Abstract
Eravacycline (formerly TP-434) was evaluated in vitro against pre-established biofilms formed by a uropathogenic Escherichia coli strain. Biofilms were eradicated by 0.5 μg/ml eravacycline, which was within 2-fold of the MIC for planktonic cells. In contrast, colistin and meropenem disrupted biofilms at 32 and 2 μg/ml, respectively, concentrations well above their respective MICs of 0.5 and 0.03 μg/ml. Gentamicin and levofloxacin eradicated biofilms at concentrations within 2-fold of their MICs.
TEXT
Many bacterial pathogens associated with chronic infections can persist as inherently antibiotic- and host defense-tolerant biofilms, embedded in complex extracellular matrices attached to inert surfaces, dead or living tissue, and medical devices during mild or serious infections (1–5). Biofilm-like intracellular bacterial communities have also been observed within infection sites, such as bladder cells in urinary tract infections and epithelial cells in respiratory infections (6–8). Restricted antibiotic diffusion across the extracellular matrix, upregulation of intrinsic efflux pumps, generally lower metabolic activity, and the presence of persister cells are all thought to be significant factors contributing to increased antimicrobial tolerance of bacteria growing in biofilms (5, 7, 9 10).
Eravacycline is a novel broad-spectrum fluorocycline with in vitro activity against emerging multidrug-resistant Gram-negative pathogens, including carbapenem-resistant and extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae and Acinetobacter baumannii (11). Eravacycline is currently in phase 3 clinical studies for complicated urinary tract infections and complicated intra-abdominal infections. The present study was undertaken to characterize the activity in vitro of eravacycline, and several other antibiotics commonly used in treatment of infections caused by Gram-negative bacteria against biofilms formed by a uropathogenic, tetracycline-resistant, β-lactamase-producing Escherichia coli isolate, EC200 (ATCC BAA-1161) [tet(B) blaTEM].
For biofilm assays, cells from a fresh tryptic soy agar (TSA; BBL BD no. 221283) plate grown overnight at 35°C were suspended in 0.9% saline to a 0.5 McFarland standard, diluted 10-fold into tryptic soy broth (TSB)–1% yeast extract (YE) medium (Bacto BD no. 211825 [TSB] and no. 210929 [YE]) and grown at 35°C to log phase for 2 h. The culture was diluted 1/100 in TSB-YE to ∼106 CFU/ml, and 500 μl of culture was added to 5-ml round-bottom polystyrene tubes (BD Falcon no. 352054; BD, Franklin Lakes, NJ). After 24 h of stationary incubation at 35°C, the EC200 biofilm formed as a ring of growth on the walls of the tube at the liquid-air interface (Fig. 1A). All subsequent manipulations of tubes for biofilm staining and CFU quantification (see below) were done with care to avoid disrupting the adhered biofilm. After 24 h of growth, planktonic cells were aseptically aspirated, and 600 μl of fresh TSB-YE, with or without antibiotic, was added. Tubes were incubated upright in a test tube rack, with loosened caps, for an additional 24 h at 35°C in ambient air without shaking to determine antibiotic activity on the pre-established biofilm and planktonic growth derived from the biofilm; a new planktonic culture was seeded from the pre-established biofilm, and the biofilm itself either remained intact or was replenished after another 24 h of incubation at 35°C (48 h total incubation) (Fig. 1B). “Planktonic MICs” were defined as the lowest concentration that completely inhibited visible turbidity in compound-treated tubes inoculated with a biofilm that had been established previously for 24 h.
FIG 1.
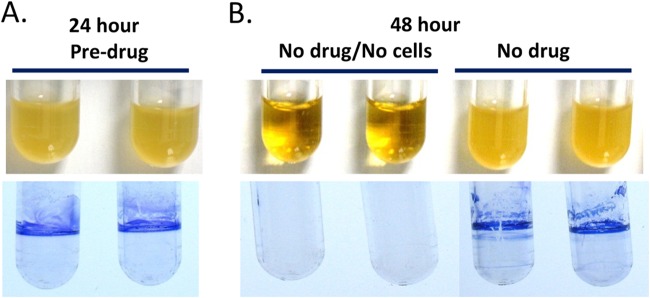
Strain EC200 biofilm formation at 24 and 48 h. (A) (Top) Turbid duplicate cultures inoculated with ∼106 CFU and grown at 35°C for 24 h. (Bottom) Associated biofilms at 24 h stained with crystal violet. (B) (Top) Duplicate pairs with no drug and no cells incubated at 35°C in parallel to 48-h no-drug biofilms (24-h biofilms were aseptically aspirated to remove planktonic cells, fresh medium was added, and cells were grown for an additional 24 h). (Bottom) Associated biofilms at 48 h stained with crystal violet.
