Abstract
Production of the OXA-23 carbapenemase is the most common reason for the increasing carbapenem resistance in Acinetobacter spp. This study was conducted to reveal the genetic basis of blaOXA-23 dissemination in Acinetobacter spp. in China. A total of 63 carbapenem-resistant OXA-23-producing Acinetobacter sp. isolates, representing different backgrounds, were selected from 28 hospitals in 18 provinces for this study. Generally, two patterns of plasmids carrying blaOXA-23 were detected according to S1-nuclease pulsed-field gel electrophoresis and Southern blot hybridization. A ca. 78-kb plasmid, designated pAZJ221, was found in 23 Acinetobacter baumannii and three Acinetobacter nosocomialis isolates, while a novel ca. 50-kb plasmid was carried by only two other A. baumannii isolates. Three of these isolates had an additional copy of blaOXA-23 on the chromosome. Transformation of the two plasmids succeeded, but only pAZJ221 was conjugative. Plasmid pAZJ221 was sequenced completely and found to carry no previously known resistance genes except blaOXA-23. The blaOXA-23 gene of the remaining 35 isolates was chromosome borne. The blaOXA-23 genetic environments were correlated with Tn2009 in 57 isolates, Tn2008 in 5 isolates, and Tn2006 in 1 isolate. The MIC values for the carbapenems with these isolates were not significantly associated with the genomic locations or the copy numbers of blaOXA-23. Overall, these observations suggest that the plasmid pAZJ221 and Tn2009 have effectively contributed to the wide dissemination of blaOXA-23 in Acinetobacter spp. in China and that horizontal gene transfer may play an important role in dissemination of the blaOXA-23 gene.
INTRODUCTION
The increasing trend of carbapenem resistance in bacteria worldwide has become a great concern in recent years since it dramatically limits the range of therapeutic alternatives (1, 2). In Acinetobacter spp., especially Acinetobacter baumannii isolates, the production of carbapenem-hydrolyzing class D β-lactamases, such as OXA-23, OXA-24, and OXA-58, has contributed frequently to carbapenem resistance (1, 3).
The blaOXA-23 gene was first identified in an A. baumannii isolate from Scotland. However, the progenitor of blaOXA-23 was found to be Acinetobacter radioresistens, a nonpathogenic and environmental Acinetobacter species, which was carbapenem susceptible and thus was considered a silent source of blaOXA-23 (4). Now, the blaOXA-23 gene is the most common acquired gene for carbapenem resistance among carbapenem-resistant A. baumannii (CRAB) (3, 5, 6). Moreover, the blaOXA-23 gene has also been detected in non-baumannii Acinetobacter spp. (7, 8). Transposable elements have played an essential role in the dissemination of the blaOXA-23 gene between different genomic locations within one bacterium or between different isolates (5).
Four main transposons, Tn2006, Tn2007, Tn2008, and Tn2009, were found to be associated with the transfer of blaOXA-23 in A. baumannii (5, 9–11). Besides sharing a common region (OXA-23-ΔATPase), all the transposons have ISAba1 upstream from blaOXA-23 except Tn2007, which is correlated with ISAba4 (5, 9–11). Tn2006 and Tn2009 were also identified in non-baumannii species of Acinetobacter (7, 8). Furthermore, the expression of blaOXA-23 has probably been enhanced by the strong promoters provided on the upstream insertion sequences of the transposons (12, 13).
The blaOXA-23 gene has mostly been identified on plasmids, although a chromosomal location has also been reported (5, 10, 14). The complete genome sequencing of Acinetobacter spp. showed that, even for isolates sharing the same genetic background that were collected from the same country and the same time period, the locations of blaOXA-23 can be different (10, 15). This phenomenon suggests that frequent horizontal transmission of this carbapenemase-encoding gene is possible, which is distinct from the simple clonal spread we previously anticipated(14). In addition, whether the location of the blaOXA-23 gene is influential to the susceptibility of a carbapenem is still unknown.
Our previous study showed that production of the OXA-23 carbapenemase was the predominant mechanism contributing to carbapenem resistance in Acinetobacter spp. in China (16, 17). In 2011, we reported the first genome sequence of a multidrug-resistant (MDR) A. baumannii strain in China, MDR-ZJ06, which belonged to clonal complex 92 (CC92) (10). In this strain, blaOXA-23 is carried on the composite transposon Tn2009 and is located on the chromosome. However, the genetic characteristics of blaOXA-23 in other carbapenem-resistant Acinetobacter sp. isolates in China are still unclear. Therefore, this study was performed to investigate the genetic basis of the dissemination of the blaOXA-23 gene in carbapenem-resistant Acinetobacter sp. isolates from different backgrounds in China.
MATERIALS AND METHODS
Bacterial isolates.
