Abstract
Dalbavancin is an intravenous lipoglycopeptide with activity against Gram-positive pathogens and an MIC90 for Staphylococcus aureus of 0.06 μg/ml. With a terminal half-life of >14 days, dosing regimens with infrequent parenteral administration become available to treat infectious diseases such as osteomyelitis and endocarditis that otherwise require daily dosing for many weeks. In order to support a rationale for these novel regimens, the pharmacokinetics over an extended dosing interval and the distribution of dalbavancin into bone and articular tissue were studied in two phase I trials and pharmacokinetic modeling was performed. Intravenous administration of 1,000 mg of dalbavancin on day 1 followed by 500 mg weekly for seven additional weeks was well tolerated and did not demonstrate evidence of drug accumulation. In a separate study, dalbavancin concentrations in cortical bone 12 h after infusion of a single 1,000-mg intravenous infusion were 6.3 μg/g and 2 weeks later were 4.1 μg/g. A two-dose, once-weekly regimen that would provide tissue exposure over the dalbavancin MIC for Staphylococcus aureus for 8 weeks, maximizing the initial exposure to treatment while minimizing the frequency of intravenous therapy, is proposed.
INTRODUCTION
Osteomyelitis is an infection of the bone associated with either hematogenous dissemination or direct inoculation as a consequence of trauma or infection from contiguous tissues. Its presentation may be either acute or chronic. The most common pathogen responsible for acute infection in adults is Staphylococcus aureus, with Pseudomonas aeruginosa, Serratia marcescens, and Escherichia coli also involved in traumatic infections and chronic presentations. Infection in children occurs less frequently and is predominantly a result of bacteremia with S. aureus and infection in the growth plate (1). Treatment regimens extend for 4 to 6 weeks, with durations as long as 8 weeks recommended for treatment of infection due to methicillin-resistant S. aureus (MRSA) (2). Commonly used therapies include cefazolin, oxacillin, or vancomycin for coverage of Gram-positive pathogens and cephalosporins, carbapenems, and the β-lactamase inhibitor agents for treatment of Gram-negative pathogens (2, 3). No other therapies in recent times have received FDA approval.
Because the incidence of MRSA in the community in the United States is as high as 40% (4), empirical treatment of osteomyelitis now requires consideration of antimicrobial coverage of this organism, typically with vancomycin. Vancomycin has been demonstrated to be active in animal models of osteomyelitis (5). Concentrations in bone from 2.7 to 9.3 μg/ml have been documented (6, 7), exceeding the vancomycin MIC90 for S. aureus of 1 μg/ml (8). Because of the wide variety of underlying etiologies and comorbidities associated with osteomyelitis, it is difficult to generalize the rates of clinical success from clinical trials, but one study documented a clinical response in 10/15 patients after continuous intravenous (i.v.) infusion of vancomycin (9). Long-term dosing with vancomycin typically requires placement of a peripherally inserted central catheter (PICC), monitoring of serum drug levels to avoid toxicity, and logistical constraints involving delivery of twice-daily dosing in an outpatient setting for extended periods of time.
Dalbavancin is a novel lipoglycopeptide with a terminal half-life of 14.4 days that inhibits peptidoglycan cross-linking in the cell wall. The MIC90 of dalbavancin for S. aureus is 0.06 μg/ml, with 99.9% of organisms inhibited at a concentration of 0.12 μg/ml (8). The mean bactericidal concentrations in plasma are achievable at therapeutically achievable concentrations (10, 11). Dalbavancin has been studied in the treatment of acute bacterial skin and skin structure infections and has received regulatory approval for that indication with a dosing regimen of 1,000 mg i.v. over 30 min followed 1 week later by a 500-mg infusion (12).
The long half-life of dalbavancin, activity against MRSA, and animal drug distribution studies (13) with promising bone penetration suggest that it might be considered for treatment of patients with osteomyelitis. The potential for infrequent dosing and the generally favorable emerging safety profile are encouraging in this regard. In order to evaluate the pharmacokinetics of dalbavancin in bone and articular tissue and model a potential dosing regimen suitable for longer-term dosing, two phase I studies in healthy volunteers were conducted.
MATERIALS AND METHODS
Two phase I studies were conducted: a bone penetration study (DUR001-105) done in 30 patients enrolled in six separate cohorts of five patients each and an extended-duration dosing study (DUR001-104) in 18 subjects divided into three cohorts of six patients each. Data derived from the bone penetration study were included in a pharmacokinetic (PK) modeling analysis. Both studies were approved by the institutional review boards (IRBs) at the participating centers, including, for DUR001-104, the PRACS Institute, Ltd., IRB, and, for DUR001-105, Western IRB, Olympia, WA, the Sharp HealthCare IRB, San Diego, CA, and the Liberty IRB, Deland, FL.
Bone and articular tissue penetration.