For visualizing biofilms after the second overnight incubation, with or without drug, planktonic cells were removed by aspiration, and 750 μl tap water was gently added to biofilm-containing tubes, which were allowed to sit for 1 min at room temperature. After aspiration of the wash liquid, 750 μl 0.1% crystal violet (no. C3886-100G0; Sigma-Aldrich, St. Louis, MO) prepared in deionized water was gently added. After approximately 5 min at room temperature, the dye was aspirated, and the tubes were rinsed with 1 ml of tap water and allowed to dry prior to photography. Results for antibiotic treatments presented here are representative of at least two independent experiments (Fig. 2). “Biofilm MICs” were defined as the lowest compound concentration that produced a significant reduction in the biofilm by two criteria (i.e., the biofilm ring was no longer visualized by crystal violet staining, plus there was a >90% reduction in biofilm CFU [see below]).
FIG 2.
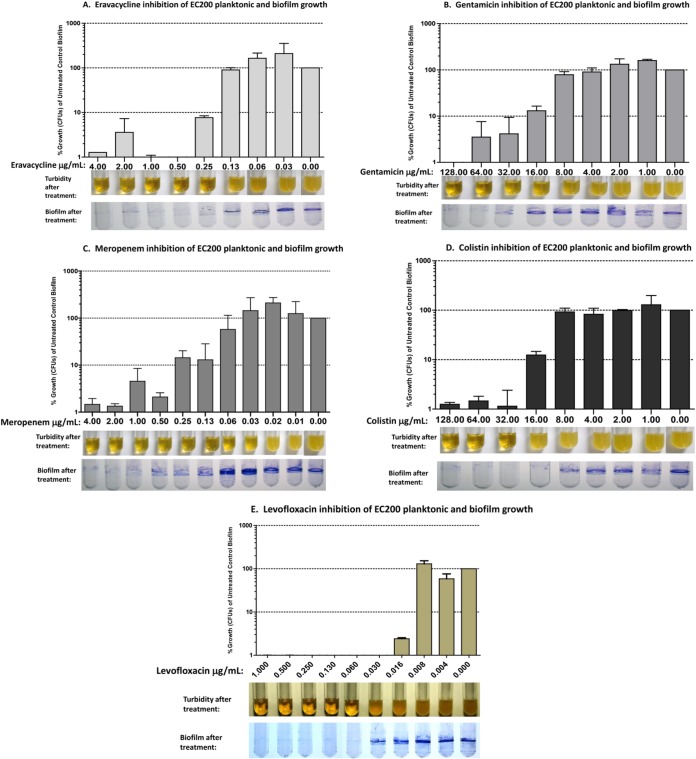
Antibiotic susceptibility of pre-established (24-h) biofilms from uropathogenic E. coli strain EC200. Tubes containing 24-h pre-established biofilms were aseptically aspirated to remove planktonic cells, medium containing drug was added, and tubes were incubated for an additional 24 h. (A) Eravacycline, 0.03 to 4 μg/ml; (B) gentamicin, 1 to 128 μg/ml; (C) meropenem, 0.01 to 4 μg/ml; (D) colistin, 1 to 128 μg/ml; (E) levofloxacin, 0.004 to 1 μg/ml. Each panel shows average CFU released from biofilms by sonication of antibiotic-treated biofilms relative to untreated biofilms (bar graph), turbidity of planktonic cultures after antibiotic treatment (broth tubes), and staining of antibiotic-treated biofilms with crystal violet (stained tubes). Error bars represent standard deviations.