Between January 2009 and December 2010, 844 nonrepetitive OXA-23-producing CRAB isolates were collected from 28 hospitals in 18 provinces of China (6). Multilocus sequence typing analysis was performed according to Bartual et al. (6, 18). In addition, six OXA-23-producing Acinetobacter nosocomialis isolates were detected from 20 carbapenem-resistant non-baumannii Acinetobacter spp. in the same period, including Acinetobacter calcoaceticus isolates (9 isolates), A. nosocomialis (8 isolates), Acinetobacter soli (2 isolates), and Acinetobacter haemolyticus (1 isolate). All isolates were identified to the species level by PCR amplification of the blaOXA-51-like gene and sequence analysis of the rpoB gene (19, 20). According to molecular typing and provincial distributions, 60 A. baumannii isolates and 3 A. nosocomialis isolates were selected for further study.
Antimicrobial susceptibility testing.
Susceptibilities to piperacillin, ampicillin-sulbactam, piperacillin-tazobactam, cefepime, ceftazidime, amikacin, gentamicin, ciprofloxacin, aztreonam, and minocycline (Oxoid, United Kingdom) were determined by the disc diffusion method. The MICs for imipenem and meropenem were determined by the broth microdilution method (Oxoid, United Kingdom). Escherichia coli strain ATCC 25922 was used as the quality control. Manipulation and interpretation were in accordance with Clinical and Laboratory Standards Institute (CLSI) 2013 procedures (21).
PFGE and Southern blot analysis.
To determine the plasmid location of the blaOXA-23 gene, genomic DNA digested with S1-nuclease (TaKaRa, Japan) was electrophoresed on a CHEF-mapper XA pulsed-field gel electrophoresis (PFGE) system (Bio-Rad, USA) for 18 h at 14°C with run conditions of 6 V/cm and pulse times from 2.16 s to 63.8 s. The DNA fragments were transferred to a positive-charged nylon membrane (Millipore, USA) and then hybridized with a digoxigenin-labeled blaOXA-23-specific probe. The fragments then were detected using an NBT/BCIP color detection kit (Roche, Germany) (7). In order to identify plasmid patterns, genomic DNA of isolates with the plasmid-borne blaOXA-23 gene, digested with BamHI or EcoRI (TaKaRa, Japan), was separated and hybridized, as described above.
Furthermore, genomic DNA digested by ApaI (TaKaRa, Japan) was electrophoresed and then hybridized, as described above, to reveal the chromosomal locations of the blaOXA-23 gene through comparison of hybridization signals with S1-PFGE. ApaI-digested DNA was electrophoresed with a switch time from 5 to 20 s for 20 h. The XbaI-digested DNA of Salmonella enterica serotype Braenderup H9812 was electrophoresed as the size marker (22).
PCR amplification and DNA sequencing.
PCR mapping was used to detect the occurrence of Tn2006, Tn2007, Tn2008, and Tn2009. The common region of those transposons (OXA-23-ΔATPase) was amplified using primers P3 and P5 (23). PCR targeting of ISAba1 or ISAba4 upstream from blaOXA-23 was performed, as previously described (9, 24). However, PCR mapping for Tn2006 and Tn2009 was performed using different primers (listed in Table 1).
TABLE 1.
Primers designed in this study
| Primer | DNA sequence (5′ to 3′) | Target | Length (bp) |
|---|---|---|---|
| Z5 | CGACTTATTTGATGGCTGACG | Tn2009 | 2,431 |
| Z6 | CTTGTGGATGCAACTCGGTAT | ||
| Z7 | GTAAGGTTGAGCCTGAAGT | Tn2009 | 2,025 |
| Z8 | TTTCTTTCCGATGCTTATTCC | ||
| Z9 | ATGCTCGCAATCGTTTATCGT | Tn2009 | 1,949 |
| Z10 | TCGCCAACTTCTTTGACTTCTG | ||
| Rep-F | ACTCATCAAGGAATAAGACAGC | repAZJ221 | 959 |
| Rep-R | ATCACACTCGCACATACAAT |
Plasmid extraction, sequencing, and analysis.
Plasmid DNA was extracted using a Qiagen plasmid midi kit (Qiagen, Germany). The plasmid DNA was further sequenced using HiSeq 2000 (Illumina, Inc., USA) technology following the 2× 100-bp paired-end protocol. The derived reads were assembled using the Velvet program version 1.1 (25). Gaps were filled by primer walking using plasmid DNA as a template. The plasmid sequence was annotated by the RAST server (26), and all of the predicted proteins were further compared against the NCBI nonredundant protein database using the BLASTP program. The CGview server was used to generate a circular map of plasmid pAZJ221 (27). In addition, plasmid types were identified by PCR-based replicon typing according to the A. baumannii PCR-based replicon typing (AB-PBRT) method (28).
Transferability of the blaOXA-23 gene.
Filter mating was performed using a spontaneous rifampin-resistant mutant of Acinetobacter baylyi ADP1 (ADP1-rifr) as the recipient strain (7). The transconjugants were selected based on growth on agar supplemented with imipenem (2 mg/liter) and rifampin (256 mg/liter).
The plasmid DNA was extracted as previously described and then electrotransformed into A. baumannii ATCC 17978 (7). The transformants were selected on agar plates containing imipenem (2 mg/liter). The transconjugants and transformants were confirmed as blaOXA-23 positive by PCR analysis.