The bone penetration study was an open-label, single-dose, safety, tolerability, and PK study of bone, synovium, synovial fluid, skin, and plasma concentrations of dalbavancin. Thirty subjects with five enrolled into each of six cohorts received 1,000 mg of dalbavancin, infused for 30 min at 0.5, 1, 3, 7, 10, or 14 days before elective orthopedic surgery. Subjects were aged 18 years or older and were scheduled for an elective orthopedic procedure unrelated to infection from which an adequate sample of bone and adjacent tissues could be obtained. Subjects were assigned to treatment groups to populate the cohorts. Plasma pharmacokinetic sampling for all subjects who received dalbavancin was performed 1 h, 4 h, 12 h, 336 h, 720 h, and 1,080 h postdose, regardless of the cohort.
The dalbavancin plasma, bone, and concomitant skin and synovium concentrations were measured using a validated liquid chromatography-tandem mass spectrometry method.
Bioanalytical methods were used according to the bioanalytical laboratory's standard operating procedures (ICON Development Solutions, Whitesboro, NY) and Food and Drug Administration guidance. A full validation of a sensitive assay for the appropriate analytes in each biological matrix, including precision, accuracy, reproducibility, limit of quantitation, recovery, and selectivity, was completed and approved prior to sample analysis. In plasma, the analysis methodology was validated from 1.00 to 100.0 μg/ml, with a lower limit of quantification of 1.00 μg/ml. For dalbavancin quality control samples, the overall precision was ≤7.35%, as measured by the percent coefficient of variation, and their overall accuracy (percent deviation from nominal) ranged from −5.67% to −4.67%, as measured by percent relative error. The precision and accuracy for the 10-fold dilution integrity quality control samples were 7.49% and −3.83%, respectively.
For bone, the validation range was 0.20 to 25.0 μg/mg of tissue, and the assay precision and accuracy were similar to those for plasma.
Extended-duration dosing.
An open-label, multiple-dose study was performed to assess the safety, tolerability, and pharmacokinetics of dalbavancin delivered over increasing weekly dosing durations. The total duration of the study, from screening through study exit, was up to 15 weeks with at least a 7-day period between doses. Six subjects were enrolled into each of three cohorts: subjects in cohort I received 4 weekly doses of dalbavancin, subjects in cohort II received 6 weekly doses of dalbavancin, and subjects in cohort III received 8 weekly doses of dalbavancin. Subjects reported to the clinical site at least 20 h prior to each dosing and were required to stay for 1 h after each dosing. Blood sample collections on day 1 were obtained at the end of the infusion and at 1, 2, 3, 4, 6, 8, 10, and 12 h after the start of the infusion. On days 8 and 15, blood samples were obtained within 15 min prior to each subject's scheduled dose time (0 h) and at the end of the infusion (approximately 0.5 h). Blood samples were also obtained within 15 min prior to each subject's last scheduled dose time (0 h), at the end of the infusion (approximately 0.5 h), and at 1, 2, 3, 4, 6, 8, 10, and 12 h after the start of the last infusion. The last such schedule of blood draws occurred on day 22 for cohort I, day 36 for cohort II, and day 50 for cohort III. A single blood draw was performed an additional 4 weeks after the conclusion of the intense PK sampling.
Pharmacokinetic analyses. (i) Population pharmacokinetic analysis of bone and articular tissues.
The PK analysis for dalbavancin was conducted using the plasma and bone concentration-time data obtained from the subjects enrolled in the bone penetration study. The population PK analysis was conducted using the nonlinear mixed-effects modeling software NONMEM, version 7.2 (ICON Development Solutions, Ellicott City, MD).
In the first analysis stage, the PK of dalbavancin in plasma was evaluated. Both two- and three-compartment structural population PK models were evaluated in the current analysis to describe the time course of dalbavancin concentrations in plasma in this population following i.v. infusion of dalbavancin (1 g) over 30 min as a single dose prior to surgery. The model parameters total clearance (CL), central volume of distribution (Vc), peripheral volume of distribution for both the first and second peripheral compartments (Vp1 and Vp2, respectively), and distribution clearance between the central and either the first or second peripheral compartment (CLd1 and CLd2, respectively) were all estimated in the three-compartment model. Interindividual variability (ω2) was modeled for CL and Vc using exponential error models. Residual variability (σ2) was estimated using a constant coefficient of variability (CCV) model.
After an acceptable base structural population PK model was derived, a formal covariate analysis was performed in NONMEM using stepwise univariate forward selection (α = 0.01) and backward elimination (α = 0.001) procedures. The following subject covariates were evaluated as potential predictors of dalbavancin plasma PK: age, gender, height, body weight, ideal body weight, body mass index (BMI), body surface area (BSA), albumin, and creatinine clearance (CLcr) normalized to a BSA of 1.73 m2 to help account for body size differences in the estimation of renal function.
