Abstract
Pseudomonas aeruginosa is the most common pathogen infecting the lower respiratory tract of cystic fibrosis (CF) patients, where it forms tracheobronchial biofilms. Pseudomonas biofilms are refractory to antibacterials and to phagocytic cells with innate immunity, leading to refractory infection. Little is known about the interaction between antipseudomonal agents and phagocytic cells in eradication of P. aeruginosa biofilms. Herein, we investigated the capacity of three antipseudomonal agents, amikacin (AMK), ceftazidime (CAZ), and ciprofloxacin (CIP), to interact with human polymorphonuclear leukocytes (PMNs) against biofilms and planktonic cells of P. aeruginosa isolates recovered from sputa of CF patients. Three of the isolates were resistant and three were susceptible to each of these antibiotics. The concentrations studied (2, 8, and 32 mg/liter) were subinhibitory for biofilms of resistant isolates, whereas for biofilms of susceptible isolates, they ranged between sub-MIC and 2 × MIC values. The activity of each antibiotic alone or in combination with human PMNs against 48-h mature biofilms or planktonic cells was determined by XTT [2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] assay. All combinations of AMK with PMNs resulted in synergistic or additive effects against planktonic cells and biofilms of P. aeruginosa isolates compared to each component alone. More than 75% of CAZ combinations exhibited additive interactions against biofilms of P. aeruginosa isolates, whereas CIP had mostly antagonistic interaction or no interaction with PMNs against biofilms of P. aeruginosa. Our findings demonstrate a greater positive interaction between AMK with PMNs than that observed for CAZ and especially CIP against isolates of P. aeruginosa from the respiratory tract of CF patients.
INTRODUCTION
Pseudomonas aeruginosa causes acute and chronic infections predominantly associated with compromised innate host defenses (1, 2). In patients with cystic fibrosis (CF), chronic P. aeruginosa infection of the lower respiratory tract is associated with excessive morbidity and mortality, as more than 80% of these patients succumb to respiratory failure (3, 4). A critical virulence trait of this pathogen that dominates in the CF lung is its capacity to form biofilms, a characteristic that has been linked to antimicrobial resistance and host defense evasion (5, 6). Chronic pulmonary infections arise because host responses are ineffective against biofilms (7). However, virulence factors that depend on the cell-to-cell communication system of P. aeruginosa are shown to have a major role as a defense against polymorphonuclear leukocytes (PMNs) (8–10).
Biofilms are surface-attached structured networks of aggregated bacteria embedded in a self-produced matrix composed of polysaccharides, protein, and DNA. These aggregates can resist high concentrations of antimicrobial agents that would efficiently eliminate the single-cell planktonic phenotype (11–13).
Exposure of bacteria to subinhibitory antibiotic concentrations (sub-MICs) results in regression of certain virulence factors that control cell morphology, adherence, or enzymatic secretion, eventually leading to alterations in the biofilm architecture. Such alterations could interfere with the ability of the pathogens to colonize susceptible hosts and develop an infectious process (14–17).
To date, several studies have investigated the effects of antimicrobial agents on P. aeruginosa biofilm formation or the response of the innate immune cells to P. aeruginosa biofilms (6, 18). However, to our knowledge, no study has yet addressed the combined effect of antibiotics with PMNs against established biofilms of P. aeruginosa. We hypothesized that antipseudomonal antimicrobial agents could interact with PMNs to enhance biofilm destruction. To this end, we evaluated the pharmacodynamic effects of representative antibiotics of three classes of antimicrobial agents: an aminoglycoside (amikacin [AMK]), cephalosporin (ceftazidime [CAZ]), and fluoroquinolone (ciprofloxacin [CIP]), alone or in combination with PMNs, against established biofilms and planktonic cells of susceptible or resistant P. aeruginosa strains isolated from patients chronically infected with CF.
MATERIALS AND METHODS
Bacterial isolates and growth conditions.
