Abstract
Mycobacterium tuberculosis, the causative agent of tuberculosis, is protected from toxic solutes by an effective outer membrane permeability barrier. Recently, we showed that the outer membrane channel protein CpnT is required for efficient nutrient uptake by M. tuberculosis and Mycobacterium bovis BCG. In this study, we found that the cpnT mutant of M. bovis BCG is more resistant than the wild type to a large number of drugs and antibiotics, including rifampin, ethambutol, clarithromycin, tetracycline, and ampicillin, by 8- to 32-fold. Furthermore, the cpnT mutant of M. bovis BCG was 100-fold more resistant to nitric oxide, a major bactericidal agent required to control M. tuberculosis infections in mice. Thus, CpnT constitutes the first outer membrane susceptibility factor in slow-growing mycobacteria. The dual functions of CpnT in uptake of nutrients and mediating susceptibility to toxic molecules are reflected in macrophage infection experiments: while loss of CpnT was detrimental for M. bovis BCG in macrophages that enable bacterial replication, presumably due to inadequate nutrient uptake, it conferred a survival advantage in macrophages that mount a strong bactericidal response. Importantly, the cpnT gene showed a significantly higher density of nonsynonymous mutations in drug-resistant clinical M. tuberculosis strains, indicating that CpnT is under selective pressure in human tuberculosis and/or during chemotherapy. Our results indicate that the CpnT channel constitutes an outer membrane gateway controlling the influx of nutrients and toxic molecules into slow-growing mycobacteria. This study revealed that reducing protein-mediated outer membrane permeability might constitute a new drug resistance mechanism in slow-growing mycobacteria.
INTRODUCTION
Almost 10 million new cases of tuberculosis (TB) are reported each year (1). The worldwide spread of multidrug-resistant (MDR) and the emergence of extensively (XDR) and totally (TDR) drug-resistant strains of Mycobacterium tuberculosis (2) have made TB an almost untreatable disease with prohibitive costs for health care systems in developing countries (3). Unfortunately, our understanding of the genetic basis of drug resistance in M. tuberculosis is limited to a few genes of known functions (4, 5), and a large number of new genes of mostly unknown functions are associated with drug resistance, as revealed by genomic sequencing of MDR and XDR M. tuberculosis strains (6). In order to design more effective drugs, we need a better understanding of the molecular basis of mycobacterial growth in vivo and the mechanisms by which M. tuberculosis becomes tolerant and resistant to drugs currently used in TB chemotherapy (7).
Major mechanisms that contribute more or less unspecifically to the intrinsic drug resistance of M. tuberculosis are the very low outer membrane permeability toward the vast majority of small molecules (8, 9) and the manifold drug efflux pumps (10, 11). However, both uptake and efflux processes across the mycobacterial cell envelope are poorly understood transport processes (12). In addition, these intrinsic drug resistance mechanisms render most in vitro screening approaches ineffective and hamper the discovery of new TB drugs (13).
The mycobacterial cell wall contains an outer membrane of low permeability (14–16). Outer membrane integrity is required for protection of M. tuberculosis from toxic compounds (8) and is essential for virulence and pathogenicity of M. tuberculosis (17). Additionally, nutrient molecules must be able to permeate the outer membrane to sustain M. tuberculosis viability and replication. Uptake of small, hydrophilic solutes across the outer membrane of Gram-negative bacteria is mediated by water-filled protein channels called porins (18). Influx of solutes through pore proteins is driven by a concentration gradient and primarily depends on the size, charge, hydrophobicity, and shape of the solute (19). Many drugs and toxic solutes exploit the porins to enter bacterial cells (20). It has been shown that channel-forming proteins exist in slow-growing mycobacteria such as M. tuberculosis, but their low channel activity prevented their identification (21, 22).
MspA is the best characterized outer membrane channel protein of mycobacteria. However, it is present only in fast-growing species, such as Mycobacterium smegmatis (23). MspA is, to date, the only mycobacterial protein whose β-barrel structure is consistent with that of an integral outer membrane protein (24), and is widely used as an outer membrane marker protein in mycobacteria (25–27). MspA is the major porin of M. smegmatis (28) and mediates diffusion of small, hydrophilic solutes across the outer membrane both in M. smegmatis (29) and upon heterologous overexpression in M. tuberculosis (30).
In this study, we examined the role of the recently discovered outer membrane protein CpnT in drug susceptibility of M. tuberculosis (31). CpnT consists of two domains. The oligomeric N-terminal domain (NTD) forms an outer membrane channel and is required for efficient glycerol uptake. The toxic C-terminal domain (CTD) is cleaved, secreted, and induces necrosis of eukaryotic cells by a currently unknown mechanism. Thus, CpnT promotes replication of M. tuberculosis in macrophages, probably by increasing nutrient uptake, and escape from the macrophage by inducing a necrosis-like cell death through the secreted toxin (31). The domain organization of CpnT and its function in secreting its own C-terminal domain resemble those of autotransporters in Gram-negative bacteria (32). It was proposed that the N-terminal domain remains in the outer membrane as an open channel after cleavage and secretion of the C-terminal toxin. Such a functional role of the β-domain of autotransporters in nutrient uptake has not been observed in Gram-negative bacteria, most likely because their outer membranes contain up to 1,000-fold more porins than M. tuberculosis (30).
While the function of the CpnT pore domain in glycerol uptake has been established (31), the involvement of this protein in other transport processes across the outer membrane of M. tuberculosis has not been examined yet. In this study, we showed that CpnT confers susceptibility of slow-growing mycobacteria to structurally different antibiotics and TB drugs and to toxic immune factors, such as nitric oxide (NO). Furthermore, genome sequencing studies of drug-resistant clinical isolates indicate that CpnT mutations may constitute a novel molecular drug resistance mechanism of M. tuberculosis.
MATERIALS AND METHODS
Chemicals and enzymes.
Hygromycin B (Hyg) was purchased from Calbiochem. All other chemicals were purchased from Merck or Sigma at the highest purity available.
Bacterial strains, media, and growth conditions.
Mycobacterium bovis BCG strains (Strain Institute Pasteur) were grown in Middlebrook 7H9 medium (Difco) supplemented with 0.2% glycerol, 0.02% tyloxapol, and 10% oleic acid-albumin-dextrose-catalase (OADC) (Remel) or on Middlebrook 7H10 plates supplemented with 0.5% glycerol and 10% OADC (Remel). Antibiotics were used when required at the following concentrations: hygromycin, 50 mg/liter; and kanamycin, 30 mg/liter. The M. bovis BCG strains and plasmids used in this work are described in reference 31. Briefly, ML383 is a wild-type (wt) derivative of M. bovis BCG with the L5 integrated empty vector pML2008 containing a hygromycin resistance marker, ML1012 is a transposon mutant of M. bovis BCG disrupted with an insertion in cpnT, ML386 is an ML1012 derivative containing pML2008, ML387 is an ML1012 derivative complemented with mspA, ML388 is an ML1012 derivative complemented with cpnT, and ML805 is an ML1012 derivative complemented with cpnTNTD, which encodes the channel-forming N-terminal domain (NTD) of CpnT.