In the second analysis stage, the population PK model developed to characterize the time course of dalbavancin concentrations in plasma was expanded by one additional compartment (e.g., the three-compartment model was modified to be a four-compartment model) in order to simultaneously characterize the PK of dalbavancin in plasma and distribution into bone. The assayed bone dalbavancin concentrations (in μg/g bone tissue) were converted to mg amounts in total body bone for use in this analysis. An allometric relationship between bone mass and total body weight, which was obtained from the literature, was used to determine the bone mass for each subject (14). The amount of dalbavancin in bone (the dependent variable for the population PK model) was then calculated using the observed bone concentration and the estimated bone mass. In addition to the PK parameters described previously to characterize the plasma dalbavancin concentrations, this expanded structural PK model estimated the first-order transfer rate constants from plasma to bone (kcb) and from bone to plasma (kbc). Interindividual variability was additionally modeled for kbc using an exponential error model. Residual variability was estimated separately for the plasma and bone PK data using separate CCV models. With the final population PK model from this stage of the analysis, the area under the curve (AUC) was generated in plasma and bone using numerical integration and used to calculate a bone/plasma total-drug area under the curve penetration ratio for each patient.
In order to better understand the importance of the variability in plasma and bone PK on the time course of bone concentrations after various dalbavancin dosing regimens, simulations were conducted to generate plasma and bone concentration-time profiles for 1,000 subjects with typical CLcr and BSA values. The variability in the simulated profiles was therefore completely dependent on the interindividual variability in the population PK parameters (CL, Vc, and kbc).
(ii) Extended-duration study pharmacokinetics.
For the subjects enrolled in the extended-duration study, the maximum and minimum plasma dalbavancin concentrations (Cmax and Cmin, respectively) were determined by direct inspection of the plasma concentration-time profiles for every intensive sampling period. Tmax was defined as the time after the start of infusion at which Cmax occurred. The AUC from the start of the infusion to the time of the next dose (AUC0–τ) for every intensive sampling period was calculated using the trapezoidal rule. The accumulation ratio (R) was calculated as (AUC0–τ [cohort I/days 22 to 29; cohort II/days 36 to 43; and cohort III/days 50-57] over AUC0–τ days 1 to 8).
RESULTS
Extended-duration dosing.
Eighteen patients were enrolled, with six patients in each of the three cohorts. The mean age was 38.1 years (range, 21 to 55 years) and 50% of subjects were male. The mean BMI was 27 kg/m2 (range, 22 to 32 kg/m2).
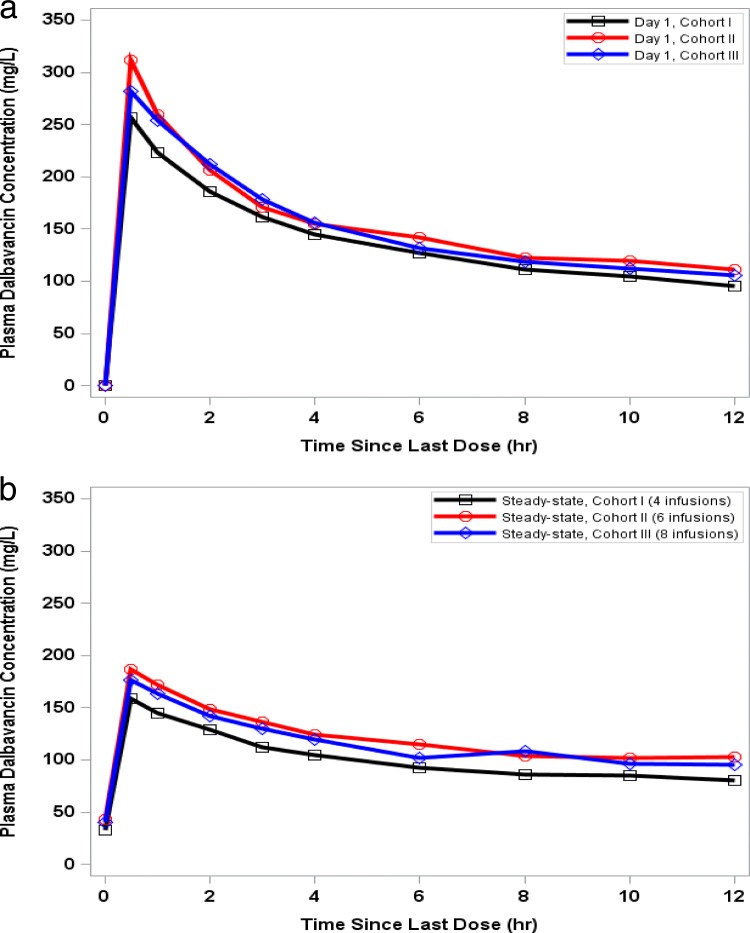
Cmin, Cmax, and AUC0–τ were slightly higher, even at day 8, in cohorts II and III than in cohort I, reflecting some minor variability among the populations of these three groups (Tables 1 and 2). The Cmin at day 22 was similar to the Cmin at day 8 within each of the three cohorts. The accumulation ratios were comparable between all cohorts, and no apparent accumulation was observed. There were no significant differences observed for Cmin, Cmax, and AUC0–τ in comparing the administration of 6 and 8 total weekly infusions in cohorts II and III. For all three cohorts, the observed predose plasma concentrations (Cmin) were consistent with steady state being achieved by day 8 (Table 2). A display of the concentrations in serum for the first 12 h after dosing, by cohort, is provided for day 1 and the last day of dosing (Fig. 1a and b, respectively).