Nucleotide sequence accession number.
The nucleotide sequence of plasmid pAZJ221 has been submitted to the EMBL/GenBank database under the accession number KM922672.
RESULTS AND DISCUSSION
Bacterial isolates and susceptibility testing.
The 60 OXA-23-producing CRAB isolates that were selected for further study belonged to 36 different sequence types (STs) (6). Forty-five of the CRAB isolates were grouped into CC92, and ST92, ST138, and ST75 were the most common STs. The other 15 CRAB isolates were of diverse genetic backgrounds, and only 3 of them belonged to the same CC (CC254). The three OXA-23-producing carbapenem-resistant A. nosocomialis (CRAN) isolates, which were collected from three hospitals in two provinces, belonged to three different PFGE patterns and were included in this study.
Among the 63 isolates, the MIC values for meropenem ranged from 32 to 256 mg/liter, and the imipenem MICs ranged from 16 to 128 mg/liter. All of the isolates showed high resistance rates (>90%), except to ceftazidime (82.5%), to the penicillins, monobactams, quinolones, and extended-spectrum cephalosporins. The rates of resistance to gentamicin and amikacin were 85.7% and 71.4%, respectively. Minocycline (66.7% susceptibility) was the most active antimicrobial tested.
Location of the blaOXA-23 gene.
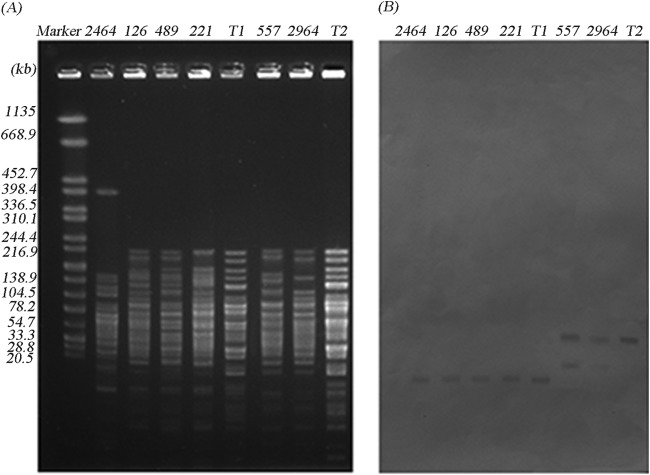
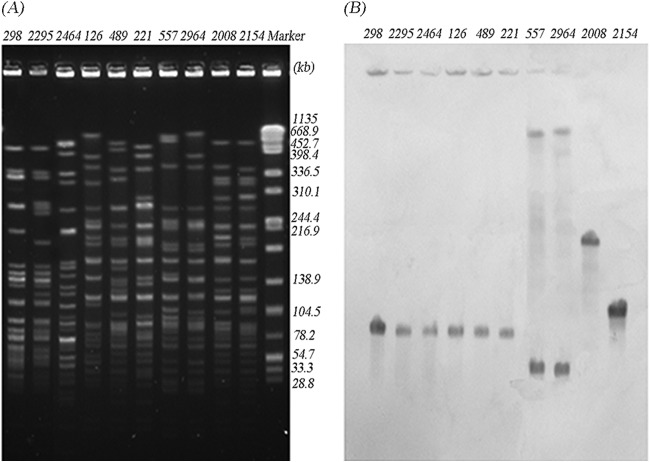
Acinetobacter radioresistens was found to be the progenitor of blaOXA-23, and plasmids played an essential role in the mobilization process of blaOXA-23 from A. radioresistens to A. baumannii (4). By S1-nuclease digestion and Southern blot analysis, the blaOXA-23 gene was found to be plasmid encoded in 25 CRAB isolates and in all 3 CRAN isolates in this study. According to the hybridization signals of CRAB, the blaOXA-23 gene was located on ca. 78-kb plasmids in 23 isolates and on ca. 50-kb plasmids in 2 isolates, assigned into 15 STs and 2 STs, respectively. Interestingly, three CRAN isolates were found to be associated with the same ca. 78-kb plasmid as well (Fig. 1). Furthermore, digestion by BamHI (Fig. 2) or EcoRI (not shown) classified these plasmids into two distinct patterns, suggesting that these blaOXA-23-carrying plasmids belonged to only two plasmid types.
FIG 1.
Analysis of the localization of blaOXA-23 using the S1 nuclease-PFGE method. Shown are PFGE profiles after S1 nuclease digestion (A) and Southern blot hybridization with a blaOXA-23 probe (B). The 10 isolates displayed were clustered into one of four groups: Acinetobacter nosocomialis isolates (298, 2295, and 2464), A. baumannii isolates carrying the 78-kb plasmid (126, 489, and 221), A. baumannii isolates carrying the 50-kb plasmid (557 and 2964), and A. baumannii isolates of the same clone with chromosomal blaOXA-23 (2008 and 2154). Salmonella enterica serotype Braenderup strain H9812 DNA digested by XbaI was used as a molecular marker (in kb).
FIG 2.