Antibiotic susceptibility measurements.
The MIC was determined using a microplate alamarBlue assay (33) with modifications (34, 35). The final drug concentrations were as follows: isoniazid and ethambutol, 0.03125 to 1 mg/liter; streptomycin and rifampin, 0.04 to 1.28 mg/liter; levofloxacin, ofloxacin, and clarithromycin, 0.0625 to 2 mg/liter; p-aminosalicylic acid (PAS) and moxifloxacin, 0.25 to 8 mg/liter; ciprofloxacin and norfloxacin, 0.5 to 16 mg/liter; chloramphenicol and cycloserine, 1 to 32 mg/liter; vancomycin, 2.5 to 80 mg/liter; erythromycin A and tetracycline, 8 to 256 mg/liter; and ampicillin, 30 to 960 mg/liter. The alamarBlue assays were performed twice in triplicate. The nitrate reductase assay was performed to determine MICs of M. tuberculosis as previously described (36).
Susceptibility to nitric oxide.
M. bovis BCG strains were grown in Middlebrook 7H9-OADC medium containing 0.01% tyloxapol to an optical density at 600 nm (OD600) of 2. Cultures were pelleted by centrifugation, washed, and resuspended in 1/10 of the original volume. Aliquots (0.1 ml) of suspensions were added to 0.9 ml of fresh medium containing 0, 5, 25, and 100 mM sodium nitroferricyanide(III) dehydrate (SNP [Sigma]). Each suspension was incubated at 37°C (200 rpm) for 0, 1, 3, or 7 days. Serial dilutions of the cultures were plated on Middlebrook 7H10-OADC-Hyg plates and incubated at 37°C for 3 weeks, after which colony-forming units (CFU) were counted. The data are displayed as the percentage of survival: (no. of CFU exposed at day x/no. of CFU unexposed at day x) × 100%. Each strain was tested in triplicate; the assay was repeated three times independently.
Macrophage infection experiments.
The human acute monocytotic leukemia cell line THP-1 (ATCC TIB202) and the mouse macrophage cell line J774A.1 (ATTC TIB-67) were maintained as described previously (37). Differentiation of THP-1 monocytes into macrophages was induced overnight with 50 nM phorbol 12-myristate 13-acetate (PMA). THP-1 cells were infected at a multiplicity of infection (MOI) of 5. In each experiment, 3 h after infection, cells were washed three times with phosphate-buffered saline (PBS) to remove noninternalized bacteria. At different time points after infection (3 h and 1, 3, 5, and 7 days), cells were washed with PBS and lysed with 1% IGEPAL (Sigma) solution in water. Serial dilutions were done in water and plated on Middlebrook 7H10-OADC plates. CFU were counted after 2 weeks of incubation at 37°C. When required, macrophages were treated with murine gamma interferon (IFN-γ) (100 IU) overnight before infection.
Statistical analysis.
Data are presented as the mean ± standard deviation (SD). P values were calculated using the Student t test, and a P value of <0.05 was considered to be significant.
RESULTS
Absence of CpnT confers high-level drug resistance to M. bovis BCG.
The permeability barrier of the outer membrane is a major drug resistance determinant in slow-growing mycobacteria (30). Since lack of porins in Gram-negative bacteria and M. smegmatis confers low-level resistance to some small, hydrophilic drugs, in particular β-lactam antibiotics (35, 38, 39), we hypothesized that channel proteins might be important for uptake of drugs through the otherwise impermeable outer membrane of M. tuberculosis. Thus far, such proteins are unknown. However, the porin MspA of M. smegmatis increases drug susceptibility when expressed in slow-growing mycobacteria (30). As CpnT is required for efficient transport of glycerol in slow-growing mycobacteria (31) we thought to test its role for drug resistance. To this end, we determined the drug susceptibility of an M. bovis BCG cpnT::Tn mutant strain using the microplate alamarBlue assay. Resistance to small, hydrophilic antibiotics (Table 1) was drastically increased in the absence of cpnT by up to 32-fold. Resistance to the antituberculosis drugs ethambutol, isoniazid, and p-aminosalicylic acid (PAS) was increased more than 4-fold. Expression of mspA or full-length cpnT fully restored the susceptibility of the cpnT::Tn mutant (Table 1), providing evidence that insertion of a pore protein in the outer membrane is sufficient to mediate susceptibility to these antibiotics, presumably by increasing drug uptake. Remarkably, MICs to large and hydrophobic antibiotics, such as erythromycin and rifampin, were also increased in the cpnT::Tn mutant by at least 16-fold (Table 1). The lack of CpnT also made M. bovis BCG more resistant to large hydrophilic antibiotics, such as streptomycin, and to several fluoroquinolones (Table 1). Importantly, expression of both mspA and cpnT restored the susceptibility of the cpnT::Tn strain to wt levels. These results indicate that the outer membrane protein CpnT is an important drug susceptibility factor of M. bovis BCG.
TABLE 1.
Antibiotic resistance of the cpnT mutants of M. bovis BCG
| Antibiotic by classa | MIC for M. bovis BCG (μg/ml)b |
Resistance factorc | |||
|---|---|---|---|---|---|
| wt | Mutation/complementation |
||||
| cpnT::Tn | cpnT::Tn/mspA | cpnT::Tn/cpnT | |||
| A | |||||
| Ampicillin | 30 | 960 | 60 | 60 | 32 |
| Tetracycline | 31.25 | >250 | 62.5 | 62.5 | >8 |
| Chloramphenicol | 4 | 64 | 8 | 8 | 16 |
| Ethambutol | 0.125 | >1 | 0.125 | 0.125 | >8 |
| Isoniazid | 0.25 | >1 | 0.25 | ND | >4 |
| p-Aminosalicylic acid | 2 | >8 | 2 | 2 | >4 |
| Cycloserine | 4 | 8 | 4 | 4 | 2 |
| B | |||||
| Vancomycin | 5 | 20 | 10 | 10 | 4 |
| Streptomycin | 0.16 | 0.64 | 0.32 | 0.32 | 4 |
| C | |||||
| Clarithromycin | 0.125 | >2 | 0.5 | 0.25 | >16 |
| Erythromycin A | 16 | >256 | 32 | 16 | >16 |
| Rifampin | 0.008 | >0.128 | 0.032 | 0.032 | 16 |
| D | |||||
| Ciprofloxacin | 1 | 2 | 2 | 2 | 2 |
| Ofloxacin | 0.25 | 0.5 | 0.25 | 0.25 | 2 |
| Levofloxacin | 0.125 | 0.5 | 0.125 | 0.125 | 4 |
| Moxifloxacin | 0.5 | 1 | 0.5 | 0.5 | 2 |
| Norfloxacin | 1 | 4 | 2 | 2 | 4 |
TB drugs and antibiotics were grouped into four classes: A, small, hydrophilic antibiotics (102 to 444 g/mol), B large, hydrophilic antibiotics (484 to 1,450 g/mol); C, large, hydrophobic antibiotics (612 to 823 g/mol); and D, fluoroquinolones (319 to 402 g/mol).