TABLE 1.
Dalbavancin PK parameters after multiple dosinga
| Parameter | Results in: |
||
|---|---|---|---|
| Cohort I (4th infusion) | Cohort II (6th infusion) | Cohort III (8th infusion) | |
| AUC0–τ (μg · h/ml) | 10,203 (14.9) | 12,292.79 (17.8) | 12,173 (17.7) |
| Cmax (μg/ml) | 160.0 (14.0) | 187.0 (13.1) | 179.7 (11.0) |
| Cmin (μg/ml) | 33.0 (19.3) | 42.9 (17.4) | 40.2 (20.0) |
| Tmax (median [range]) (h) | 0.5 (0.5–1.0) | 0.5 (0.5–0.5) | 0.5 (0.5–1.0) |
| Accumulation ratio (R) | 0.89 (10.7) | 0.96 (12.5) | 0.91 (17.0) |
On day 1, subjects received 1,000 mg of dalbavancin i.v. over 30 min, followed by 500 mg i.v. over 30 min weekly for a total of 4 weekly infusions (cohort I), 6 weekly infusions (cohort II), or 8 weekly infusions (cohort III). Results are means (standard deviations [SD]) unless otherwise indicated.
TABLE 2.
Minimum serum concentrations on day of weekly dose
| Day |
Cmin (mean μg/ml [% CVa]) in: |
||
|---|---|---|---|
| Cohort I | Cohort II | Cohort III | |
| 8 | 33.0 (18.5) | 38.1 (10.7) | 40.4 (18.7) |
| 15 | 31.9 (16.3) | 39.7 (15.4) | 38.7 (16.8) |
| 22 | 33.1 (18.9) | 39.0 (16.4) | 36.5 (17.7) |
| 29 | NAb | 38.7 (13.9) | 38.2 (17.7) |
| 36 | NA | 43.2 (17.9) | 38.0 (16.2) |
| 43 | NA | NA | 38.5 (18.3) |
| 50 | NA | NA | 40.6 (19.7) |
CV, coefficient of variation.
NA, not applicable.
FIG 1.
Mean dalbavancin plasma concentrations on day 1 (a) and at steady state (b).
Adverse events occurred in 2 or 3 patients per cohort (Table 3). Among all 18 subjects, two adverse events possibly related to the drug were reported: one episode of transient urticaria and one of mild pain in the forearm.
TABLE 3.
Summary of treatment-emergent adverse events
| TEAEa | Results (no. [%] of patients) in: |
|||
|---|---|---|---|---|
| Cohort I (n = 6) | Cohort II (n = 6) | Cohort III (n = 6) | Total (n = 18) | |
| At least 1 | 2 (33.3) | 2 (33.3) | 3 (50.0) | 7 (38.9) |
| Related to the study drug | 1 (16.7) | 1 (16.7) | 0 (0) | 2 (11.1) |
Treatment-emergent adverse events.
Bone penetration.
Thirty-five patients were screened, and 31 received dalbavancin. The mean age was 66.7 years (range, 47 to 82 years), 45% of subjects were male, and the mean BMI was 32.1 kg/m2 (range, 22.4 to 43.4 kg/m2). Bone samples were taken from 22 patients receiving knee replacements and 8 patients receiving hip replacements.
