Abstract
Ceftazidime and the β-lactamase inhibitor avibactam constitute a new, potentially highly active combination in the battle against extended-spectrum-β-lactamase (ESBL)-producing bacteria. To determine possible clinical use, it is important to know the pharmacokinetic profiles of the compounds related to each other in plasma and the different compartments of infection in experimentally infected animals and in humans. We used a neutropenic murine thigh infection model and lung infection model to study pharmacokinetics in plasma and epithelial lining fluid (ELF). Mice were infected with ca. 106 CFU of Pseudomonas aeruginosa intramuscularly into the thigh or intranasally to cause pneumonia and were given 8 different (single) subcutaneous doses of ceftazidime and avibactam in various combined concentrations, ranging from 1 to 128 mg/kg of body weight in 2-fold increases. Concomitant samples of serum and bronchoalveolar lavage fluid were taken at up to 12 time points until 6 h after administration. Pharmacokinetics of both compounds were linear and dose proportional in plasma and ELF and were independent of the infection type, with estimated half-lives (standard deviations [SD]) in plasma of ceftazidime of 0.28 (0.02) h and of avibactam of 0.24 (0.04) h and volumes of distribution of 0.80 (0.14) and 1.18 (0.34) liters/kg. The ELF-plasma (area under the concentration-time curve [AUC]) ratios (standard errors [SE]) were 0.24 (0.03) for total ceftazidime and 0.27 (0.03) for unbound ceftazidime; for avibactam, the ratios were 0.20 (0.02) and 0.22 (0.02), respectively. No pharmacokinetic interaction between ceftazidime and avibactam was observed. Ceftazidime and avibactam showed linear plasma pharmacokinetics that were independent of the dose combinations used or the infection site in mice. Assuming pharmacokinetic similarity in humans, this indicates that similar dose ratios of ceftazidime and avibactam could be used for different types and sites of infection.
INTRODUCTION
Ceftazidime (CAZ) is a potent β-lactam antibiotic against Gram-negative bacteria in particular (1). However, since more and more Gram-negative bacteria have emerged that carry extended-spectrum β-lactamases (ESBLs) (2, 3) and class C β-lactamases (4), resistance has led to difficulty in identifying β-lactam therapies that would minimize the risk of resistance-related failure (5). Moreover, Klebsiella pneumoniae carbapenemase (KPC) and OXA-48 carbapenemase are narrowing treatment options against Gram-negative bacteria even further (6–8). For this reason, alternatives have been sought. The use of β-lactamase inhibitors seems to be a reasonable approach, and combinations consisting of a β-lactam agent and a β-lactamase inhibitor, such as piperacillin-tazobactam and amoxicillin-clavulanic acid, are widely used. AstraZeneca and Actavis (formerly Forest-Cerexa) are developing a combination of ceftazidime (CAZ) with avibactam, a new promising β-lactamase inhibitor, to overcome resistance caused by β-lactamases (9, 10). This combination has an extended spectrum of activity and is active against Ambler class A extended-spectrum β-lactamases (ESBLs), KPC class A enzymes, class C (AmpC) enzymes, and some class D enzymes (11–15). In vitro studies have shown that the drug MIC values for resistant clinical isolates, including Pseudomonas aeruginosa, were drastically reduced in the presence of this inhibitor and that the isolates thereby became susceptible to ceftazidime (11, 16–19). Activity of the inhibitor in vivo has been shown as well (see, e.g., reference 13).
As pneumonia is one of the leading causes of death in humans (20) and treatments using several new compounds have failed in patients with lower respiratory tract infections (21, 22), it is important to understand the mechanism of activity, including the pharmacokinetic/pharmacodynamic (PK/PD) properties of the drugs used for this indication. For the combination ceftazidime-avibactam, concentrations of ceftazidime and avibactam in the lungs relative to each other might be different from the relative concentrations in plasma and therefore might result in bacterial responses in lung infection that are different from those in infections in other tissues.
In the present study, we determined the pharmacokinetics of ceftazidime and avibactam and concentration-time profiles of the two compounds relative to each other in plasma and epithelial lining fluid (ELF) of infected neutropenic mice. Both thigh infection and lung infection models were used, to determine whether different kinds of infections would have different impacts on the pharmacokinetic profiles of each compound. The pharmacokinetic parameter estimates and the penetration of the two compounds reported in this study are intended to serve as a basis to determine exposure-response relationships.
(The results of this study were presented in part at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Denver, CO, 10 to 13 September 2013.)
MATERIALS AND METHODS
Drugs.