For quantification of biofilm CFU after the second overnight incubation, with or without drug, planktonic cells were aseptically removed by aspiration, 750 ml sterile 0.9% saline was gently added for 1 min and then aspirated, and 1 ml of sterile 0.9% saline was added to each tube along with two sterile 6-mm borosilicate glass beads (Kimax no. 89001-520; VWR, Arlington Heights, IL). Replicate tubes were sonicated in a Branson 5510 water bath at room temperature for 1 min and then placed on ice. Preliminary time course experiments showed that 1 min of sonication was sufficient to release a maximum of CFU from tubes, with no increase or decrease in CFU for sonication up to 5 min. The sonicates containing cells dispersed from the biofilm were serially diluted, plated on TSA plates, and incubated at 35°C for CFU quantification. Untreated and drug-treated tubes at each concentration were run at least in duplicate, and the results presented here are average percent CFU relative to untreated control values at each drug concentration. Typically, untreated biofilms produced ∼5 × 107 CFU. Representative results of at least two independent assays are shown in Fig. 2.
Macrodilution MIC assays were performed according to the methods described by the Clinical and Laboratory Standards Institute (CLSI) (12) with the exception that log-phase cultures grown in TSB-YE medium were diluted 1/100 in fresh TSB-YE medium containing 2-fold serial dilutions of antibacterial compound to ∼106 CFU/ml, and 500 μl of culture was added to 5-ml round-bottom polystyrene tubes. Eravacycline was synthesized as described by Xiao et al. (13). Gentamicin, meropenem, levofloxacin, and colistin were obtained from Sigma-Aldrich (St. Louis, MO).
Biofilms exposed to ≥0.25 μg/ml eravacycline showed reduced crystal violet staining; the eravacycline biofilm MIC was 0.5 μg/ml, resulting in ≤1% of the CFU seen with the untreated control; the eravacycline macrodilution MIC was 0.25 μg/ml (Fig. 2A and Table 1). It is noteworthy that EC200 planktonic cells and biofilms were both insensitive to tetracycline at 128 μg/ml, the highest concentration tested (data not shown), consistent with the requirement of intracellular antimicrobial activity to eradicate the biofilms of this tetracycline-resistant tet(B) isolate. For eravacycline, the macrodilution, planktonic, and biofilm MICs against EC200 were all similar (0.25 to 0.5 μg/ml). Similar to other tetracycline antibiotics, eravacycline inhibits protein translation by binding to the 30S ribosomal subunit and blocking the entry of tRNA molecules to the A site of the ribosome (14); however, it remains to be determined if eravacycline's mechanism of action against biofilm cells is the same as that against planktonic cells.
TABLE 1.
MICs in macrodilution assays, biofilm-derived planktonic cultures, and biofilms
| Compound | MIC (μg/ml) |
% CFU remaining at biofilm MIC vs no-drug control | ||
|---|---|---|---|---|
| Macrodilution | Planktonic | Biofilm | ||
| Eravacycline | 0.25 | 0.25 | 0.5 | 0.9 |
| Gentamicin | 32 | 64 | 64 | 3.5 |
| Colistin | 0.5 | 32 | 32 | 1.1 |
| Meropenem | 0.03 | 0.13 | 2 | 1.3 |
| Levofloxacin | 0.06 | 0.06–0.13 | 0.06–0.13 | 0.01 |
Biofilms treated with meropenem and colistin showed reduced crystal violet staining after 24 h of exposure to ≥0.13 μg/ml (Fig. 2C) and ≥16 μg/ml (Fig. 2D), respectively, with biofilm MICs of 2 μg/ml and 32 μg/ml, respectively, compared to macrodilution MICs of 0.03 μg/ml and 0.5 μg/ml, respectively (Table 1). Planktonic MICs, derived from a 24-h biofilm inoculum, for meropenem and colistin were also elevated at 0.13 μg/ml and 32 μg/ml, respectively, compared to the corresponding macrodilution MICs (Table 1). While the reason for the elevated planktonic and biofilm MICs compared to macrodilution MICs is not known, several factors, such as an increased inoculum from biofilm, the physiology of the cells initially released from the biofilm upon introduction of fresh medium, and the ability of the compound to penetrate the biofilm, may play a role in susceptibility to these compounds. For gentamicin and levofloxacin, both the biofilm MIC and the planktonic MICs were within 2-fold of their macrodilution MICs of 32 μg/ml and 0.06 μg/ml, respectively (Fig. 2B and E and Table 1). Results with comparator antibiotics were similar to findings reported for Pseudomonas aeruginosa biofilms (15–17).
