T. rubrum (PAS stain, ×20 magnification). (A) Untreated control; (B) HPCH-based nail solution; (C) isopropyl alcohol; (D) and urea.
FIG 6.
Microscopy of hooves at day 15 postexposure to T. rubrum (PAS stain, ×20 magnification). (A) Untreated control; (B) HPCH-based nail solution; (C) isopropyl alcohol; (D) and urea.
DISCUSSION
The population at risk for developing fungal nail infection includes those with nail dystrophy, brittle or psoriatic nails, and mechanical or chemical nail injury. These various nail conditions highlight the importance of nail integrity in preventing the ability of dermatophytes and nondermatophyte nail pathogens from establishing an infection. Although it is commonly known that aggressive chemicals, normally used for cosmetic or therapeutic purposes, can result in weakening the nails, to our knowledge this is the first time that it was experimentally demonstrated. The use of isopropyl alcohol to remove previously applied solution layers of some nail formulations or the use of urea as a nail pretreatment to improve the penetration of antifungals is not supported by our data. In fact, adverse changes in the physical characteristics of the hoof translated into a higher susceptibility to fungal nail permeation. This may account for the treatment failure of topical products for onychomycosis, which contain penetration enhancers (6). On the contrary, strengthening the nail may improve the natural barrier to infection, as shown by our investigation with an HPCH-based nail solution.
The composite results of this study indicate that the application of the HPCH-based nail solution may improve nail barrier characteristics, as demonstrated by the enhanced hoof hardness and tensile strength and by protection against nail abrasion. Taken together, these findings may translate into a more effective barrier against fungal nail infection. In conclusion, the results obtained in this study suggest that repeated application of the HPCH-based nail solution may reinforce the nail structure and thus prevent fungal nail colonization and infection.
ACKNOWLEDGMENT
This work was supported by Polichem S.A., Lugano, Switzerland.
REFERENCES
- 1.Scher RK, Baran R. 2003. Onychomycosis in clinical practice: factors contributing to recurrence. Br J Dermatol 149(Suppl 65):S5–S9. [DOI] [PubMed] [Google Scholar]
- 2.Haneke E. 1991. Fungal infections of the nail. Semin Dermatol 10:41–53. [PubMed] [Google Scholar]
- 3.Jesudanam TM, Rao GR, Laksmi DJ, Kumari GR. 2002. Onychomycosis: a significant medical problem. Indian J Dermatol Venerol Leprol 68:326–329. [PubMed] [Google Scholar]
- 4.Boonchai W, Kulthanan K, Maungprasat C, Suthipinittham P. 2003. Clinical characteristics and mycology of onychomycosis in autoimmune patients. J Med Assoc Thai 86:995–1000. [PubMed] [Google Scholar]
- 5.Ghannoum MA, Hajjeh RA, Scher R, Konnikov N, Gupta AK, Summerbell R, Sullivan S, Daniel R, Krusinski P, Fleckman P, Rich P, Odom R, Aly R, Pariser D, Zaiac M, Rebell G, Lesher J, Gerlach B, Ponce-de-Leon GF, Ghannoum A, Warner J, Isham N, Elewski B. 2000. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal. J Am Acad Dermatol 43:641–648. doi: 10.1067/mjd.2000.107754. [DOI] [PubMed] [Google Scholar]
- 6.Elewski BE, Rich P, Tosti A, Pariser DM, Scher R, Daniel RC, Gupta AK. 2013. Onychomycosis: an overview. J Drugs Dermatol 12:s96–s103. [PubMed] [Google Scholar]
- 7.Tasic S, Stojanovic S, Poljacki M. 2001. Etiopathogenesis, clinical picture and diagnosis of onychomycosis. Med Pregl 54:45–51. (In Croatian.) [PubMed] [Google Scholar]
- 8.Cantoresi F, Sorgi P, Arcese A, Bidoli A, Bruni F, Carnevale C, Calvieri S. 2009. Improvement of psoriatic onychodystrophy by a water-soluble nail solution. J Eur Acad Dermatol Venereol 23:832–834. doi: 10.1111/j.1468-3083.2009.03209.x. [DOI] [PubMed] [Google Scholar]
- 9.Cantoresi F, Caserini M, Bidoli A, Maggio F, Marino R, Carnevale C, Sorgi P, Palmieri R. 2014. Randomized controlled trial of a water-soluble nail solution based on hydroxypropyl-chitosan (HPCH), in the management of nail psoriasis. Clin Cosmet Investig Dermatol 7:185–190. doi: 10.2147/CCID.S61659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monti D, Saccomani L, Chetoni P, Burgalassi S, Tampucci S, Mailland F. 2011. Validation of bovine hoof slices as a model for infected human toenails: in vitro ciclopirox transungual permeation. Br J Dermatol 165:99–105. doi: 10.1111/j.1365-2133.2011.10303.x. [DOI] [PubMed] [Google Scholar]
- 11.Smith RL, Sandland GE. 1922. An accurate method of determining the hardness of metals, with particular reference to those of a high degree of hardness. Proc Inst Mech Eng 102:623–641. doi: 10.1243/PIME_PROC_1922_102_033_02. [DOI] [Google Scholar]
- 12.Czichos H. 2006. Springer handbook of materials measurement methods, p 303–304. Springer, Berlin, Germany. [Google Scholar]
- 13.Hodgkinson JM. 2000. Mechanical testing of advanced fibre composites, p 132–133. Woodhead Publishing, Ltd., Cambridge, United Kingdom. [Google Scholar]