Ceftazidime (CAZ; lot no. G263770; sodium carbonate blend; potency, 77.2%) and avibactam (AVI; lot no. AFCH005151 [07113P028]; potency, 91.7%) were provided by AstraZeneca Pharmaceuticals LP, Waltham, MA, USA. The drugs were reconstituted in sterile water to a stock solution of 5,120 mg/liter, and further solutions were prepared in Mueller-Hinton broth (Difco/Brunschwig Chemie, Amsterdam, The Netherlands).
Bacterial strains.
Two P. aeruginosa strains (strains 7 and 19) were used in the experiments. Both had ceftazidime MICs of 64 mg/liter and ceftazidime-avibactam MICs of 4 mg/liter (with AVI at 4 mg/liter) as defined in earlier checkerboard experiments (23).
Animals.
Outbred female CD-1 mice (Charles River, The Netherlands), 7 to 8 weeks old and weighing 20 to 25 g, were used in the experiments. Neutropenia was induced by two doses of cyclophosphamide injected intraperitoneally 4 days (150 mg/kg of body weight) and 1 day (100 mg/kg) before inoculation. The animals were housed under standard conditions with drink and feed supplied ad libitum and were examined once daily before and 2 to 3 times per day after immunosuppression. The animal studies were conducted in accordance with the recommendations of the European Community (Directive 86/609/EEC, 24 November 1986), and all animal procedures were approved by the Animal Welfare Committee of Radboud University (RU-DEC 2012-003).
Infection model and treatment.
Pharmacokinetics were determined in a thigh infection model (2 P. aeruginosa strains per animal, one inoculated in the left thigh and the other in the right) and a lung infection model (1 strain per animal). In both cases, 0.05 ml of a bacterial suspension consisting of approximately 106 to 107 bacteria was inoculated intramuscularly (thigh) or intranasally with a syringe, the latter under conditions of light anesthesia with isoflurane. Ceftazidime and avibactam were subcutaneously administered 2 h after infection with a single dose of 0.1 ml.
Eight dose combinations were used. For the thigh-infected animals, the combinations of ceftazidime and avibactam were 16/4, 8/1, 64/32, and 2/128 mg/kg. For the lung-infected mice, combinations of 32/16, 4/2, 128/8, and 1/64 mg/kg of the respective constituents were used. These combinations were chosen in order to detect possible pharmacokinetic interactions between the two compounds and to cover a wide range of doses of each compound.
Concomitant samples of serum and bronchoalveolar lavage (BAL) fluid were taken at 12 time points before (0 min) and after (5, 10, 20, 30, 45, 60, 90, 120, 180, 240, and 360 min) administration of the combination of ceftazidime and avibactam. Bronchoalveolar lavage (BAL) fluid and blood were obtained immediately after mice were humanely sacrificed. ELF was obtained using a technique described previously (24). In short, after mice were sacrificed under conditions of isoflurane anesthesia followed by cervical dislocation, they were secured on a plastic platform and the trachea was exposed by a 1-cm-long incision on the ventral neck skin for insertion of the cannula, which was sutured in place. Lungs were instilled 4 times with 0.5 ml of sterile 0.9% saline solution, and the fluid was aspirated immediately. The aspirates thus recovered were pooled, directly placed on ice, and subsequently stored at −80°C.
Antibiotic concentration measurements.
Plasma was separated from blood using a cooled centrifuge. Samples were split and stored at −80°C. Concentrations of ceftazidime and avibactam were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) as described previously in detail (25), with lower limits of quantitation (LLQ) of 1.5 ng/ml for ceftazidime and 1.8 ng/ml for avibactam. For ceftazidime, the accuracy in plasma was 96.9%, with 2.53% precision (percent relative standard deviation [%RSD]); the corresponding values in ELF were 100.4% and 3.84%, respectively. For avibactam, these values were 106.4% accuracy with 7.33% precision in plasma and 98.7% accuracy with 6.57% precision in ELF. Protein binding in plasma was 10% for ceftazidime as described before (26) and seen in previous studies at our department (not published); it was 8% for avibactam as determined in the equilibrium dialysis chamber and analyzed via LC-MS/MS (27, 28). In ELF, protein binding was considered negligible (29).
Concentrations of ceftazidime and avibactam in ELF were determined by using the ratio of the urea concentration in BAL fluid to the concentration in plasma as measured with a modified enzymatic assay (QuantiChrom urea assay kit DIUR-500; BioAssay Systems).