Six clinical isolates of P. aeruginosa were collected from sputa of six CF patients monitored at Hippokration Hospital, Salonika, Greece, from 2007 to 2012. Isolate identity was determined using a Vitek II automated system (bioMérieux, Marcy l'Etoile, France) according to the manufacturer's instructions. Stock cultures were divided into small portions and maintained at −35°C in a solution containing 25% glycerol and 75% peptone. All isolates were revived from frozen stock cultures on cation-adjusted (50 mg/liter Ca2+, 25 mg/liter Mg2+ [pH 7.2]) Mueller-Hinton (MH) agar (Scharlau Chemie, S.A., Barcelona, Spain) plates after growth at 37°C for 12 h. Five to 10 colonies from each isolate were subsequently transferred to 20 ml of cation-adjusted MH broth and incubated at 37°C overnight on a rocking table. The grown cultures were harvested by centrifugation at 2,000 rpm for 20 min, washed twice with 10 ml of phosphate-buffered saline (PBS) (0.02 M phosphate, 0.15 M NaCl [pH 7.2]), and resuspended in RPMI 1640 (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) buffered to pH 7.2 with 3-(N-morpholino) propanesulfonic acid (Sigma-Aldrich Chemie GmbH). Three of the clinical isolates were resistant to each of the agents amikacin (AMKr), ceftazidime (CAZr), and ciprofloxacin (CIPr), while the other three isolates were susceptible to these antibiotics (AMKs, CAZs, and CIPs). The isolates studied were classified as resistant or susceptible following the CLSI interpretive standards of breakpoints for resistance: AMKr, ≥32 mg/liter, and AMKs, ≤16 mg/liter; CAZr, ≥32 mg/liter, and CAZs, ≤8 mg/liter; CIPr, ≥4 mg/liter, and CIPs, ≤1 mg/liter (19). For all experiments, the organisms were used at a final concentration of 106/ml.
Antimicrobial agents.
AMK was obtained from Vianex S.A. (Athens, Greece), CAZ from GlaxoSmithKline S.A. (Athens, Greece), and CIP from Bayer Hellas S.A. (Athens, Greece). Each antibiotic was dissolved in distilled water to final concentrations of 1,000 mg/ml for AMK and CAZ and 2,000 mg/ml for CIP. They were then maintained as a stock solution at −35°C for up to 1 month.
Biofilm formation.
Bacterial biofilms were produced in RPMI 1640 growth medium using 96-well microtiter polystyrene plates as described previously with minor modifications (20). Briefly, for each isolate, 100 μl of a suspension of 106 cells/ml was added to each well and incubated at 37°C for 48 h under constant linear shaking in order to promote biofilm formation. Mature biofilm production was evaluated by staining the polysaccharide structure of the extracellular matrix of biofilms with safranin. Mature biofilms were first washed twice with PBS in order to remove nonadherent cells and then stained with 200 μl of 0.1% safranin for 5 min. The optical absorbance at 492 nm was measured using a microplate reader (ChroMate 4300; Awareness Technology, Inc., Palm City, FL). All six isolates were strong biofilm producers.
Planktonic and biofilm drug susceptibilities.
The MICs of the three antibiotics for planktonic cells were determined using both the broth microdilution method, according to Clinical and Laboratory Standards Institute (CLSI) guidelines, and the XTT [2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] colorimetric assay (21). Antibiotic susceptibility of mature biofilms was also determined by the XTT assay. Briefly, antimicrobial agents were added to corresponding wells of biofilms and free-living planktonic cell cultures at serial 2-fold dilutions with concentrations ranging from 0.5 to 512 mg/liter. The microtiter plates were incubated at 37°C for 24 h with shaking. Drug-free wells containing only growth medium served as controls. At the end of the incubation period, antimicrobial susceptibility was determined by the XTT assay, as described below. MICs were determined as the lowest antibiotic concentration at which a prominent decrease in turbidity was observed either microscopically or colorimetrically, corresponding to 50% bacterial damage compared to that of untreated controls.
Isolation of human PMNs.
Heparinized whole blood was obtained from healthy adult volunteers, and PMNs were isolated by dextran sedimentation and Ficoll centrifugation as previously described (22). Following hypotonic lysis of red blood cells, the purified PMNs were resuspended in Hanks' balanced salt solution without Ca2+ and Mg2+ (Gibco, BRL, Life Technologies, Ltd., Paisley, Scotland, United Kingdom) and counted with a hemocytometer. The cell concentration was adjusted to 106 cells/ml.