The MICs for the wt, mutant (cpnT::Tn), and complemented (cpnT::Tn/mspA or cpnT::Tn/cpnT) strains were measured using the microplate alamarBlue assay as described previously 34. ND, not determined.
The resistance factor is defined as the ratio of the MIC of the cpnT::Tn mutant to the MIC of the wt strain.
Considering the important role of CpnT in drug susceptibility of M. bovis BCG, we determined the MICs of wt M. tuberculosis, the unmarked cpnT mutant, and the mutant complemented with a full-length or an N-terminal pore domain of CpnT (31). We did not detect a significant difference in drug susceptibility of these strains (not shown).
CpnT is required for susceptibility of M. bovis BCG to nitric oxide.
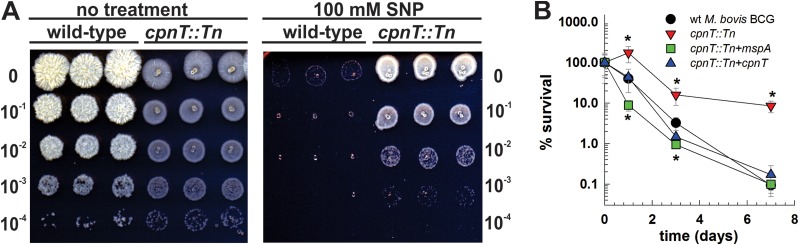
Next, we examined whether the CpnT channel plays a role in susceptibility of M. bovis BCG to other, physiologically more relevant toxic compounds. Nitric oxide (NO) plays a crucial role in control of M. tuberculosis infections in mice (40). Furthermore, previously it was shown that porins mediate susceptibility of M. smegmatis to NO and that aquaporin-1 is required for NO permeability in eukaryotic cells (41, 42), suggesting that CpnT of M. bovis BCG might have a similar function. To this end, wt M. bovis BCG, the cpnT::Tn mutant, and the mutant complemented with either cpnT or mspA were exposed to NO, and serial dilutions were applied as spots to agar plates (Fig. 1A). All strains were killed by NO in a time-dependent manner (Fig. 1B). A rapid decrease in viability of the wt and both complemented cpnT mutant strains was observed after 24 h of exposure, with the majority of bacteria being killed after 3 days. In contrast, the cpnT::Tn mutant grew during the first 24 h and was approximately 100-fold more resistant to killing by NO at later time points than the wt M. bovis BCG strain and the complemented mutant strains (Fig. 1). Thus, CpnT might play an important role in susceptibility of slow-growing mycobacteria toward toxic molecules produced by host immune cells. The observation that the susceptibility of the cpnT::Tn mutant to NO is restored by expression of mspA indicates that the channel function and/or outer membrane integration of CpnT and other pore proteins mediates NO susceptibility by increasing the permeability of the outer membrane to this molecule. Furthermore, overexpression of mspA in the cpnT::Tn mutant resulted in almost complete clearance of the strain after 24 h of treatment (8.9% ± 2.5% survival for the mspA-expressing strain versus 173% ± 75% for the cpnT::Tn strain), thus suggesting that MspA-mediated uptake of toxic compounds reduced survival of the strain under adverse conditions.
FIG 1.
Absence of CpnT in M. bovis BCG renders high-level resistance to nitric oxide. (A) Survival of wt M. bovis BCG and the cpnT::Tn mutant after 7 days of exposure to 100 mM sodium nitroferricyanide(III) dehydrate (SNP). Serial dilutions were spotted on Middlebrook 7H10-OADC agar. (B) Survival of wt M. bovis BCG (circles), cpnT::Tn mutant (inverted triangles), and the cpnT::Tn mutant complemented with mspA (squares) or cpnT (triangles) after exposure to 100 mM SNP for different times. The CFU of the original inoculum of each strain were set as 100% survival. The experiment was done in triplicate and repeated three times. Survival of the wt versus mutant or complemented strains was analyzed using Student's t test. *, P < 0.05 was deemed significant.
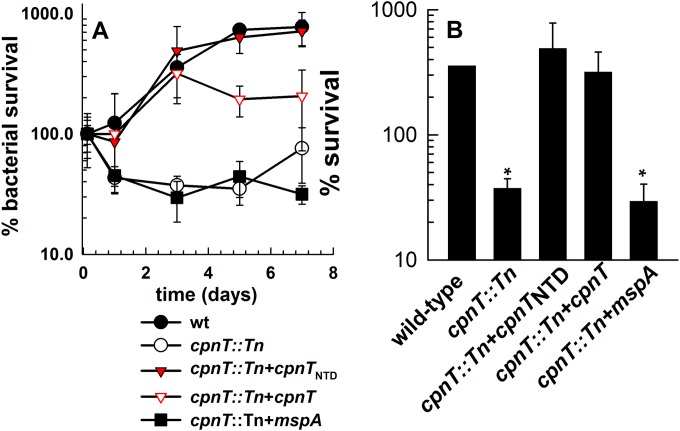
The channel-forming N-terminal domain of CpnT is required for growth and survival of M. bovis BCG in macrophages.
Previously, we observed that both the channel and the toxin domain of CpnT contribute to the survival of M. tuberculosis in macrophages (31). This made it difficult to determine the individual roles of these CpnT domains during infection of host cells. M. bovis BCG does not permeabilize phagosomes and does not escape from phagosomes, in contrast to M. tuberculosis (43, 44). Thus, the C-terminal toxin domain of CpnT is capable of leaving the phagosome during M. tuberculosis infection to induce necrosis in cytosol, while it is likely trapped in phagosomes during M. bovis BCG infection, where it is incapable of inducing necrosis. This assumption is supported by the virtual absence of cytotoxicity of M. bovis BCG or M. tuberculosis mutants that lack the RD1 region, a primary virulence locus of M. tuberculosis that is also missing in M. bovis BCG (45, 46). Hence, we sought to exploit this feature of M. bovis BCG to examine the role of the CpnT channel domain in the absence of confounding effects of the toxin domain. Here we show that the absence of cpnT drastically reduced intracellular growth of M. bovis BCG in the undifferentiated human monocytic cell line THP-1 (Fig. 2). This is consistent with the results obtained for M. tuberculosis-infected THP-1 macrophages (31) and underlines the important role of CpnT for survival of slow-growing mycobacteria in macrophages. Growth of M. bovis BCG was fully restored by expression of cpnTNTD encoding the channel-forming N-terminal domain (NTD) of CpnT. This observation confirmed that the toxin domain is indeed dispensable for normal growth of M. bovis BCG in macrophages and that only the pore domain is needed for uptake of solutes and growth. In contrast, CpnTNTD is only capable of restoring wt growth of M. tuberculosis in THP-1 macrophages during the first 24 h, while the toxic CpnTCTD protein is required for normal growth of M. tuberculosis at later time points (31).