PFGE files of BamHI-digested genomic DNA of isolates with plasmid-borne blaOXA-23 (A) and Southern blot hybridization with a blaOXA-23 probe (B). T1 and T2 indicate the transformants associated with the 78-kb and 50-kb plasmids, respectively. The two isolates carrying the 50-kb plasmid have an additional copy of blaOXA-23 on the chromosome; thus, the donors have one more hybridization signal than the transformants. Detailed information of other isolates is given in the Fig. 1 legend. Salmonella enterica serotype Braenderup strain H9812 DNA digested by XbaI was used as a molecular marker (in kb).
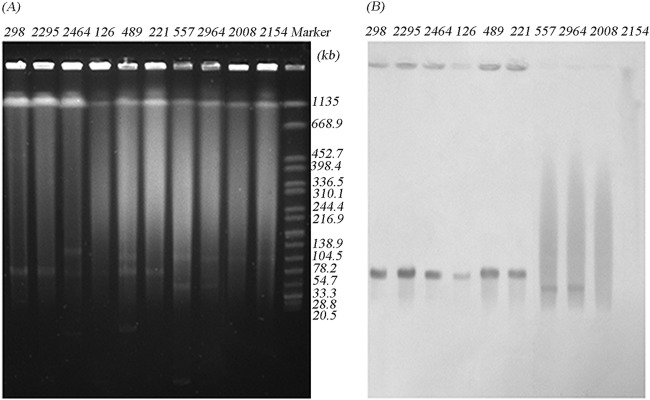
To reveal the chromosomal location of blaOXA-23, ApaI-PFGE and hybridization were performed on all 63 isolates. Through comparison with the hybridization signals of S1-PFGE, ApaI-PFGE and hybridization with the probe of blaOXA-23 revealed that 38 isolates harbored the blaOXA-23 gene on the chromosome (Fig. 3). Chromosomal locations of blaOXA-23 make it less likely for the bacteria to lose carbapenem resistance. Therefore, clonal spread might make the dissemination of blaOXA-23 wider. Moreover, the carbapenem resistance of Acinetobacter spp. will still exist for a long period of time, even without the antimicrobial selective pressure found in hospital settings. In particular, four of the isolates harbored two chromosomal copies of the blaOXA-23 gene on different fragments. Three isolates had one copy on the chromosome and another copy on a plasmid; in two of these three isolates, the 50-kb plasmid carried blaOXA-23, which had an additional hybridization band compared to their transformants examined through BamHI/EcoRI-PFGE and hybridization (Fig. 2). In the 45 CC92 isolates, the PFGE bands were similar, as expected, but the blaOXA-23 hybridization signals appeared at different bands, ranging from ca. 33.3 kb to 336.5 kb. Different insertion sites of blaOXA-23 on the chromosomal fragments can be considered another piece of evidence that supports horizontal gene transfer instead of clonal spread. Details about the locations and genetic surroundings of blaOXA-23 are shown in Table 2.
FIG 3.
Analysis of the localization of blaOXA-23 using the ApaI-PFGE method. Shown are PFGE profiles after ApaI digestion (A) and Southern blot hybridization with a blaOXA-23 probe (B). The 10 isolates displayed are in the same order as in Fig. 1. Salmonella enterica serotype Braenderup strain H9812 DNA digested by XbaI was used as a molecular marker (in kb).
TABLE 2.
Features of the blaOXA-23 gene
| Species | Molecular pattern | Isolate no. | Provincea | Transposon | Location | Copy no. |
|---|---|---|---|---|---|---|
| A. baumannii | ST75 (CC92) | 6 | XJ, BJ | Tn2009 | Plasmid, 78 kb | 1 |
| HB | Tn2009 | Plasmid, 50 kb | 1 | |||
| Chromosome | 1 | |||||
| GD, SH | Tn2009 | Chromosome | 1 | |||
| SC | Tn2008 | Chromosome | 1 | |||
| ST76 (CC92) | 1 | HLJ | Tn2009 | Chromosome | 1 | |
| ST88 (CC92) | 1 | SC | Tn2008 | Chromosome | 1 | |
| ST90 (CC92) | 2 | SH, HB | Tn2009 | Chromosome | 1 | |
| ST91 | 3 | XJ, ZJ, HN1 | Tn2009 | Chromosome | 1 | |
| ST92 (CC92) | 7 | XJ, BJ, HLJ, NMG | Tn2009 | Plasmid, 78 kb | 1 | |
| HB, ZJ | Tn2009 | Chromosome | 1 | |||
| SC | Tn2008 | Chromosome | 1 | |||
| ST118 (CC92) | 1 | SX1 | Tn2009 | Plasmid, 78 kb | 1 | |
| ST136 (CC92) | 1 | GD | Tn2009 | Plasmid, 50 kb | 1 | |
| Chromosome | 1 | |||||
| ST137 (CC92) | 1 | HLJ | Tn2009 | Plasmid, 78 kb | 1 | |
| ST138 (CC92) | 7 | LN, SC, HLJ | Tn2009 | Plasmid, 78 kb | 1 | |
| XJ | Tn2009 | Plasmid, 78 kb | 1 | |||