The apparent ELF volume was estimated by using urea as an endogenous marker of ELF dilution and was calculated as described previously (24, 30, 31). The drug concentration in ELF was subsequently calculated as follows: drug concentrationELF = drug concentrationBAL fluid × urea concentrationplasma/urea concentrationBAL fluid.
Pharmacokinetic analysis.
Concentrations of ceftazidime and avibactam in both plasma and ELF were plotted against time using Graphpad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). PK parameters were estimated using Phoenix WinNonlin 2.1 (Certara, St. Louis, MO, USA). Noncompartmental and one- and two-compartment models were explored. The AUC was calculated using the log-linear trapezoidal rule without extrapolation to infinity because of the very low detection limit. Dose proportionality was determined following the standard methods by determining the relationship between log (dose) and log (AUC) in both the lung model and the thigh model following the power model approach. Significant differences in pharmacokinetics between the thigh model and the lung model were tested by comparing the slopes and the intercepts of the regression lines of the relationships of the log (dose) to the log (AUC).
RESULTS
Plasma concentrations of ceftazidime and avibactam were determined in 192 mice, and ELF concentrations could be determined in 189 of these. No BAL fluid could be acquired from 3 mice because of technical reasons. Urea levels could be determined in all other samples. The mean dilution factor in BAL fluid was 11.6 (range, 4.3 to 144.2).
Figure 1 shows two examples of pharmacokinetic profiles of ceftazidime in plasma and ELF from thigh- and lung-infected mice after administration of doses of 16 and 32 mg/kg, respectively, and Fig. 2 shows two similar examples of avibactam in plasma and ELF. The curves for avibactam are comparable to those for ceftazidime. From visual inspection of these and the analogous graphs from all analyses, the results for both ceftazidime and avibactam indicated linear pharmacokinetics and no systematic differences in the pharmacokinetic profiles of the thigh- and lung-infected animals.
FIG 1.
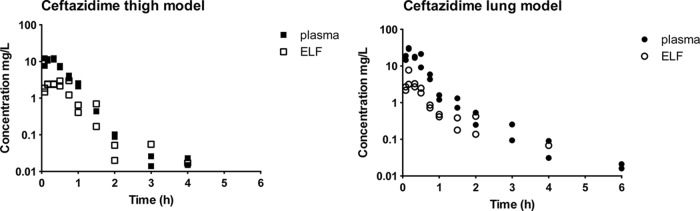
Example of pharmacokinetic profiles of 2 different single doses of ceftazidime (codosed with avibactam) in plasma and ELF of neutropenic mice with thigh infection (left panel, 16 mg/kg dose) or lung infection (right panel, 32 mg/kg dose). Filled and open squares, concentrations in plasma and ELF, respectively, in the thigh model; filled and open circles, concentrations in plasma and ELF, respectively, in the lung model (each dose group consisted of two mice).
FIG 2.
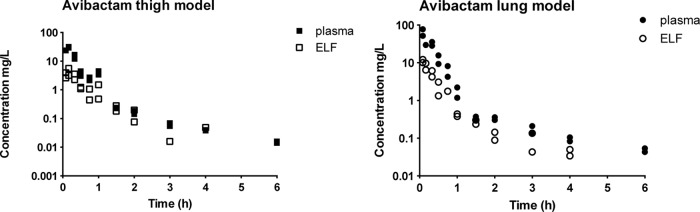
Example of pharmacokinetic profiles of 2 different single doses of avibactam (codosed with ceftazidime) in plasma and ELF of neutropenic mice with thigh infection (left panel, 32 mg/kg dose) or lung infection (right panel, 64 mg/kg). Filled and open squares, concentrations in plasma and ELF, respectively, in the thigh model; filled and open circles, concentrations in plasma and ELF, respectively, in the lung model (each dose group consisted of two mice).
Table 1 summarizes the pharmacokinetic parameter estimates for ceftazidime and avibactam in plasma and ELF. Concentrations in ELF after the administration of 1, 2, and 4 mg/kg were relatively low or below the LLQ for most time points; estimates were therefore either not possible or not very accurate. The mean estimated half-life in plasma of ceftazidime in the terminal phase was 0.28 h (SD, 0.02 h), and that of avibactam was 0.24 h (SD, 0.04 h). Volumes of distribution were 0.80 liters/kg (SD, 0.14 liters/kg) and 1.18 liters/kg (SD, 0.34 liters/kg), respectively (data not shown).
TABLE 1.