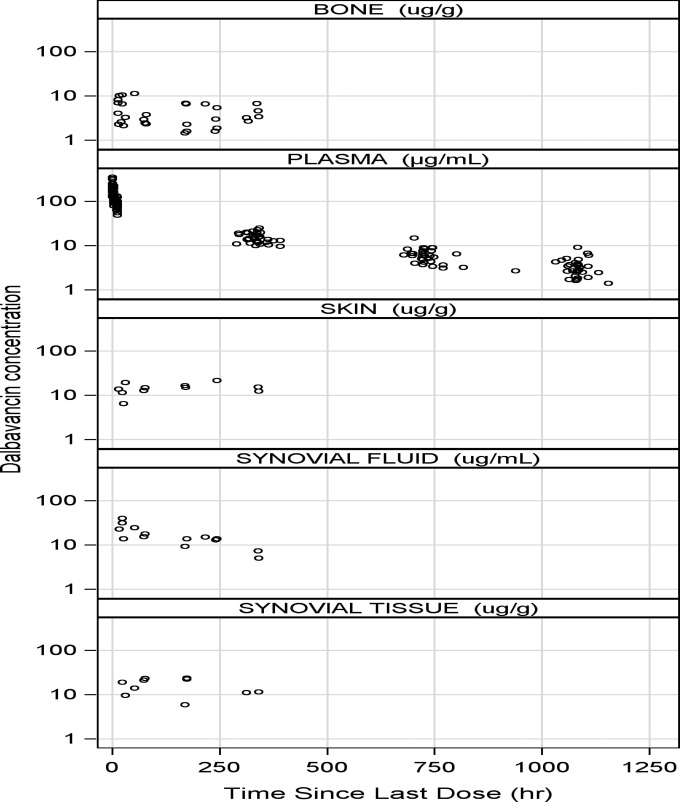
Dalbavancin concentrations in cortical bone 12 h after infusion of a single 1,000-mg intravenous infusion were 6.3 μg/g and 2 weeks later were 4.1 μg/g. Levels at 12 h postinfusion and 2 weeks postdose, respectively, in synovial tissue were 25.0 μg/g and 15.9 μg/g, in synovial fluid were 22.9 μg/ml and 6.2 μg/ml, in skin were 19.4 μg/g and 13.8 μg/g, and in plasma were 85.3 μg/ml and 15.3 μg/ml (Table 4). Semilog scatterplots of dalbavancin concentrations in plasma, bone, synovium, synovial fluid, and skin are provided in Fig. 2.
TABLE 4.
Dalbavancin tissue concentrations (safety population)
| Tissue | Dalbavancin concn (mean [SD]; no. of samples) at hours (days) postdose that samples were collected: |
|||||
|---|---|---|---|---|---|---|
| 12 (0.5) | 24 (1) | 72 (3) | 168 (7) | 240 (10) | 336 (14) | |
| Plasma (μg/ml)a | 85.3 (18.9); 31 | NDb | ND | ND | ND | 15.3 (4.1); 31 |
| Synovium (μg/g)c | 25.0 (0); 3 | 17.9 (7.8); 3 | 19.5 (4.9); 3 | 19.2 (8.9); 4 | 25.0 (0); 2 | 15.9 (7.9); 3 |
| Synovial fluid (μg/ml)c | 22.9; 1 | 27.4 (10.8); 4 | 19.2 (4.9); 3 | 11.6 (3.3); 2 | 13.9 (1.0); 3 | 6.2 (1.7); 2 |
| Bone (μg/g) | 6.3 (3.1); 5 | 5.0 (3.5); 5 | 4.6 (3.8); 5 | 3.8 (2.7); 5 | 3.7 (2.2); 5 | 4.1 (1.6); 5 |
| Skin (μg/g)c | 19.4 (7.9); 2 | 12.5 (6.5); 3 | 13.8 (1.4); 2 | 15.7 (1.0); 2 | 21.6; 1 | 13.8 (2.1); 2 |
Mean (SD) plasma concentrations in 31 subjects at 772 and 1,080 h were 6.2 (2.4) and 3.4 (1.7), respectively.
ND, not detected.
Concentrations above the upper limit of quantification are reported as 25 μg/unit.
FIG 2.
Dalbavancin concentrations in plasma, bone, and related tissues. Semilog scatterplots: one skin concentration and eight synovial tissue concentrations were greater than the upper limit of quantification of the assay and do not appear in these plots.
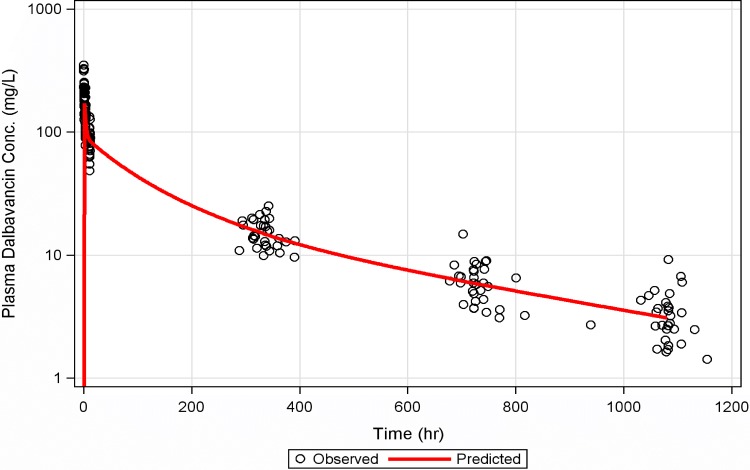
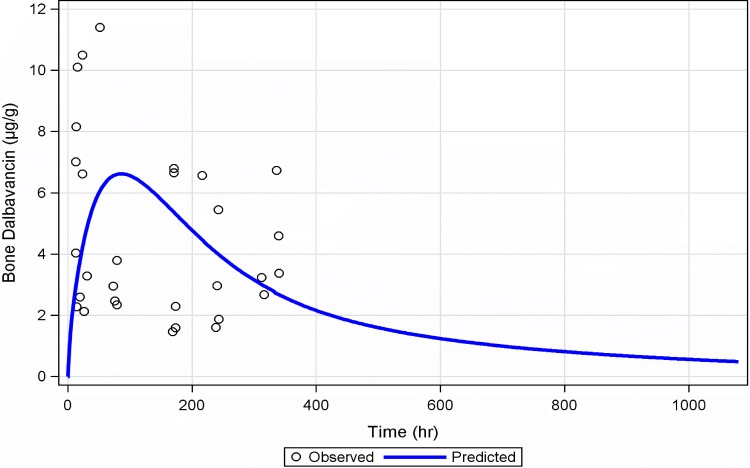
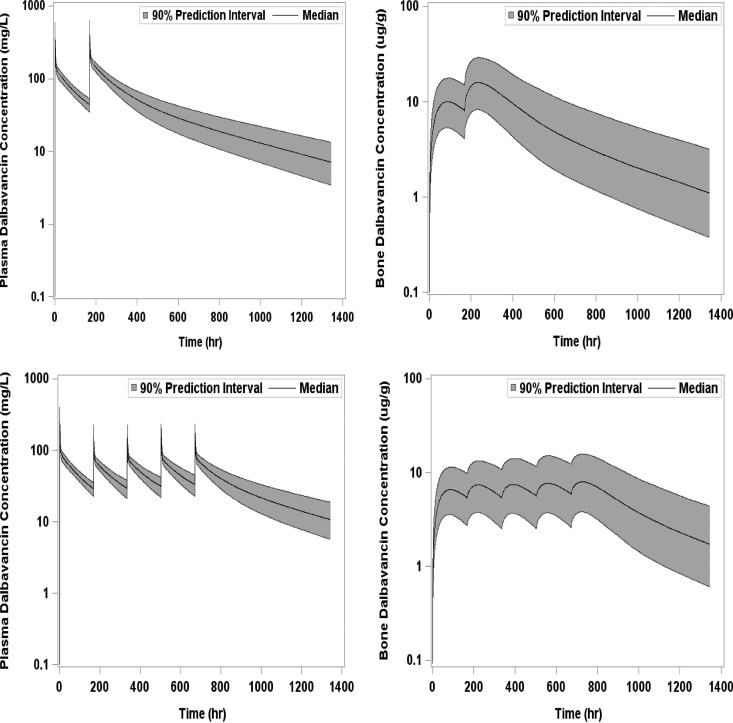