FIG 2.
Survival of M. bovis BCG in human macrophages. (A) THP-1 cells were infected with wt M. bovis BCG (filled circles), the cpnT::Tn mutant (open circles), or the cpnT::Tn mutant complemented with cpnTNTD (filled triangles), cpnT (open triangles), or mspA (squares) at an MOI of 5. The CFU of the original inoculum of each strain was set as 100% survival. (B) Bar graph representing survival of M. bovis BCG in THP-1 cells 3 days after infection. The CFU of the original inoculum of each strain was defined as 100% survival. Survival of the wt versus mutant or complemented strains was analyzed using Student's t test. *, P < 0.05 was deemed significant.
Interestingly, expression of mspA did not rescue the growth defect of the cpnT::Tn mutant even in the growth-permissive THP-1 cells (Fig. 2), although full complementation of all phenotypes was observed in vitro (Table 1 and Fig. 1). These results indicate that the CpnT channel mediates sufficient nutrient influx but provides better protection against toxic host immune factors than the wide open MspA channel when expressed in slow-growing mycobacteria (24).
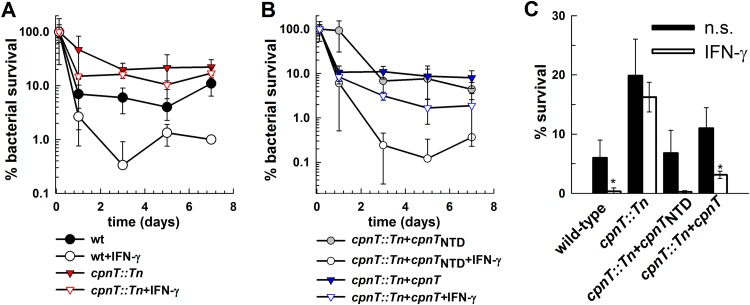
The absence of CpnT protects M. bovis BCG from killing in macrophages and from the bactericidal response of stimulated macrophages.
We showed that CpnT facilitates uptake of nutrients (31), but it also enables toxic molecules to enter the bacterial cell (Fig. 1 and Table 1). Hence, we wondered which of these properties of CpnT would prevail in vivo when the uptake of nutrients for bacterial replication might be less important than protection from toxic compounds, such as NO, which utilize the CpnT channel to enter mycobacterial cells. To this end, we used J774 mouse macrophages in which M. bovis BCG does not replicate and is efficiently eliminated (37). We hypothesized that the requirement for uptake of nutrients through porins under these conditions is minimal, while resistance to toxic compounds produced by macrophages plays a crucial role in clearance of bacteria. As expected, the bacterial burden for all strains decreased during infection of J774 cells (Fig. 3). However, the cpnT::Tn strain was more resistant to killing than the wt or complemented strains. Furthermore, stimulation of J774 with IFN-γ, a potent inducer of nitric oxide and other reactive nitrogen intermediates, reduced survival of wt M. bovis BCG but did not affect the cpnT::Tn strain (Fig. 3). These experiments suggest that the inertness of the cpnT::Tn mutant to the bactericidal response of macrophages upon IFN-γ stimulation is due to the mutant's high level of resistance to NO, a potent effector molecule of mouse macrophages (40), and possibly other toxic compounds produced by macrophages.
FIG 3.
Survival of M. bovis BCG in mouse macrophages. J774 cells were infected with wt M. bovis BCG (circles), the cpnT::Tn mutant (inverted triangles) (A), and the cpnT::Tn mutant complemented with cpnTNTD (squares) or cpnT (triangles) (B) at an MOI of 5. J774 cells treated with murine IFN-γ (100 IU) overnight before infection are marked with open symbols. The CFU of the original inoculum of each strain was set as 100% survival. (C) Bar graph representing survival of M. bovis BCG in J774 cells 3 days postinfection. When required, J774 cells were treated with murine IFN-γ (100 IU) overnight before infection (open bars). n.s., nonstimulated J774 cells (solid bars). The CFU of the original inoculum of each strain was defined as 100% survival. Analysis of variance (ANOVA) with a one-parameter test was performed, with post hoc analysis done using the Holm-Sidak test. *, P < 0.05 was deemed significant.
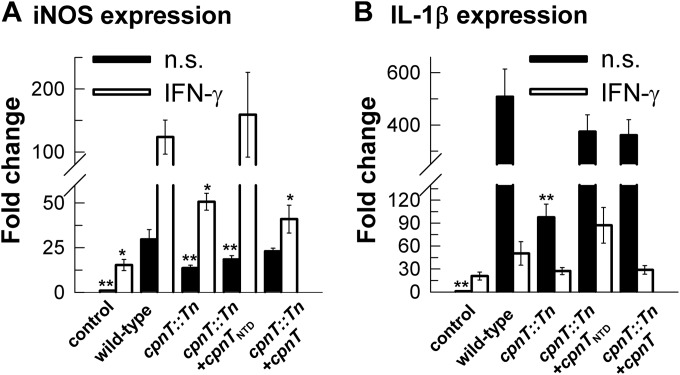
The absence of CpnT reduces the proinflammatory response of macrophages infected with M. bovis BCG.
In some cases, porins are highly antigenic and are recognized by the host immune system (47, 48). Hence, porins can induce inflammation in mice, activation of B cells, and release of cytokines (49, 50). Thus, an alternative explanation for the increased resistance of the cpnT mutant to killing by macrophages might be a lower proinflammatory response of macrophages that could result in reduced bacterial killing (51). To test this hypothesis, we analyzed the expression of the proinflammatory interleukin 1β (IL-1β) gene and of the gene encoding the inducible isoform of nitric oxide synthase (iNOS) during infection of J774 mouse macrophages with M. bovis BCG. J774 mouse macrophages were chosen because survival experiments indicated that the intracellular environment in J774 is less permissive for M. bovis BCG growth than human THP-1 macrophages (Fig. 2 and 3). Thus, a stronger proinflammatory response was expected for mouse macrophages. We focused on IL-1β and iNOS as key markers of M. tuberculosis immune responses due to their essential roles for pulmonary infection with this pathogen. Specifically, M. tuberculosis infection results in robust initial production of IL-1β in a mouse model of infection. This is followed by the induction of adaptive immune responses, including production of iNOS, which generates nitric oxide, a molecule found to be essential not only for antibacterial control, but also for modulation of inflammatory immune responses through alteration of the NLRP3 inflammasome (52). We observed 2- and 5-fold-lower iNOS and IL-1β mRNA levels, respectively, after infection of macrophages with the cpnT::Tn mutant compared to the wt strain (Fig. 4). This difference was independent of the presence of IFN-γ. Expression of IL-1β and iNOS was partially restored in the cpnT mutant complemented by the full-length cpnT gene and was fully complemented by the cpnTNTD construct. Thus, the lower levels of proinflammatory response of macrophages in response to infection with the cpnT::Tn mutant are likely a contributing factor to its ability to survive better in macrophages with a strong bactericidal response. Taken together, these results suggest that the absence of CpnT confers a survival advantage in the presence of a strong bactericidal response of macrophages to mycobacterial infection, while the nutrient uptake function of CpnT appears to dominate in macrophages under conditions that enable bacterial replication.