PK parameter estimates in plasma and ELF and ELF/plasma penetration ratios of ceftazidime and avibactam after a single subcutaneous dose in neutropenic mice with thigh or lung infectiona
| Dose (mg/kg) | Infection | ELF AUC (mg · h/liter) | ELF t1/2 (h) | Plasma AUC (mg · h/liter) | Plasma t1/2 (h) | Plasma Cl/F (liters/h) | ELF/plasma ratio |
|
|---|---|---|---|---|---|---|---|---|
| Total | Freec | |||||||
| Ceftazidime | ||||||||
| 1 (64) | Lung | 0.04b | 0.51 | 0.30 | 1.95 | |||
| 4 (2) | Lung | 0.11b | 0.51b | 1.60 | 0.29 | 2.50 | ||
| 32 (16) | Lung | 2.41 | 0.45 | 13.66 | 0.29 | 2.34 | 0.18 | 0.20 |
| 128 (8) | Lung | 15.50 | 0.41 | 68.52 | 0.25 | 1.87 | 0.23 | 0.25 |
| 2 (128) | Thigh | 0.08b | 2.29b | 1.25 | 0.30 | 1.60 | ||
| 8 (1) | Thigh | 1.31 | 0.27 | 4.37 | 0.27 | 1.83 | 0.30 | 0.33 |
| 16 (4) | Thigh | 2.27 | 0.27 | 7.64 | 0.24 | 2.09 | 0.30 | 0.33 |
| 64 (32) | Thigh | 7.54 | 0.54 | 36.40 | 0.29 | 1.76 | 0.21 | 0.23 |
| Mean (SD) | All | 0.39 (0.12) | 0.28 (0.02) | 1.99 (0.30) | ||||
| Mean (SE) | All | 0.24 (0.03) | 0.27 (0.03) | |||||
| Avibactam | ||||||||
| 2 (4) | Lung | 0.04b | 0.346b | 0.48 | 0.31 | 4.19 | ||
| 8 (128) | Lung | 0.54 | 0.26 | 2.64 | 0.27 | 3.02 | 0.20 | 0.22 |
| 16 (32) | Lung | 1.01 | 0.41 | 4.35 | 0.27 | 3.68 | 0.23 | 0.25 |
| 64 (1) | Lung | 4.12 | 0.32 | 19.41 | 0.24 | 3.29 | 0.21 | 0.23 |
| 1 (8) | Thigh | 0.10b | 0.291b | 0.26 | 0.18 | 3.87 | ||
| 4 (16) | Thigh | 0.40b | 0.237b | 1.31 | 0.19 | 3.05 | 0.30 | 0.33 |
| 32 (64) | Thigh | 2.31 | 0.40 | 10.66 | 0.27 | 3.00 | 0.22 | 0.24 |
| 128 (2) | Thigh | 7.99 | 0.30 | 51.46 | 0.23 | 2.49 | 0.16 | 0.17 |
| Mean (SD) | All | 0.34 (0.07) | 0.24 (0.04) | 3.32 (0.55) | ||||
| Mean (SE) | All | 0.22 (0.02) | 0.24 (0.02) | |||||
Doses in parentheses in column 1 represent the dose of the alternative compound (avibactam in the case of ceftazidime; ceftazidime in the case of avibactam). AUC, area under the concentration-time curve; ELF, epithelial lining fluid; Cl/F, total body clearance relative to bioavailability; t1/2, half-life of the compound in the corresponding body fluid sample.
Values uncertain because of a low number of data points due to the LLQ in ELF. Those values were excluded for calculation of the mean.
The proportion of protein binding for ceftazidime in plasma was 10% and in ELF was 0%; the proportion of protein binding for avibactam in plasma was 8% and in ELF was 0%.