A three-compartment population PK model with zero-order i.v. input and first-order elimination best described the time course of dalbavancin in plasma. The covariate analysis identified CLcr and BSA as statistically significant predictors of dalbavancin CL and Vc, respectively. The three-compartment model used to describe the time course of dalbavancin in plasma was expanded to a four-compartment model to account for the transfer of dalbavancin between plasma and bone tissue when the PK data from these matrices were simultaneously fitted. This model provided a robust fit to the bone concentration data. The mean bone/plasma area under the curve penetration ratio was subsequently determined to be 13.1%. A linear plot of the population mean predicted amount of dalbavancin in serum versus time, overlaid upon the observed data, is provided in Fig. 3. A similar plot for bone, overlaid upon the observed data, is provided in Fig. 4. Figure 5 provides the simulated median (90% confidence interval) plasma and bone dalbavancin concentration-time profiles after a 1,500-mg infusion on day 1 and day 8 in adults as well as the profiles resulting from a 1,000-mg infusion on day 1 followed by four weekly 500-mg infusions.
FIG 3.
Linear plot of the population mean predicted amount of dalbavancin in serum versus time, overlaid upon the observed data.
FIG 4.
Linear plot of the population mean predicted amount of dalbavancin in bone versus time, overlaid upon the observed data.
FIG 5.
Simulated mean concentration-time profiles with 1,500 mg i.v. on days 1 and 8 in plasma and bone (upper panels) and with 1,000 mg i.v. on day 1 and 500 mg i.v. weekly in plasma and bone (lower panels).
In total, 18 subjects experienced at least 1 treatment-emergent adverse event, and 1 subject experienced a treatment-related adverse event of dizziness. There were no treatment-related serious adverse events, treatment-emergent adverse events leading to withdrawal, or serious adverse events resulting in early discontinuation.
DISCUSSION
The most common Gram-positive pathogen associated with osteomyelitis is S. aureus, and typical treatment durations for this infection are recommended to be at least 4 weeks but as long as 8 weeks if the pathogen is MRSA (2). Concentrations in bone of an antibiotic used for treatment of osteomyelitis would be expected to be at or above the MIC for a significant proportion of the treatment period.