| Chromosome | 1 | |||||
| ZJ, HB, BJ | Tn2009 | Plasmid, 78 kb | 1 | |||
| ST189 (CC92) | 1 | XJ | Tn2009 | Plasmid, 78 kb | 1 | |
| ST223 (CC92) | 1 | HN1 | Tn2009 | Plasmid, 78 kb | 1 | |
| ST254 | 1 | GD | Tn2009 | Chromosome | 2 | |
| ST346 (CC92) | 1 | SX1 | Tn2009 | Chromosome | 2 | |
| ST365 (CC92) | 1 | GD | Tn2006 | Chromosome | 1 | |
| ST381 (CC92) | 4 | GD, SC, ZJ | Tn2009 | Chromosome | 1 | |
| HB | Tn2009 | Chromosome | 2 | |||
| ST395 (CC92) | 1 | ZJ | Tn2009 | Chromosome | 1 | |
| ST492 (CC92) | 1 | AH | Tn2009 | Chromosome | 1 | |
| ST517 | 1 | GD | Tn2008 | Chromosome | 1 | |
| ST520 | 2 | BJ, HLJ | Tn2009 | Plasmid, 78 kb | 1 | |
| ST522 | 1 | XJ | Tn2009 | Chromosome | 1 | |
| ST523 (CC92) | 1 | XJ | Tn2009 | Chromosome | 1 | |
| ST524 | 1 | HN1 | Tn2009 | Plasmid, 78 kb | 1 | |
| ST525 (CC92) | 1 | SX | Tn2009 | Plasmid, 78 kb | 1 | |
| ST526 | 1 | NMG | Tn2009 | Plasmid, 78 kb | 1 | |
| ST527 (CC92) | 1 | HN | Tn2009 | Plasmid, 78 kb | 1 | |
| ST528 | 1 | SH | Tn2009 | Chromosome | 1 | |
| ST529 | 1 | JS | Tn2009 | Chromosome | 1 | |
| ST531 | 1 | GD | Tn2008 | Chromosome | 2 | |
| ST532 (CC92) | 1 | BJ | Tn2009 | Plasmid, 78 kb | 1 | |
| ST533 (CC92) | 1 | GD | Tn2009 | Chromosome | 1 | |
| ST668 | 1 | SD | Tn2009 | Plasmid, 78 kb | 1 | |
| ST669 (CC92) | 1 | HLJ | Tn2009 | Plasmid, 78 kb | 1 | |
| ST670 (CC92) | 1 | HN1 | Tn2009 | Chromosome | 1 | |
| ST671 | 1 | JX | Tn2009 | Chromosome | 1 | |
| ST736 (CC92) | 1 | SH | Tn2009 | Chromosome | 1 | |
| A. nosocomialis | PFGE clone A | 1 | BJ | Tn2009 | Plasmid, 78 kb | 1 |
| PFGE clone B | 1 | BJ | Tn2009 | Plasmid, 78 kb | 1 | |
| PFGE clone C | 1 | HN1 | Tn2009 | Plasmid, 78 kb | 1 |
AH, Anhui; BJ, Beijing; GD, Guangdong; HB, Hubei; HLJ, Heilongjiang; HN, Hunan; HN1, Henan; LN, Liaoning; JS, Jiangsu; JX, Jiangxi; NMG, Inner Mongolia; SC, Sichuan; SD, Shandong; SH, Shanghai; SX, Shanxi; SX1, Shaanxi; XJ, XinJiang; ZJ, Zhejiang.
The carbapenem resistance of isolates with different locations or copy numbers of blaOXA-23 was further studied. The 63 isolates were divided into three groups according to the locations of the blaOXA-23 gene, i.e., plasmid, chromosome, and both locations (plasmid and chromosome). The MIC50 and MIC90 values for meropenem and imipenem fluctuated between 64 and 128 mg/liter in the three groups. In addition, for isolates with only one copy of the blaOXA-23 gene, the MIC50 and MIC90 values for the two carbapenems tested were 64 and 128 mg/liter, respectively. For isolates with two copies of the blaOXA-23 gene, the MIC50 and MIC90 for meropenem were 64 and 128 mg/liter, respectively; however, the two values for imipenem were the same (64 mg/liter). These facts revealed that the carbapenem susceptibility was not significantly different among isolates carrying blaOXA-23 on different genetic locations. Moreover, the increased number of copies of blaOXA-23 does not obviously change the carbapenem susceptibility. Different locations or different copies of blaOXA-23 may only be pieces of evidence revealing the evolution process.
Genetic environment of the blaOXA-23 gene.
In this study, we found that 54 of 60 CRAB and all three CRAN isolates were associated with Tn2009, while five CRAB isolates were correlated with Tn2008. Tn2009 was detected in all 18 provinces included in this study, while Tn2008 was found only in the Beijing, Sichuan, and Guangdong provinces. In addition, one isolate of ST365, collected from the Guangdong province, carried chromosome-borne blaOXA-23 on Tn2006. Tn2007 was not detected in these isolates.