FIG 4.
Proinflammatory gene expression is reduced in the absence of CpnT. qRT-PCR quantification of iNOS (A) and IL-β (B) gene expression in J774 macrophages 24 h after infection with M. bovis BCG strains. Data are represented as the mean ± standard deviation (SD) relative to uninfected cells (control). When required, J774 cells were treated with murine IFN-γ (100 IU) overnight before infection (open bars). n.s., nonstimulated J774 cells (solid bars). ANOVA with a one-parameter test was performed, with post hoc analysis done using Holm-Sidak test. *, P < 0.01; **, P < 0.001.
DISCUSSION
Role of CpnT in drug susceptibility of M. bovis BCG and M. tuberculosis.
The outer membrane permeability barrier is of utmost importance for survival of M. tuberculosis under harsh conditions in vivo (8, 17) and is a key component of its intrinsic drug resistance (53). In this study, we showed that the cpnT mutant of M. bovis BCG exhibited high-level resistance to a wide range of antibiotics and TB drugs. The resistance factors for most of these compounds were much higher than those previously observed for a single porin mutant of M. smegmatis (39) or for Gram-negative bacteria (38). In particular, the cpnT mutant of M. bovis BCG became more resistant to the TB drugs isoniazid and ethambutol, while their MICs were not affected by the deletion of the main porin gene mspA of M. smegmatis (39). This difference might be explained by the larger number of porins in the outer membrane of M. smegmatis compared to M. bovis BCG. While the number of porins is reduced from 2,400 to 800 by the deletion of mspA in M. smegmatis (29), no open pores were detected by electron microscopy in M. bovis BCG and M. tuberculosis (30). Thus, the loss of CpnT probably has a much larger effect on permeability of M. bovis BCG than the loss of the porin MspA in M. smegmatis. The effect of a single porin deletion in Gram-negative bacteria on drug resistance is even less pronounced as they contain 100- to 1,000-fold more porins than M. smegmatis (54).
Our results show that CpnT mediates the susceptibility of M. bovis BCG to small, hydrophilic antituberculosis drugs ethambutol, isoniazid, and p-aminosalicylic acid. This is consistent with the hypothesis that these drugs diffuse through the CpnT channel. Surprisingly, we also found that absence of CpnT in M. bovis BCG resulted in high-level resistance to large and hydrophobic drugs, such as erythromycin and rifampin. While we do not assume that these antibiotics diffuse directly through the CpnT pore, deletion of an outer membrane protein may indirectly reduce drug susceptibility. A similar phenomenon has been observed in M. smegmatis: deletion of the major porin MspA not only reduced the permeability of M. smegmatis for hydrophilic compounds but also reduced it for hydrophobic compounds, such as chenodeoxycholate, and concomitantly increased resistance to large, hydrophobic antibiotics (39), in contrast to porin mutants of Escherichia coli and other Gram-negative bacteria (55). Such membrane changes might be caused by bilayer deformations upon protein insertions (56, 57) resulting in increased solute permeability at the lipid-protein interface as previously proposed (39). Further experiments utilizing porins with closed channels that can integrate into the outer membrane but are deficient for solute transport will enable the testing of this hypothesis.
The lack of CpnT did not increase drug resistance of M. tuberculosis in contrast to M. bovis BCG. This might be explained by lower protein levels of CpnT in M. tuberculosis versus M. bovis BCG during in vitro growth. Indeed, RNA sequencing revealed that transcript levels of cpnT are very low in in vitro-grown M. tuberculosis cells (58). Much higher CpnT levels were observed in M. tuberculosis after infection of macrophages (J. Sun and M. Niederweis, unpublished data), supporting the hypothesis that CpnT primarily functions in vivo and is largely silent under in vitro growth conditions (31). Another possible explanation is that the phenotype of cpnT deletion in M. tuberculosis in our experiments is concealed by the presence of other, currently unknown channel-forming proteins that do not exist or are not produced by M. bovis BCG. Such differences might result from the absence of regulatory proteins in M. bovis BCG compared to M. tuberculosis, whose genes were deleted with the loss of “regions of difference” (RD) during attenuation of M. bovis BCG (59, 60). Thus, it is possible that cpnT mutations in combination with mutations of other genes produce a drug resistance phenotype in M. tuberculosis similar to that observed for the M. bovis BCG cpnT mutant and may contribute to the stepwise acquisition of drug resistance by M. tuberculosis (6, 61).
CpnT mutations appear to be associated with drug resistance in clinical M. tuberculosis strains.
To our knowledge, CpnT has not been previously associated with drug-resistant tuberculosis. However, resistance in approximately 5 to 30% of clinical isolates cannot be explained by mutations in any known genes associated with resistance to a particular drug (62) suggesting that other undefined mechanisms are involved. Indeed, cpnT (rv3903c) was identified as a gene with a significantly higher density of nonsynonymous single nucleotide polymorphisms in a genome sequencing study of drug-resistant clinical M. tuberculosis strains in China (6). These data indicate that CpnT is under selective pressure during infection, supporting a significant role of CpnT in human tuberculosis and/or chemotherapy. Since the cpnT mutant of M. tuberculosis H37Rv did not show a significant resistance phenotype in vitro, M. bovis BCG strains lacking wt cpnT and containing designed cpnT alleles with the mutations found in clinical isolates of M. tuberculosis are needed to establish a causal relationship between these CpnT mutants of clinical M. tuberculosis strains and drug resistance.
Role of CpnT in resistance to nitric oxide and other toxic compounds in macrophages.
This study provides the first evidence that an outer membrane protein is a major drug susceptibility determinant of slow-growing mycobacteria. In addition, the absence of CpnT renders M. bovis BCG 100-fold more resistant to nitric oxide than the wt strain. This is consistent with previous observations that the MspA porin of M. smegmatis mediates susceptibility to nitric oxide in mycobacteria (41, 63), but to our knowledge, porins of Gram-negative bacteria were never implicated in this process. This is likely due to the very large number of various channel-forming proteins in the outer membrane of these bacteria (64). Thus, Gram-negative bacteria with porin mutations still have a relatively large number of channels remaining in the outer membrane, which often prevents detection of phenotypes in susceptibility and/or transport experiments.