Two studies in healthy subjects have provided data to help inform a dose regimen of dalbavancin for treatment of osteomyelitis by examining the safety and serum pharmacokinetics of extended durations of dosing and then measuring the concentrations in serum as well as in bone and articular tissue. After administration of a single 1,000-mg dose, serum concentrations of >3 μg/ml were observed through day 45. In the extended-duration dosing study, 500-mg weekly administrations of dalbavancin following an initial 1,000-mg infusion achieved minimum total dalbavancin concentrations that were >30 μg/ml from day 8 through day 50. A steady state was reached by day 8 without accumulation through day 50, likely as a result of the loading dose of 1,000 mg given on day 1. The level in bone 14 days after a single 1,000-mg i.v. infusion of dalbavancin was 4.1 μg/ml. The proposed dosing regimen of two 1,500-mg intravenous infusions 1 week apart, derived from these data and population PK modeling, should result in dalbavancin exposure at or above the S. aureus MIC99.9 for dalbavancin of 0.12 μg/ml for the entire treatment duration.
While drug concentrations above the MIC are reassuring, the PK/pharmacodynamic (PD) parameter most likely to predict the efficacy of dalbavancin is the AUC/MIC (15). A regimen of 1,500 mg i.v. given on day 1 and again on day 8 is expected to achieve an AUC similar to that for a 1,000-mg initial dose, followed by four subsequent 500-mg weekly doses. Although the same total AUC should provide similar outcomes, experimental data suggest that the efficacy of drugs with a long half-life, such as dalbavancin, is enhanced by providing higher doses earlier in the course of therapy. This finding has been observed in animal studies with dalbavancin in which better outcomes were observed when the same total dose was delivered in larger amounts earlier and less frequently (15). With translation of these animal data to humans, we anticipate that the two-dose 1,500-mg regimen would be more likely to achieve clinical success.
A 1,500-mg single dose of dalbavancin is expected to be well tolerated. The safety of a single 1,500-mg dose was studied in 50 patients in the course of conducting a thorough QT interval study and found to have an adverse-event profile similar to that of the 1,000-mg single infusion (16). In addition, the safety profile of the weekly regimen for 4 weeks of dosing in 18 subjects and 8 weeks of dosing in 6 subjects was also well tolerated, suggesting that longer-term exposures can be safely studied.
The concentrations of dalbavancin in bone are expected to be relevant to treatment of osteomyelitis. Dalbavancin in the serum is 93% protein bound, mostly to albumin, and the bone/plasma ratio is 13%. The drug levels measured in bone are very similar to the calculated simultaneous free drug concentrations of dalbavancin in serum, and as a result, the bone concentrations are expected to be free and available for antimicrobial activity. In addition, the concentrations of dalbavancin in bone were from cortical bone in uninfected subjects with degenerative joint disease. Concentrations in infected bone and potentially in medullary bone may be higher than those measured in these otherwise healthy patients (12, 17, 18).
The concentrations of dalbavancin in other tissues were also assessed. In synovium and synovial fluid, the levels of the drug were even higher than those in bone. Due to the limited number of samples of evaluable synovial fluid, synovial tissue, and skin concentrations, it was not possible to model the time course of dalbavancin concentrations or the penetration ratio for these matrices. However, patients who provided samples for these matrices exhibited significant dalbavancin concentrations, suggesting that dalbavancin does penetrate into these fluids/tissues. The proposed dalbavancin dosing regimens would be anticipated to provide drug in the joint space that would be above the MIC for S. aureus over a 6- to 8-week treatment duration. Similarly, the level in skin at 2 weeks was 13.8 μg/ml, providing additional support for the efficacy of dalbavancin demonstrated in recent clinical trials of acute bacterial skin and skin structure infections (19).
There are limitations to the pharmacokinetic modeling. It should be noted that there were not sufficient data to clearly depict the removal of dalbavancin from bone, given the sparse PK sampling strategy utilized, and, thus, the model parameters should be interpreted with caution. Also, the number of patients at each sampling point for the skin and articular tissues was small. Even so, redistribution of drug from the synovial fluid over time was expected, and the consistency across the measurements in the synovium and skin at each time point was reassuring. Although the study provides evidence of the penetration of dalbavancin into bone, it does not provide evidence of pharmacodynamic activity of dalbavancin in the bone.
These data provide evidence that dalbavancin distributes in the bone, skin, and articular tissue at concentrations that are expected to exceed the MIC for S. aureus for extended periods of time after a significantly shortened dosing regimen. Pharmacokinetic modeling provides direction to the selection of a therapeutic dosing regimen for treatment of infections in these tissues and justifies further study in prospective clinical trials.
ACKNOWLEDGMENTS
We acknowledge the contributions of the subjects who participated in these studies, of Li Zhang, who provided support for the PK modeling, and of Karen Griffith, who oversaw the bone level measurements
The principal investigators for study DUR001-105 are Mark Gottfried (Olympia, WA), William O'Riordan (San Diego, CA), Hayes Williams (Olympia, WA), J. Richard Rhodes (Deland, FL), and Craig R. Sprenger (Fargo, ND).