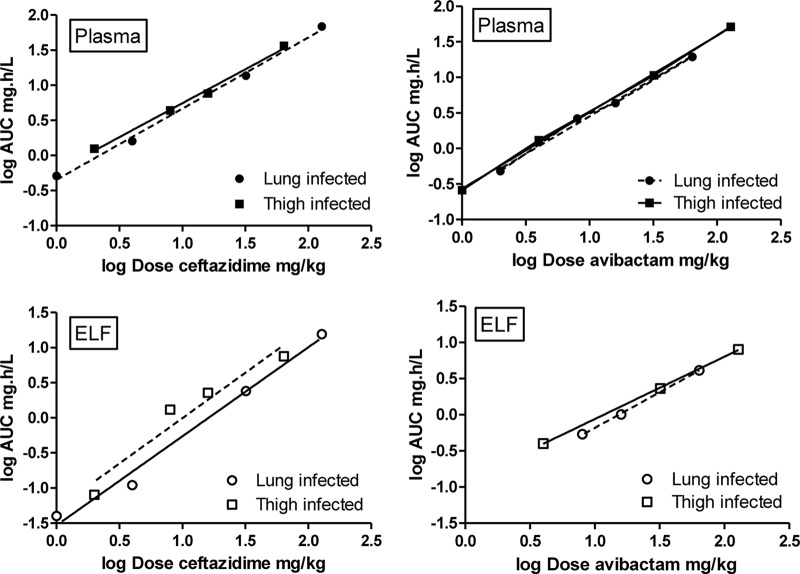
Figure 3 shows the dose proportionality of ceftazidime and avibactam in the lung model and the thigh model and comparisons of the two models in plots of log dose versus log AUC following the power model approach. Dose linearity and dose proportionality results were similar for the two compounds; significant differences between thigh- and lung-infected animals in dose proportionality were not found for ceftazidime or for avibactam, either in plasma or in ELF. In addition, there were no significant differences between the intercepts of the thigh and lung regression lines for the plasma concentrations of the two drugs in the two models, regardless of the combination of doses chosen, further confirming the absence of a difference in the pharmacokinetics of the two compounds in the two infection models.
FIG 3.
Dose proportionality of ceftazidime (left panels) and avibactam (right panels) in plasma and ELF of infected mice after single doses. Filled symbols represent plasma concentrations and open symbols concentrations in ELF. Squares represent concentrations in the thigh model, circles represent concentrations in the lung model, and every symbol represents two mice. AUC, area under the concentration-time curve; Dose, a single dose, administered subcutaneously; ELF, epithelial lining fluid.
Since no significant pharmacokinetics differences between the thigh model and lung model were observed, an overall relationship between dose (mg/kg) and plasma AUC (mg · h/liter) after a single dose of ceftazidime could be described as follows: log AUC = −0.294 + 0.9988 × log (dose ceftazidime). The overall relationship for avibactam was as follows: log AUC = −0.5896 + 1.070 × log (dose avibactam).
Summarizing, the pharmacokinetics are linear and dose proportional for both compounds and there are no significant differences between the thigh- and lung-infected animals in plasma pharmacokinetics.
In general, concentrations in ELF were found to be around 4-fold lower than those in plasma (Fig. 1 and 2 and Table 1). The concentration-time curves in ELF followed a pattern of linear pharmacokinetics for both ceftazidime and avibactam. In similarity to the findings in plasma, there was dose linearity as well as dose proportionality for both ceftazidime and avibactam in ELF. Based on the AUCs of the compounds in plasma and ELF, the overall “penetration ratio” of ceftazidime in ELF (Table 1), was 0.24 (0.03) for the total drug concentrations. The ratios for the 1, 2, and 4 mg/kg doses were excluded, because the AUCs in ELF could be determined only up to 1 h and therefore did not represent the same time span as or a time span comparable to that in plasma. Taking into account the protein binding of ceftazidime of 10% in plasma (and no binding in ELF), the penetration ratio of free drug was 0.27 (0.03) and was independent of the infection model. The penetration ratio seemed to be slightly higher in the thigh infection model, but this was not significant. The values of the penetration ratio for avibactam (Table 1) were 0.20 and 0.22, respectively, comparable to those of ceftazidime, and no significant differences between the thigh- and lung-infected models were found.
DISCUSSION
In the present study, we studied the pharmacokinetic properties of the combination of ceftazidime and avibactam in infected mice, whether thigh or lung infected, to be able to compare pharmacokinetics in two different types of infections. The aim was to look for possible dose linearity and dose proportionality, the possible influence of the pharmacokinetic behavior of each of the two compounds on the other, and the possible influence of the type of infection and the extent of penetration of the compounds in ELF compared to plasma. The pharmacokinetics of both ceftazidime and avibactam were linear and dose proportional as judged by graphical plots of the data and by the outcomes of linear regression analysis. The similarity of the profiles in plasma and ELF potentially reflects passive diffusion from plasma to ELF. For this reason, plasma could be used as a surrogate for target attainment, although the value of the pharmacodynamic target could be relatively high for that reason.
Although a formal drug-drug interaction study was not performed, based on the results of several different dose combinations we used encompassing virtually the whole dose range of both compounds, we did not find any evidence of drug-drug interactions between ceftazidime and avibactam. Together with the dose proportionality results, this indicates that exposures to both compounds can be determined for pharmacodynamic analysis in murine infection models in a relatively straightforward manner without the need to include interactions between the two compounds.