Interestingly, tetracycline has been shown to efficiently penetrate E. coli biofilms, suggesting that other tetracycline antibiotics may have an advantage in targeting cells growing in biofilms (18). In conclusion, eravacycline shows promising activity in vitro against E. coli biofilms, and if confirmed in vivo, this activity would be advantageous for the treatment of antibiotic-resistant chronic biofilm infections, including complicated urinary tract infections.
REFERENCES
- 1.Fux CA, Costerton JW, Steward PS, Stoodley P. 2005. Survival strategies of infectious biofilms. Trends Microbiol 13:34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Costerton JW, Veeh R, Shirtliff M, Pasmore M, Post C, Ehrlich G. 2003. The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest 112:1466–1477. doi: 10.1172/JCI20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung C, Zhou Y, Pinkner JS, Dodson KW, Crowley JR, Heuser J, Chapman MR, Hadjifrangiskou M, Henderson JP, Hultgren SJ. 2013. Escherichia coli biofilms have an organized and complex extracellular matrix structure. mBio 4:e00645–13. doi: 10.1128/mBio.00645-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costerton JW, Steward PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 5.Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blango MG, Mulvey MA. 2010. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob Agents Chemother 54:1855–1863. doi: 10.1128/AAC.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall-Stoodley L, Stoodley P. 2009. Evolving concepts in biofilm infections. Cell Microbiol 11:1034–1043. doi: 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 8.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 9.Lewis K. 2001. Riddle of biofilm resistance. Antimicrob Agents Chemother 45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K. 2004. Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett 230:13–18. doi: 10.1016/S0378-1097(03)00856-5. [DOI] [PubMed] [Google Scholar]
- 11.Sutcliffe JA, O'Brien W, Fyfe C, Grossman TH. 2013. Antibacterial activity of eravacycline (TP-434), a novel fluorocycline, against hospital and community pathogens. Antimicrob Agents Chemother 57:5548–5558. doi: 10.1128/AAC.01288-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CLSI. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed CLSI document M07-A9 CLSI, Wayne, PA. [Google Scholar]
- 13.Xiao X-Y, Hunt DK, Zhou J, Clark RB, Dunwoody N, Fyfe C, Grossman TH, O'Brien WJ, Plamondon L, Ronn M, Sun C, Zhang W-Y, Sutcliffe JA. 2012. Fluorocyclines. 1. 7-Fluoro-9-pyrrolidinoacetamido-6-demethyl-6-deoxytetracycline: a potent, broad spectrum antibacterial agent. J Med Chem 55:597–605. doi: 10.1021/jm201465w. [DOI] [PubMed] [Google Scholar]
- 14.Grossman TH, Starosta AL, Fyfe C, O'Brien W, Rothstein DM, Mikolajka A, Wilson DN, Sutcliffe JA. 2012. Target- and resistance-based mechanistic studies with TP-434, a novel fluorocycline antibiotic. Antimicrob Agents Chemother 56:2559–2564. doi: 10.1128/AAC.06187-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowler LL, Zhanel GG, Blake Ball T, Saward LL. 2012. Mature Pseudomonas aeruginosa biofilms prevail compared to young biofilms in the presence of Ceftazidime. Antimicrob Agents Chemother 56:4976–4979. doi: 10.1128/AAC.00650-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haagensen JAJ, Kalusen M, Ernst RK, Miller SI, Folkesson A, Tolker-Nielsen T, Molin S. 2007. Differentiation and distribution of colistin- and sodium dodecyl sulfate-tolerant cells in Pseudomonas aeruginosa biofilms. J Bacteriol 189:28–37. doi: 10.1128/JB.00720-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida H, Ishida Y, Kurosaka Y, Otani T, Sato K, Kobayashi H. 1998. In vitro and in vivo activities of levofloxacin against biofilm-producing Pseudomonas aeruginosa. Antimicrob Agents Chemother 42:1641–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone G, Wood P, Dixon L, Keyhan M, Matin A. 2002. Tetracycline rapidly reaches all the constituent cells of uropathogenic Escherichia coli biofilms. Antimicrob Agents Chemother 46:2458–2461. doi: 10.1128/AAC.46.8.2458-2461.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]