REFERENCES
- 1.Yeo A, Ramachandran M. 2014. Acute haematogenous osteomyelitis in children. BMJ 348:g66. doi: 10.1136/bmj.g66. [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Ryback MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 3.Fraimow HS. 2009. Systemic antimicrobial therapy in osteomyelitis. Semin Plast Surg 23:90–99. doi: 10.1055/s-0029-1214161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David MZ, Daum RS, Bayer AS, Chambers HF, Fowler VG, Miller LG, Ostrowsky B, Baesa A, Boyle-Vavra S, Eells SJ, Garcia-Houchins S, Gialanella P, Macias-Gil R, Rude TH, Ruffin F, Sieth JJ, Volinski J, Spellberg B. 2014. Staphylococcus aureus bacteremia at 5 US academic medical centers, 2008-2011: significant geographic variation in community-onset infections. Clin Infect Dis 59:798–807. doi: 10.1093/cid/ciu410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnea Y, Carmeli Y, Kuzmenko B, Navon-Venezia S. 2008. Staphylococcus aureus mediastinitis and sternal osteomyelitis following median sternotomy in a rat model. J Antimicrob Chemother 62:1339–1343. doi: 10.1093/jac/dkn378. [DOI] [PubMed] [Google Scholar]
- 6.Garazzino S, Aprato A, Baietto L, D'Avolio A, Maiello A, De Rosa FG, Aloj D, Siccardi M, Biasibetti A, Massè A, Di Perri G. 2008. Glycopeptide bone penetration in patients with septic pseudoarthrosis of the tibia. Clin Pharmacokinet 47:793–805. doi: 10.2165/0003088-200847120-00004. [DOI] [PubMed] [Google Scholar]
- 7.Massias L, Dubois C, de Lentdecker P, Brodaty O, Fischler M, Farinotti R. 1992. Penetration of vancomycin in uninfected sternal bone. Antimicrob Agents Chemother 36:2539–2541. doi: 10.1128/AAC.36.11.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones RN, Sader HS, Flamm RK. 2013. Update of dalbavancin spectrum and potency in the U S A: report from the SENTRY Antimicrobial Surveillance Program (2011). Diagn Microbiol Infect Dis 75:304–307. doi: 10.1016/j.diagmicrobio.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 9.Lazzarini L, Lipsky BA, Mader JT. 2005. Antibiotic treatment of osteomyelitis: what have we learned from 30 years of clinical trials? Int J Infect Dis 9:127–138. doi: 10.1016/j.ijid.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Lopez S, Hackbarth C, Trias Joaquim R G, Jabes D, Goldstein BP. 2005. In vitro antistaphylococcal activity of dalbavancin, a novel glycopeptide. J Antimicrob Chemother 55Suppl S2:ii21–ii24. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein BP, Draghi DC, Sheehan DJ, Hogan P, Sahm DF. 2007. Bactericidal activity and resistance development profiling of dalbavancin. Antimicrob Agents Chemother 51:1150–1154. doi: 10.1128/AAC.00620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feigin RD, Pickering LK, Anderson D, Keeney RE, Shackleford PF. 1975. Clindamycin treatment of osteomyelitis and septic arthritis in children. Paediatrics 55:213–223. [PubMed] [Google Scholar]
- 13.Solon EG, Dowell JA, Lee J, King SP, Damle BD. 2007. Distribution of radioactivity in bone and related structures following administration of [14C]dalbavancin to New Zealand White rabbits. Antimicrob Agents Chemother 51:3008–3010. doi: 10.1128/AAC.00020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Deurenberg P, Wang W, Pietrobelli A, Baumgartner RN, Heymsfield SB. 1999. Hydration of fat-free body mass: new physiological modeling approach. Am J Physiol 276:E995–E1003. [DOI] [PubMed] [Google Scholar]
- 15.Andes D, Craig WA. 2007. In vivo pharmacodynamic activity of the glycopeptide dalbavancin. Antimicrob Agents Chemother 51:1633–1642. doi: 10.1128/AAC.01264-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunne MW, Zhou M, Darpo B. 22 January 2015. A thorough QT study with dalbavancin: a novel lipoglycopeptide antibiotic for the treatment of acute bacterial skin and skin structure infections. Int J Antimicrobial Agents doi: 10.1016/j.ijantimicag.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Baird P, Hughes S, Sullivan M, Willmot I. 1978. Penetration into bones and tissues of clindamycin phosphate. Postgrad Med J 54:65–67. doi: 10.1136/pgmj.54.628.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicholas P, Meyers BR, Levy RN, Hirschman SZ. 1975. Concentration of clindamycin in human bone. Antimicrob Agents Chemother 8:220–221. doi: 10.1128/AAC.8.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. 2014. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med 370:2169–2178. doi: 10.1056/NEJMoa1310480. [DOI] [PubMed] [Google Scholar]