Interestingly, expression of mspA did not rescue the growth defect of the cpnT::Tn mutant even in growth-permissive THP-1 cells (Fig. 2), while full complementation of all phenotypes was observed in vitro (Table 1 and Fig. 1). These results indicate that the CpnT channel mediates sufficient nutrient influx in M. bovis BCG but provides much better protection against toxic solutes in vivo than the wide open and potentially unregulated MspA channel (24). Although MspA provides an efficient uptake pathway for nutrients, the apparently unregulated uptake of toxic compounds by MspA leads to reduction of M. bovis BCG survival in macrophages. These observations suggest that in order to survive and grow in macrophages, a tight balance between nutrient acquisition and minimization of the influx of toxic compounds is essential, and disturbance of this equilibrium in either direction might be detrimental during infection. These results also indicated that increasing the efficiency of channel protein-mediated outer membrane permeability may indeed be an attractive approach for developing new TB drugs with an alternative mechanism of action as proposed earlier (15).
CpnT is a novel susceptibility factor of M. bovis BCG.
The important role of CpnT in susceptibility to drugs and NO implies that it constitutes a gateway protein in the outer membrane of M. bovis BCG. This is consistent with the observation that other outer membrane channel proteins control entry of small, hydrophilic solutes, including antibiotics, into cells of Gram-negative bacteria and mycobacteria (20, 35). Not surprisingly, porin mutations often cause drug resistance of clinical isolates of Gram-negative bacterial pathogens. However, it needs to be noted that CpnT does not belong to a group of classical porins found in Gram-negative bacteria, whose physiological function is only to mediate influx of small, hydrophilic solutes through their water-filled channels across the outer membrane (38). The primary function of CpnT in M. tuberculosis appears to be the secretion of a novel necrosis-inducing toxin located in the C terminus of the protein by utilizing its N-terminal domain in an autotransporter-like mechanism (31). Although details of this secretion process are unknown, it is clear that the channel-forming activity of the N-terminal domain of CpnT is the main molecular determinant mediating drug susceptibility and nutrient transport in M. bovis BCG. It is interesting that the C-terminal domain does not seem to interfere with the channel activity, indicating that perhaps the vast majority of CpnT proteins release the toxin and only the N-terminal channel domain remains in the outer membrane of M. tuberculosis. However, we cannot exclude the possibility that the toxin domain does not interfere with transport through the CpnT channel, since full-length CpnT is also detectable in the outer membrane of M. tuberculosis (31). In this regard, it would be interesting to determine the ratio of full-length versus cleaved CpnT in M. bovis BCG and M. tuberculosis under different conditions, in addition to lipid bilayer experiments using full-length CpnT protein.
The channel activity of CpnT is a double-edged sword for M. bovis BCG in macrophages.
The macrophage experiments with M. bovis BCG revealed dual functions of CpnT. (i) In macrophages that prevent intracellular mycobacterial replication, absence of CpnT confers a survival advantage. The 100-fold-increased resistance of the cpnT mutant to nitric oxide might play a key role in this regard, although regulatory effects, as evidenced by reduced iNOS and IL-1β levels, probably contribute to the increased resistance of the cpnT mutant to killing in J774 macrophages. (ii) Under in vivo conditions that enable bacterial replication, such as in human THP-1 macrophages, the presence of CpnT is beneficial, probably because of increased nutrient uptake. These opposing functions of CpnT in different macrophage environments might provide a mechanistic explanation for the lack of a phenotype of the M. tuberculosis cpnT mutant in mouse infection experiments (31). CpnT has multiple functions in nutrient uptake across the outer membrane and susceptibility to toxic solutes due to the activity of its N-terminal domain.
The identification of the CpnT pore as the first outer membrane susceptibility factor represents a crucial step in understanding the outer membrane permeability of slow-growing mycobacteria. Knowledge of the structure and the biophysical properties of the CpnT N-terminal channel domain might help to improve drug uptake and, thereby, to overcome the outer membrane permeability barrier, a major hurdle in TB drug development.
ACKNOWLEDGMENTS
IFN-γ was provided by the Centre for AIDS Reagents, NIBSC (United Kingdom), and was donated by Genentech, Inc. This work was supported by a Senior Research Training Fellowship from the American Lung Association to O.D., by a fellowship (SFRH/BD/63747/2009) from the National Foundation for Science FCT to D.P. and grants PIC/IC/82859/2007, PTDC/BIA-BCM/102123/2008, and PTDC/SAU-MII/098024/2008 to E.A., and by National Institutes of Health grants AI63432, AI074805, and AI083632 to M.N.
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.World Health Organization. 2013. Global tuberculosis control: WHO report 2012. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Udwadia ZF. 2012. MDR, XDR, TDR tuberculosis: ominous progression. Thorax 67:286–288. doi: 10.1136/thoraxjnl-2012-201663. [DOI] [PubMed] [Google Scholar]
- 3.Dheda K, Gumbo T, Gandhi NR, Murray M, Theron G, Udwadia Z, Migliori GB, Warren R. 2014. Global control of tuberculosis: from extensively drug-resistant to untreatable tuberculosis. Lancet Respir Med 2:321–338. doi: 10.1016/S2213-2600(14)70031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanchard JS. 1996. Molecular mechanisms of drug resistance in Mycobacterium tuberculosis. Annu Rev Biochem 65:215–239. doi: 10.1146/annurev.bi.65.070196.001243. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Yew WW. 2009. Mechanisms of drug resistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis 13:1320–1330. [PubMed] [Google Scholar]
- 6.Zhang H, Li D, Zhao L, Fleming J, Lin N, Wang T, Liu Z, Li C, Galwey N, Deng J, Zhou Y, Zhu Y, Gao Y, Wang T, Wang S, Huang Y, Wang M, Zhong Q, Zhou L, Chen T, Zhou J, Yang R, Zhu G, Hang H, Zhang J, Li F, Wan K, Wang J, Zhang XE, Bi L. 2013. Genome sequencing of 161 Mycobacterium tuberculosis isolates from China identifies genes and intergenic regions associated with drug resistance. Nat Genet 45:1255–1260. doi: 10.1038/ng.2735. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg DE, Siliciano RF, Jacobs WR Jr. 2012. Outwitting evolution: fighting drug-resistant TB, malaria, and HIV. Cell 148:1271–1283. doi: 10.1016/j.cell.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan PJ, Nikaido H. 1995. The envelope of mycobacteria. Annu Rev Biochem 64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 9.Nikaido H. 2001. Preventing drug access to targets: cell surface permeability barriers and active efflux in bacteria. Semin Cell Dev Biol 12:215–223. doi: 10.1006/scdb.2000.0247. [DOI] [PubMed] [Google Scholar]