There were no differences in the pharmacokinetic parameter estimates for thigh- or lung-infected mice as evidenced by the dose proportionality analysis; thus, the type of infection the mice suffered from did not influence pharmacokinetics. The half-life values in plasma for both drugs were relatively short, as would be expected in mice, and within the same range. The half-life of ceftazidime as determined in this study was comparable to that found in other studies (32–34) and in our own laboratory in earlier experiments (35), although the half-life found by Fantin et al. (32) was slightly longer. However, the pharmacokinetic analysis in the study reported here was far more extensive than in the previous analyses, and the concentration-time curves are reproducible and clearly dose proportional. We therefore conclude that the estimates presented here are representative.
The levels of penetration of ceftazidime and avibactam in ELF were comparable and were close to 25% for both compounds. The data regarding penetration of ceftazidime and avibactam into murine ELF reported here are similar to the results reported by Housman et al. (36), who showed that simulated human exposures of ceftazidime and avibactam were bactericidal to ceftazidime-resistant P. aeruginosa infecting the lungs of neutropenic mice. The AUC-based penetration ratios were slightly lower than those found in humans by Nicolau et al. (37), which were approximately 40% for each compound. However, it is noted that these ELF/plasma AUC ratios reported for human subjects were calculated using total plasma concentrations of ceftazidime and avibactam. This means that the ratio in humans determined on the basis of free plasma concentrations would have been slightly higher than those reported, if adjusted for the proportion of compound bound to protein. ELF/plasma ratios of ceftazidime alone in humans have been measured previously; Cazzola et al. (38) found a ratio of about 10%, measured by microbiological assay, and the group of Boselli (39) found levels around 21% in critically ill human patients, measured with HPLC.
In conclusion, we found no significant differences for the pharmacokinetics of subcutaneously administered ceftazidime or avibactam in plasma and ELF in thigh- or lung-infected neutropenic mice and the pharmacokinetics of the two compounds were proportional with respect to the dose. The ratios of the concentrations of the two drugs in plasma versus ELF were close to 25% and constant, were independent of the doses administered, and, based on pharmacokinetic studies in humans as mentioned above, were therefore comparable to or lower than those measured in humans. The higher penetration in humans minimizes the risk of underdosing in patients when extrapolating from mouse data.
ACKNOWLEDGMENTS
This study was supported by an unrestricted grant from AstraZeneca and by Forest Laboratories Inc. (now a subsidiary of Actavis PLC).
W.W.N. has been an employee of AstraZeneca. J.W.M. has been employed as a consultant for and/or has received research funding from Angelini, Astra-Zeneca, Basilea, Jansen-Cilag, Merck & Co., Cubist, Pfizer, Polyphor, and Roche. J.B., M.J.M., A.C.V.M., S.S., and C. M. L. have no conflicts of interest. S.S. received travel grants from Astellas Pharma B.V. and Gilead Sciences.
REFERENCES
- 1.Klein NC, Cunha BA. 1995. Third-generation cephalosporins. Med Clin North Am 79:705–719. [DOI] [PubMed] [Google Scholar]
- 2.Bradford PA. 2001. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat Clin Microbiol Rev 14:933–951, table of contents. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhillon RH, Clark J. 2012. ESBLs: a clear and present danger? Crit Care Res Pract 2012:625170. doi: 10.1155/2012/625170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanders CC. 1987. Chromosomal cephalosporinases responsible for multiple resistance to newer beta-lactam antibiotics. Annu Rev Microbiol 41:573–593. doi: 10.1146/annurev.mi.41.100187.003041. [DOI] [PubMed] [Google Scholar]
- 5.Biehl LM, Schmidt-Hieber M, Liss B, Cornely OA, Vehreschild MJ. 4 February 2014, posting date Colonization and infection with extended spectrum beta-lactamase producing Enterobacteriaceae in high-risk patients---review of the literature from a clinical perspective. Crit Rev Microbiol doi: 10.3109/1040841X.2013.875515. [DOI] [PubMed] [Google Scholar]