Tn2009 and Tn2008 have played dominant roles in the dissemination of blaOXA-23 in China (10, 29). However, Tn2006 has been the most common transposon associated with OXA-23-producing A. baumannii in many countries (5). The genetic structures of Tn2009 and Tn2006 were similar, but Tn2009 has an additional 2-kb segment between the DEAD/DEAH box helicase gene and the ATPase gene, and the orientations of ISAba1 are different (10). In this study, the dissemination of Tn2008 was limited in three provinces, which might be due to its chromosomal location. In addition, the target site duplications were different among those transposons; thus, the blaOXA-23 gene can be inserted into different locations in isolates with the same genetic background (10). These facts suggest that isolates of Acinetobacter spp. acquire carbapenem resistance independently under antimicrobial selective pressure in different environments. Moreover, the detection of common transposable elements in isolates of different molecular patterns revealed the horizontal transfer of the blaOXA-23 gene.
Plasmid analysis.
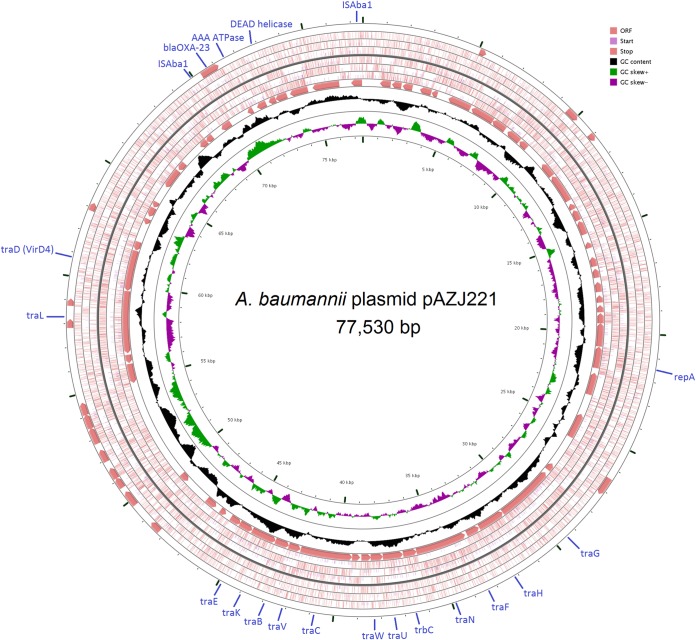
As the most common vehicle carrying the blaOXA-23 gene in China, the ca. 78-kb plasmid designated pAZJ221 was sequenced and then analyzed. In silico analysis showed that the plasmid pAZJ221 was 77,530 bp in size and contained 108 open reading frames (ORFs), with an average G+C content of 34.03%. The plasmid consisted of two main regions, a complete array of genes associated with conjugation and the composite transposon Tn2009 (Fig. 4). Moreover, this plasmid did not contain cleavage sites for the restriction endonuclease ApaI. Further analysis indicated that this plasmid shared 99% nucleotide identity with a previously described plasmid, pABTJ1, which was carried by A. baumannii MDR-TJ of global clone 2, isolated from Tianjin, China (30). These two plasmids were associated with the same replicase gene, which was designated repAZJ221 in this study, and shared 67% identity with the replicase gene of pACICU2 (30). No resistance genes other than blaOXA-23 were found in pAZJ221.
FIG 4.
Circular map of plasmid pAZJ221. The two inner circles indicate the G+C content plotted against the average G+C content of 34.03% (black circle) and GC skew information (green and purple circles). The outer circles display the open reading frames (ORFs) in opposite orientations. Regions related to conjugation, replication, and Tn2009 are marked in blue.
Neither of the plasmids were classified into any previously known replicon group using the current scheme; therefore, a pair of primers, which were added to the AB-PBRT system, was designed in this study to amplify a 959-bp region of the replicase gene of pAZJ221. Furthermore, all isolates carrying the ca. 78-kb plasmid were confirmed as carrying the same replicase gene, repAZJ221. To detect A. baumannii resistance plasmids more fully, the replicase gene associated with pAZJ221, which may represent a new homology group (GR20), was added into the multiplex 4 of the AB-PBRT system. Moreover, we were still unable to type the 50-kb blaOXA-23-carrying plasmid, which indicates that it represented a new replicon group.
Transferability of the blaOXA-23 gene.
The plasmid pAZJ221 was successfully transferred to A. baylyi ADP1-rifr through conjugation, while transfer of the 50-kb plasmid failed. The two plasmids were successfully transformed into A. baumannii ATCC 17978 through electroporation. Except for susceptibility to ceftazidime, all transformants and transconjugants had a β-lactam resistance pattern consistent with their donors, with imipenem and meropenem MICs of ≥32 mg/liter (Table 3). These isolates were susceptible to the aminoglycosides and quinolones tested. Thus, from the susceptibility profile of the transformants, we deduced that the 50-kb plasmid might also carry no resistance genes except blaOXA-23. Furthermore, the ca. 50-kb plasmid was detected in two isolates, probably indicating that the mobility of this nonconjugative plasmid was limited.