- 10.da Silva PE, Von Groll A, Martin A, Palomino JC. 2011. Efflux as a mechanism for drug resistance in Mycobacterium tuberculosis. FEMS Immunol Med Microbiol 63:1–9. doi: 10.1111/j.1574-695X.2011.00831.x. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues L, Ainsa JA, Amaral L, Viveiros M. 2011. Inhibition of drug efflux in mycobacteria with phenothiazines and other putative efflux inhibitors. Recent Patents Anti-Infect Drug Discov 6:118–127. doi: 10.2174/157489111796064579. [DOI] [PubMed] [Google Scholar]
- 12.Niederweis M. 2008. Nutrient acquisition by mycobacteria. Microbiology 154:679–692. doi: 10.1099/mic.0.2007/012872-0. [DOI] [PubMed] [Google Scholar]
- 13.Koul A, Arnoult E, Lounis N, Guillemont J, Andries K. 2011. The challenge of new drug discovery for tuberculosis. Nature 469:483–490. doi: 10.1038/nature09657. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann C, Leis A, Niederweis M, Plitzko JM, Engelhardt H. 2008. Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc Natl Acad Sci U S A 105:3963–3967. doi: 10.1073/pnas.0709530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niederweis M, Danilchanka O, Huff J, Hoffmann C, Engelhardt H. 2010. Mycobacterial outer membranes: in search of proteins. Trends Microbiol 18:109–116. doi: 10.1016/j.tim.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sani M, Houben EN, Geurtsen J, Pierson J, de Punder K, van Zon M, Wever B, Piersma SR, Jimenez CR, Daffe M, Appelmelk BJ, Bitter W, van der Wel N, Peters PJ. 2010. Direct visualization by cryo-EM of the mycobacterial capsular layer: a labile structure containing ESX-1-secreted proteins. PLoS Pathog 6:e1000794. doi: 10.1371/journal.ppat.1000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry CE. 2001. Interpreting cell wall ‘virulence factors’ of Mycobacterium tuberculosis. Trends Microbiol 9:237–241. doi: 10.1016/S0966-842X(01)02018-2. [DOI] [PubMed] [Google Scholar]
- 18.Zeth K, Thein M. 2010. Porins in prokaryotes and eukaryotes: common themes and variations. Biochem J 431:13–22. doi: 10.1042/BJ20100371. [DOI] [PubMed] [Google Scholar]
- 19.Nikaido H, Rosenberg EY. 1983. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J Bacteriol 153:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pages JM, James CE, Winterhalter M. 2008. The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat Rev Microbiol 6:893–903. doi: 10.1038/nrmicro1994. [DOI] [PubMed] [Google Scholar]
- 21.Kartmann B, Stenger S, Niederweis M. 1999. Porins in the cell wall of Mycobacterium tuberculosis. J Bacteriol 181:6543–6546. (Author correction, 181:7650.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtinger T, Heym B, Maier E, Eichner H, Cole ST, Benz R. 1999. Evidence for a small anion-selective channel in the cell wall of Mycobacterium bovis BCG besides a wide cation-selective pore. FEBS Lett 454:349–355. doi: 10.1016/S0014-5793(99)00844-3. [DOI] [PubMed] [Google Scholar]
- 23.Niederweis M. 2008. Mycobacterial porins, p 153–165. In Daffe M, Reyrat J-M (ed), The mycobacterial cell envelope. ASM Press, Washington, DC. [Google Scholar]
- 24.Faller M, Niederweis M, Schulz GE. 2004. The structure of a mycobacterial outer-membrane channel. Science 303:1189–1192. doi: 10.1126/science.1094114. [DOI] [PubMed] [Google Scholar]
- 25.Feltcher ME, Gibbons HS, Ligon LS, Braunstein M. 2012. Protein export by the mycobacterial SecA2 system is determined by the preprotein mature domain. J Bacteriol 195:672–681. doi: 10.1128/JB.02032-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bansal-Mutalik R, Nikaido H. 2011. Quantitative lipid composition of cell envelopes of Corynebacterium glutamicum elucidated through reverse micelle extraction. Proc Natl Acad Sci U S A 108:15360–15365. doi: 10.1073/pnas.1112572108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rezwan M, Laneelle MA, Sander P, Daffe M. 2007. Breaking down the wall: fractionation of mycobacteria. J Microbiol Methods 68:32–39. doi: 10.1016/j.mimet.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Stahl C, Kubetzko S, Kaps I, Seeber S, Engelhardt H, Niederweis M. 2001. MspA provides the main hydrophilic pathway through the cell wall of Mycobacterium smegmatis. Mol Microbiol 40:451–464 doi: 10.1046/j.1365-2958.2001.02394.x (Author correction, Mol Microbiol 57:1509.) [DOI] [PubMed] [Google Scholar]
- 29.Stephan J, Bender J, Wolschendorf F, Hoffmann C, Roth E, Mailänder C, Engelhardt H, Niederweis M. 2005. The growth rate of Mycobacterium smegmatis depends on sufficient porin-mediated influx of nutrients. Mol Microbiol 58:714–730. doi: 10.1111/j.1365-2958.2005.04878.x. [DOI] [PubMed] [Google Scholar]
- 30.Mailaender C, Reiling N, Engelhardt H, Bossmann S, Ehlers S, Niederweis M. 2004. The MspA porin promotes growth and increases antibiotic susceptibility of both Mycobacterium bovis BCG and Mycobacterium tuberculosis. Microbiology 150:853–864. doi: 10.1099/mic.0.26902-0. [DOI] [PubMed] [Google Scholar]
- 31.Danilchanka O, Sun J, Pavlenok M, Maueroder C, Speer A, Siroy A, Marrero J, Trujillo C, Mayhew DL, Doornbos KS, Munoz LE, Herrmann M, Ehrt S, Berens C, Niederweis M. 2014. An outer membrane channel protein of Mycobacterium tuberculosis with exotoxin activity. Proc Natl Acad Sci U S A 111:6750–6755. doi: 10.1073/pnas.1400136111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leyton DL, Rossiter AE, Henderson IR. 2012. From self sufficiency to dependence: mechanisms and factors important for autotransporter biogenesis. Nat Rev Microbiol 10:213–225. doi: 10.1038/nrmicro2733. [DOI] [PubMed] [Google Scholar]
- 33.Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, Degnan MT, Cook MB, Quenzer VK, Ferguson RM, Gilman RH. 1998. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J Clin Microbiol 36:362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danilchanka O, Mailaender C, Niederweis M. 2008. Identification of a novel multidrug efflux pump of Mycobacterium tuberculosis. Antimicrob Agents Chemother 52:2503–2511. doi: 10.1128/AAC.00298-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danilchanka O, Pavlenok M, Niederweis M. 2008. Role of porins for uptake of antibiotics by Mycobacterium smegmatis. Antimicrob Agents Chemother 52:3127–3134. doi: 10.1128/AAC.00239-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mojib N, Philpott R, Huang JP, Niederweis M, Bej AK. 2010. Antimycobacterial activity in vitro of pigments isolated from Antarctic bacteria. Antonie Van Leeuwenhoek 98:531–540. doi: 10.1007/s10482-010-9470-0. [DOI] [PubMed] [Google Scholar]