- 6.Bush K. 2013. Carbapenemases: partners in crime. J Glob Antimicrob Resist 1:7–16. doi: 10.1016/j.jgar.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch EB, Tam VH. 2010. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother 65:1119–1125. doi: 10.1093/jac/dkq108. [DOI] [PubMed] [Google Scholar]
- 8.Poirel L, Potron A, Nordmann P. 2012. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 67:1597–1606. doi: 10.1093/jac/dks121. [DOI] [PubMed] [Google Scholar]
- 9.Coleman K. 2011. Diazabicyclooctanes (DBOs): a potent new class of non-beta-lactam beta-lactamase inhibitors. Curr Opin Microbiol 14:550–555. doi: 10.1016/j.mib.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Zhanel GG, Lawson CD, Adam H, Schweizer F, Zelenitsky S, Lagace-Wiens PR, Denisuik A, Rubinstein E, Gin AS, Hoban DJ, Lynch JP III, Karlowsky JA. 2013. Ceftazidime-avibactam: a novel cephalosporin/beta-lactamase inhibitor combination. Drugs 73:159–177. doi: 10.1007/s40265-013-0013-7. [DOI] [PubMed] [Google Scholar]
- 11.Lagacé-Wiens PR, Tailor F, Simner P, DeCorby M, Karlowsky JA, Walkty A, Hoban DJ, Zhanel GG. 2011. Activity of NXL104 in combination with beta-lactams against genetically characterized Escherichia coli and Klebsiella pneumoniae isolates producing class A extended-spectrum beta-lactamases and class C beta-lactamases. Antimicrob Agents Chemother 55:2434–2437. doi: 10.1128/AAC.01722-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endimiani A, Choudhary Y, Bonomo RA. 2009. In vitro activity of NXL104 in combination with beta-lactams against Klebsiella pneumoniae isolates producing KPC carbapenemases. Antimicrob Agents Chemother 53:3599–3601. doi: 10.1128/AAC.00641-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endimiani A, Hujer KM, Hujer AM, Pulse ME, Weiss WJ, Bonomo RA. 2011. Evaluation of ceftazidime and NXL104 in two murine models of infection due to KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 55:82–85. doi: 10.1128/AAC.01198-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aktaş Z, Kayacan C, Oncul O. 2012. In vitro activity of avibactam (NXL104) in combination with beta-lactams against Gram-negative bacteria, including OXA-48 beta-lactamase-producing Klebsiella pneumoniae. Int J Antimicrob Agents 39:86–89. doi: 10.1016/j.ijantimicag.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Ehmann DE, Jahic H, Ross PL, Gu RF, Hu J, Durand-Reville TF, Lahiri S, Thresher J, Livchak S, Gao N, Palmer T, Walkup GK, Fisher SL. 2013. Kinetics of avibactam inhibition against class A, C, and D beta-lactamases. J Biol Chem 288:27960–27971. doi: 10.1074/jbc.M113.485979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levasseur P, Girard AM, Claudon M, Goossens H, Black MT, Coleman K, Miossec C. 2012. In vitro antibacterial activity of the ceftazidime-avibactam (NXL104) combination against Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother 56:1606–1608. doi: 10.1128/AAC.06064-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sader HS, Castanheira M, Flamm RK, Farrell DJ, Jones RN. 2014. Antimicrobial activity of ceftazidime-avibactam against Gram-negative organisms collected from U.S. medical centers in 2012. Antimicrob Agents Chemother 58:1684–1692. doi: 10.1128/AAC.02429-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flamm RK, Farrell DJ, Sader HS, Jones RN. 2014. Ceftazidime/avibactam activity tested against Gram-negative bacteria isolated from bloodstream, pneumonia, intra-abdominal and urinary tract infections in US medical centres (2012). J Antimicrob Chemother 69:1589–1598. doi: 10.1093/jac/dku025. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Zhang F, Zhao C, Wang Z, Nichols WW, Testa R, Li H, Chen H, He W, Wang Q, Wang H. 2014. In vitro activities of ceftazidime-avibactam and aztreonam-avibactam against 372 Gram-negative bacilli collected in 2011 and 2012 from 11 teaching hospitals in China. Antimicrob Agents Chemother 58:1774–1778. doi: 10.1128/AAC.02123-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizgerd JP. 2006. Lung infection–a public health priority. PLoS Med 3:e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freire AT, Melnyk V, Kim MJ, Datsenko O, Dzyublik O, Glumcher F, Chuang YC, Maroko RT, Dukart G, Cooper CA, Korth-Bradley JM, Dartois N, Gandjini H. 2010. Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn Microbiol Infect Dis 68:140–151. doi: 10.1016/j.diagmicrobio.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Silverman JA, Mortin LI, Vanpraagh AD, Li T, Alder J. 2005. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 191:2149–2152. doi: 10.1086/430352. [DOI] [PubMed] [Google Scholar]