TABLE 3.
Antibiotic susceptibilities of representative isolates with plasmid-borne blaOXA-23 and their transconjugants and/or transformants
| Isolate | Antibiotica susceptibility by: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/liter) |
Inhibition zone (mm) |
|||||||||||
| MEM | IPM | ATM | CAZ | FEP | PRL | SAM | TZP | AK | CIP | CN | MH | |
| A221b | 128 | 64 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 16 |
| A221-ADP1-rifr | 64 | 64 | 18 | 23 | 14 | 6 | 6 | 6 | 26 | 29 | 27 | 27 |
| A221-17978 | 64 | 32 | 13 | 19 | 10 | 6 | 6 | 6 | 20 | 27 | 19 | 23 |
| A2964b | 64 | 64 | 6 | 6 | 6 | 6 | 6 | 9 | 6 | 10 | 6 | 14 |
| A2964-17978 | 64 | 64 | 13 | 18 | 12 | 6 | 6 | 11 | 20 | 24 | 19 | 22 |
| ADP1-rifr | 0.094 | 0.094 | 18 | 25 | 25 | 24 | 24 | 28 | 27 | 30 | 27 | 24 |
| ATCC 17978 | 0.38 | 0.25 | 15 | 18 | 20 | 20 | 20 | 24 | 21 | 25 | 18 | 21 |
MEM, meropenem; IPM, imipenem; ATM, aztreonam; CAZ, ceftazidime; FEP, cefepime; PRL, piperacillin; SAM, ampicillin-sulbactam; TZP, piperacillin-tazobactam; AK, amikacin; CIP, ciprofloxacin; CN, gentamicin; MH, minocycline.
A221 and A2964 carried the 78- and 50-kb plasmids, respectively.
In conclusion, the conjugative plasmid pAZJ221 and Tn2009 may play important roles in the intra- and interspecies transfer process and further integration of blaOXA-23 in China. Such transposable elements might make the dissemination of blaOXA-23 easier.
ACKNOWLEDGMENTS
This work was supported by the State Key Program of National Natural Science of China (grant 81230039) and the Young Scholars of National Natural Science Foundation of China (grants 81301459 and 81401698).
REFERENCES
- 1.Poirel L, Nordmann P. 2006. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect 12:826–836. doi: 10.1111/j.1469-0691.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- 2.Gupta N, Limbago BM, Patel JB, Kallen AJ. 2011. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 53:60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 3.Walther-Rasmussen J, Hoiby N. 2006. OXA-type carbapenemases. J Antimicrob Chemother 57:373–383. doi: 10.1093/jac/dki482. [DOI] [PubMed] [Google Scholar]
- 4.Poirel L, Figueiredo S, Cattoir V, Carattoli A, Nordmann P. 2008. Acinetobacter radioresistens as a silent source of carbapenem resistance for Acinetobacter spp. Antimicrob Agents Chemother 52:1252–1256. doi: 10.1128/AAC.01304-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mugnier PD, Poirel L, Naas T, Nordmann P. 2010. Worldwide dissemination of the blaOXA-23 carbapenemase gene of Acinetobacter baumannii. Emerg Infect Dis 16:35–40. doi: 10.3201/eid1601.090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan Z, Chen Y, Jiang Y, Zhou H, Zhou Z, Fu Y, Wang H, Wang Y, Yu Y. 2013. Wide distribution of CC92 carbapenem-resistant and OXA-23-producing Acinetobacter baumannii in multiple provinces of China. Int J Antimicrob Agents 42:322–328. doi: 10.1016/j.ijantimicag.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Z, Du X, Wang L, Yang Q, Fu Y, Yu Y. 2011. Clinical carbapenem-resistant Acinetobacter baylyi strain coharboring blaSIM-1 and blaOXA-23 from China. Antimicrob Agents Chemother 55:5347–5349. doi: 10.1128/AAC.00425-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DH, Choi JY, Jung SI, Thamlikitkul V, Song JH, Ko KS. 2012. AbaR4-type resistance island, including the blaOXA-23 gene in Acinetobacter nosocomialis isolates. Antimicrob Agents Chemother 56:4548–4549. doi: 10.1128/AAC.00923-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corvec S, Poirel L, Naas T, Drugeon H, Nordmann P. 2007. Genetics and expression of the carbapenem-hydrolyzing oxacillinase gene blaOXA-23 in Acinetobacter baumannii. Antimicrob Agents Chemother 51:1530–1533. doi: 10.1128/AAC.01132-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou H, Zhang T, Yu D, Pi B, Yang Q, Zhou J, Hu S, Yu Y. 2011. Genomic analysis of the multidrug-resistant Acinetobacter baumannii strain MDR-ZJ06 widely spread in China. Antimicrob Agents Chemother 55:4506–4512. doi: 10.1128/AAC.01134-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams-Haduch JM, Paterson DL, Sidjabat HE, Pasculle AW, Potoski BA, Muto CA, Harrison LH, Doi Y. 2008. Genetic basis of multidrug resistance in Acinetobacter baumannii clinical isolates at a tertiary medical center in Pennsylvania. Antimicrob Agents Chemother 52:3837–3843. doi: 10.1128/AAC.00570-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mugnier PD, Poirel L, Nordmann P. 2009. Functional analysis of insertion sequence ISAba1, responsible for genomic plasticity of Acinetobacter baumannii. J Bacteriol 191:2414–2418. doi: 10.1128/JB.01258-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turton JF, Ward ME, Woodford N, Kaufmann ME, Pike R, Livermore DM, Pitt TL. 2006. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol Lett 258:72–77. doi: 10.1111/j.1574-6968.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhou H, Yang Q, Yu YS, Wei ZQ, Li LJ. 2007. Clonal spread of imipenem-resistant Acinetobacter baumannii among different cities of China. J Clin Microbiol 45:4054–4057. doi: 10.1128/JCM.00343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CC, Lin YC, Sheng WH, Chen YC, Chang SC, Hsia KC, Liao MH, Li SY. 2011. Genome sequence of a dominant, multidrug-resistant Acinetobacter baumannii strain, TCDC-AB0715. J Bacteriol 193:2361–2362. doi: 10.1128/JB.00244-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji S, Chen Y, Ruan Z, Fu Y, Ji J, Fu Y, Wang H, Yu Y. 2014. Prevalence of carbapenem-hydrolyzing class D beta-lactamase genes in Acinetobacter spp. isolates in China. Eur J Clin Microbiol Infect Dis 33:989–997. doi: 10.1007/s10096-013-2037-z. [DOI] [PubMed] [Google Scholar]
- 17.Fu Y, Zhou J, Zhou H, Yang Q, Wei Z, Yu Y, Li L. 2010. Wide dissemination of OXA-23-producing carbapenem-resistant Acinetobacter baumannii clonal complex 22 in multiple cities of China. J Antimicrob Chemother 65:644–650. doi: 10.1093/jac/dkq027. [DOI] [PubMed] [Google Scholar]
- 18.Bartual SG, Seifert H, Hippler C, Luzon MA, Wisplinghoff H, Rodriguez-Valera F. 2005. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J Clin Microbiol 43:4382–4390. doi: 10.1128/JCM.43.9.4382-4390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turton JF, Woodford N, Glover J, Yarde S, Kaufmann ME, Pitt TL. 2006. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J Clin Microbiol 44:2974–2976. doi: 10.1128/JCM.01021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gundi VA, Dijkshoorn L, Burignat S, Raoult D, La Scola B. 2009. Validation of partial rpoB gene sequence analysis for the identification of clinically important and emerging Acinetobacter species. Microbiology 155:2333–2341. doi: 10.1099/mic.0.026054-0. [DOI] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing, 23rd informational supplement. CLSI M100-S23 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 22.Hunter SB, Vauterin P, Lambert-Fair MA, Van Duyne MS, Kubota K, Graves L, Wrigley D, Barrett T, Ribot E. 2005. Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: converting the national databases to the new size standard. J Clin Microbiol 43:1045–1050. doi: 10.1128/JCM.43.3.1045-1050.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee MH, Chen TL, Lee YT, Huang L, Kuo SC, Yu KW, Hsueh PR, Dou HY, Su IJ, Fung CP. 2013. Dissemination of multidrug-resistant Acinetobacter baumannii carrying blaOXA-23 from hospitals in central Taiwan. J Microbiol Immunol Infect 46:419–424. doi: 10.1016/j.jmii.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Zhou H, Pi BR, Yang Q, Yu YS, Chen YG, Li LJ, Zheng SS. 2007. Dissemination of imipenem-resistant Acinetobacter baumannii strains carrying the ISAba1 blaOXA-23 genes in a Chinese hospital. J Med Microbiol 56:1076–1080. doi: 10.1099/jmm.0.47206-0. [DOI] [PubMed] [Google Scholar]
- 25.Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 18:821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. 2008. The RAST server: Rapid Annotations using Subsystems Technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant JR, Stothard P. 2008. The CGView server: a comparative genomics tool for circular genomes. Nucleic Acids Res 36:W181-W184. doi: 10.1093/nar/gkn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertini A, Poirel L, Mugnier PD, Villa L, Nordmann P, Carattoli A. 2010. Characterization and PCR-based replicon typing of resistance plasmids in Acinetobacter baumannii. Antimicrob Agents Chemother 54:4168–4177. doi: 10.1128/AAC.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Zong Z, Lu X. 2011. Tn2008 is a major vehicle carrying blaOXA-23 in Acinetobacter baumannii from China. Diagn Microbiol Infect Dis 69:218–222. doi: 10.1016/j.diagmicrobio.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Huang H, Yang ZL, Wu XM, Wang Y, Liu YJ, Luo H, Lv X, Gan YR, Song SD, Gao F. 2012. Complete genome sequence of Acinetobacter baumannii MDR-TJ and insights into its mechanism of antibiotic resistance. J Antimicrob Chemother 67:2825–2832. doi: 10.1093/jac/dks327. [DOI] [PubMed] [Google Scholar]