- 37.Jordao L, Bleck CK, Mayorga L, Griffiths G, Anes E. 2008. On the killing of mycobacteria by macrophages. Cell Microbiol 10:529–548. doi: 10.1111/j.1462-5822.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- 38.Nikaido H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev 67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stephan J, Mailaender C, Etienne G, Daffe M, Niederweis M. 2004. Multidrug resistance of a porin deletion mutant of Mycobacterium smegmatis. Antimicrob Agents Chemother 48:4163–4170. doi: 10.1128/AAC.48.11.4163-4170.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacMicking J, Xie QW, Nathan C. 1997. Nitric oxide and macrophage function. Annu Rev Immunol 15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 41.Fabrino DL, Bleck CK, Anes E, Hasilik A, Melo RC, Niederweis M, Griffiths G, Gutierrez MG. 2009. Porins facilitate nitric oxide-mediated killing of mycobacteria. Microbes Infect 11:868–875. doi: 10.1016/j.micinf.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Herrera M, Hong NJ, Garvin JL. 2006. Aquaporin-1 transports NO across cell membranes. Hypertension 48:157–164. doi: 10.1161/01.HYP.0000223652.29338.77. [DOI] [PubMed] [Google Scholar]
- 43.Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS. 2012. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe 11:469–480. doi: 10.1016/j.chom.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simeone R, Bobard A, Lippmann J, Bitter W, Majlessi L, Brosch R, Enninga J. 2012. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog 8:e1002507. doi: 10.1371/journal.ppat.1002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonough KA, Kress Y. 1995. Cytotoxicity for lung epithelial cells is a virulence-associated phenotype of Mycobacterium tuberculosis. Infect Immun 63:4802–4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu T, Hingley-Wilson SM, Chen B, Chen M, Dai AZ, Morin PM, Marks CB, Padiyar J, Goulding C, Gingery M, Eisenberg D, Russell RG, Derrick SC, Collins FM, Morris SL, King CH, Jacobs WR Jr. 2003. The primary mechanism of attenuation of Bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci U S A 100:12420–12425. doi: 10.1073/pnas.1635213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuusi N, Nurminen M, Saxen H, Makela PH. 1981. Immunization with major outer membrane protein (porin) preparations in experimental murine salmonellosis: effect of lipopolysaccharide. Infect Immun 34:328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabaraie B, Sharma BK, Sharma PR, Sehgal R, Ganguly NK. 1994. Evaluation of Salmonella porins as a broad spectrum vaccine candidate. Microbiol Immunol 38:553–559. doi: 10.1111/j.1348-0421.1994.tb01822.x. [DOI] [PubMed] [Google Scholar]
- 49.Vordermeier HM, Drexler H, Bessler WG. 1987. Polyclonal activation of human peripheral blood lymphocytes by bacterial porins and defined porin fragments. Immunol Lett 15:121–126. doi: 10.1016/0165-2478(87)90042-3. [DOI] [PubMed] [Google Scholar]
- 50.Galdiero F, de L'ero GC, Benedetto N, Galdiero M, Tufano MA. 1993. Release of cytokines induced by Salmonella typhimurium porins. Infect Immun 61:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mishra BB, Moura-Alves P, Sonawane A, Hacohen N, Griffiths G, Moita LF, Anes E. 2010. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell Microbiol 12:1046–1063. doi: 10.1111/j.1462-5822.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- 52.Mishra BB, Rathinam VA, Martens GW, Martinot AJ, Kornfeld H, Fitzgerald KA, Sassetti CM. 2013. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1beta. Nat Immunol 14:52–60. doi: 10.1038/ni.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jarlier V, Nikaido H. 1994. Mycobacterial cell wall: structure and role in natural resistance to antibiotics. FEMS Microbiol Lett 123:11–18. doi: 10.1111/j.1574-6968.1994.tb07194.x. [DOI] [PubMed] [Google Scholar]
- 54.Niederweis M. 2003. Mycobacterial porins—new channel proteins in unique outer membranes. Mol Microbiol 49:1167–1177. doi: 10.1046/j.1365-2958.2003.03662.x. [DOI] [PubMed] [Google Scholar]
- 55.Delcour AH. 2009. Outer membrane permeability and antibiotic resistance. Biochim Biophys Acta 1794:808–816. doi: 10.1016/j.bbapap.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bond PJ, Sansom MS. 2007. Bilayer deformation by the Kv channel voltage sensor domain revealed by self-assembly simulations. Proc Natl Acad Sci U S A 104:2631–2636. doi: 10.1073/pnas.0606822104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez-Ropero F, Fioroni M. 2012. Structural and dynamical analysis of an engineered FhuA channel protein embedded into a lipid bilayer or a detergent belt. J Struct Biol 177:291–301. doi: 10.1016/j.jsb.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 58.Cortes T, Schubert OT, Rose G, Arnvig KB, Comas I, Aebersold R, Young DB. 2013. Genome-wide mapping of transcriptional start sites defines an extensive leaderless transcriptome in Mycobacterium tuberculosis. Cell Rep 5:1121–1131. doi: 10.1016/j.celrep.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brosch R, Pym AS, Gordon SV, Cole ST. 2001. The evolution of mycobacterial pathogenicity: clues from comparative genomics. Trends Microbiol 9:452–458. doi: 10.1016/S0966-842X(01)02131-X. [DOI] [PubMed] [Google Scholar]
- 60.Brosch R, Gordon SV, Marmiesse M, Brodin P, Buchrieser C, Eiglmeier K, Garnier T, Gutierrez C, Hewinson G, Kremer K, Parsons LM, Pym AS, Samper S, van Soolingen D, Cole ST. 2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci U S A 99:3684–3689. doi: 10.1073/pnas.052548299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borrell S, Gagneux S. 2011. Strain diversity, epistasis and the evolution of drug resistance in Mycobacterium tuberculosis. Clin Microbiol Infect 17:815–820. doi: 10.1111/j.1469-0691.2011.03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Louw GE, Warren RM, Gey van Pittius NC, McEvoy CR, Van Helden PD, Victor TC. 2009. A balancing act: efflux/influx in mycobacterial drug resistance. Antimicrob Agents Chemother 53:3181–3189. doi: 10.1128/AAC.01577-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Purdy GE, Niederweis M, Russell DG. 2009. Decreased outer membrane permeability protects mycobacteria from killing by ubiquitin-derived peptides. Mol Microbiol 73:844–857. doi: 10.1111/j.1365-2958.2009.06801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kessel M, Brennan MJ, Trus BL, Bisher ME, Steven AC. 1988. Naturally crystalline porin in the outer membrane of Bordetella pertussis. J Mol Biol 203:275–278. doi: 10.1016/0022-2836(88)90108-8. [DOI] [PubMed] [Google Scholar]