- 23.Berkhout J, Melchers MJ, van Mil AC, Nichols WW, Mouton JW. 8 December 2014. In vitro activity of combinations of ceftazidime-avibactam in in vitro checkerboard assays. Antimicrob Agents Chemother doi: 10.1128/AAC.04146-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seyedmousavi S, Bruggemann RJ, Melchers WJ, Verweij PE, Mouton JW. 24 February 2014. Intrapulmonary posaconazole penetration at the infection site in an immunosuppressed murine model of invasive pulmonary aspergillosis receiving oral prophylactic regimens. Antimicrob Agents Chemother doi: 10.1128/AAC.00053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crandon JL, Schuck VJ, Banevicius MA, Beaudoin ME, Nichols WW, Tanudra MA, Nicolau DP. 2012. Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosa. Antimicrob Agents Chemother 56:6137–6146. doi: 10.1128/AAC.00851-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Callaghan CH, Acred P, Harper PB, Ryan DM, Kirby SM, Harding SM. 1980. GR 20263, a new broad-spectrum cephalosporin with anti-pseudomonal activity. Antimicrob Agents Chemother 17:876–883. doi: 10.1128/AAC.17.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toutin A. 2005. In vitro extent of binding of NXL104 to mouse, rat, rabbit, dog and human plasma. Novexel, Romainville, France. [Google Scholar]
- 28.Birks V. 2009. NXL: further investigations into the in vitro plasma protein binding in mouse, rat, rabbit, dog and human. Novexel, Romainville, France. [Google Scholar]
- 29.Kiem S, Schentag JJ. 2008. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob Agents Chemother 52:24–36. doi: 10.1128/AAC.00133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodvold KA, George JM, Yoo L. 2011. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin Pharmacokinet 50:637–664. doi: 10.2165/11594090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Von Wichert P, Joseph K, Muller B, Franck WM. 1993. Bronchoalveolar lavage. Quantitation of intraalveolar fluid? Am Rev Respir Dis 147:148–152. doi: 10.1164/ajrccm/147.1.148. [DOI] [PubMed] [Google Scholar]
- 32.Fantin B, Leggett J, Ebert S, Craig WA. 1991. Correlation between in vitro and in vivo activity of antimicrobial agents against gram-negative bacilli in a murine infection model. Antimicrob Agents Chemother 35:1413–1422. doi: 10.1128/AAC.35.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Ogtrop ML, Mattie H, Guiot HF, van Strijen E, Hazekamp-van Dokkum AM, van Furth R. 1990. Comparative study of the effects of four cephalosporins against Escherichia coli in vitro and in vivo. Antimicrob Agents Chemother 34:1932–1937. doi: 10.1128/AAC.34.10.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kita Y, Yamazaki T, Imada A. 1992. Comparative pharmacokinetics of SCE-2787 and related antibiotics in experimental animals. Antimicrob Agents Chemother 36:2481–2486. doi: 10.1128/AAC.36.11.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buijs J, Dofferhoff AS, Mouton JW, van der Meer JW. 29 March 2007, posting date Continuous administration of PBP-2- and PBP-3-specific beta-lactams causes higher cytokine responses in murine Pseudomonas aeruginosa and Escherichia coli sepsis. J Antimicrob Chemother doi: 10.1093/jac/dkm073. [DOI] [PubMed] [Google Scholar]
- 36.Housman ST, Crandon JL, Nichols WW, Nicolau DP. 2014. Efficacies of ceftazidime-avibactam and ceftazidime against Pseudomonas aeruginosa in a murine lung infection model. Antimicrob Agents Chemother 58:1365–1371. doi: 10.1128/AAC.02161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicolau DP, Siew L, Armstrong J, Edeki T, Bouw MR. 2013. Concentration of avibactam (AVI) and ceftazidime (CAZ) in plasma and epithelial lining fluid (ELF) in healthy volunteers, abstr A-1027 Abstr 53rd Intersci Conf Antimicrob Agents Chemother, Denver, CO. [Google Scholar]
- 38.Cazzola M, Gabriella Matera M, Polverino M, Santangelo G, De Franchis I, Rossi F. 1995. Pulmonary penetration of ceftazidime. J Chemother 7:50–54. doi: 10.1179/joc.1995.7.1.50. [DOI] [PubMed] [Google Scholar]
- 39.Boselli E, Breilh D, Rimmele T, Poupelin JC, Saux MC, Chassard D, Allaouchiche B. 2004. Plasma and lung concentrations of ceftazidime administered in continuous infusion to critically ill patients with severe nosocomial pneumonia. Intensive Care Med 30:989–991. doi: 10.1007/s00134-004-2171-2. [DOI] [PubMed] [Google Scholar]