Combined treatment of P. aeruginosa isolates by antibiotics and PMNs.
Biofilms and planktonic cells of resistant or susceptible P. aeruginosa isolates were incubated in the presence of each antipseudomonal agent alone or in combination with human PMNs at effector-to-target (E:T) cell ratios of 1:20 and 1:10 at 37°C in a humidified 5% CO2 incubator for 24 h. Based on the MIC of each antibiotic, the sub-MICs chosen were 2, 8, and 32 mg/liter. Due to susceptibility differences between resistant and susceptible isolates, the above concentrations ranged between the sub-MIC and 2× MIC values.
Assessment of biofilm and planktonic cell damage.
Bacterial damage induced by phagocytes and/or antimicrobial agents was assessed by using an XTT reduction assay as previously described, with minor modifications (23, 24). Briefly, 150 μl of XTT (0.25 g/liter [Sigma]) containing coenzyme Q (40 mg/liter [Sigma]) was added to microtiter plates after a washing step with PBS to remove antibacterial agents, PMNs, or growth medium. The plates were then incubated at 37°C for 20 to 30 min, and the change in color, indicating the degree of bacterial damage, was measured in a microtiter plate reader at 450 nm with a reference wavelength of 690 nm. Antibacterial activity was calculated according to the formula % bacterial damage = [1 − (X/C)] × 100, where X is the absorbance of experimental wells and C is the absorbance of control wells. The MIC for biofilms and planktonic cells was defined as the lowest antimicrobial concentration causing ≥50% bacterial damage compared to that for untreated controls.
Statistical analysis.
Each concentration of AMK, CAZ, and CIP or PMNs for every clinical isolate was tested in quadruplicate per experiment. Each experiment was performed with the cells from one PMN donor, and four independent experiments were done in total. The means of the replicate wells from each experiment were used in the data analysis to calculate the mean ± standard error (SE) from all experiments under the same conditions. The differences in the mean values of three or more groups were evaluated by repeated-measures analysis of variance (ANOVA) with Bonferroni's posttest analysis. The damage induced by the PMNs alone and the antibiotic alone was calculated and compared with the effect of the combined treatment by PMNs and antibiotic.
Synergism (SYN) was defined as an antimicrobial effect (damage) caused by the combination that was significantly greater than the effect of PMNs alone plus the effect of the antibiotic alone. An additive effect (ADD) was defined as an antimicrobial effect of the combination that was significantly greater than the effect produced by either PMNs alone or antibiotic alone but that did not reach synergism. Antagonism (ANT) was defined as an antimicrobial effect of the combination that was significantly less than the effect produced by either PMNs or antibiotic alone. Differences between biofilm and planktonic growth forms were analyzed by Student's t test after the Kolmogorov-Smirnov test of normality showed a normal distribution of data. Data were analyzed using Instat (version 3) biostatistics software (GraphPad, Inc., San Diego, CA). A P value of <0.05 indicates statistical significance.
RESULTS
Activity of antibiotics on P. aeruginosa biofilms and planktonic cells.
Planktonic MIC values obtained by the CLSI method were comparable to those obtained by the XTT assay (data not shown). As shown in Table 1, MICs of AMK, CAZ, and CIP for biofilms of resistant P. aeruginosa isolates were almost equal to MICs observed for planktonic cells. By comparison, MICs of AMK, CAZ, and CIP against biofilms of the susceptible isolates were 1× to 2× dilutions higher than those against planktonic cells (Table 1).
TABLE 1.
MICs of antibiotics for planktonic cells and biofilms of P. aeruginosa isolates
| Isolate cell type | MIC (mg/liter) of antibiotic for isolatea |
|||||
|---|---|---|---|---|---|---|
| Amikacin |
Ceftazidime |
Ciprofloxacin |
||||
| AMKr | AMKs | CAZr | CAZs | CIPr | CIPs | |
| Planktonic | 128 | 8 | 128 | 4 | 32 | 2 |
| Biofilm | 128 | 32 | 128 | 8 | 64 | 8 |
MICs for both planktonic cells and biofilms were determined as the lowest antibiotic concentration at which a prominent decrease in turbidity was observed colorimetrically, corresponding to 50% bacterial damage compared to untreated controls. r, resistant; s, susceptible.
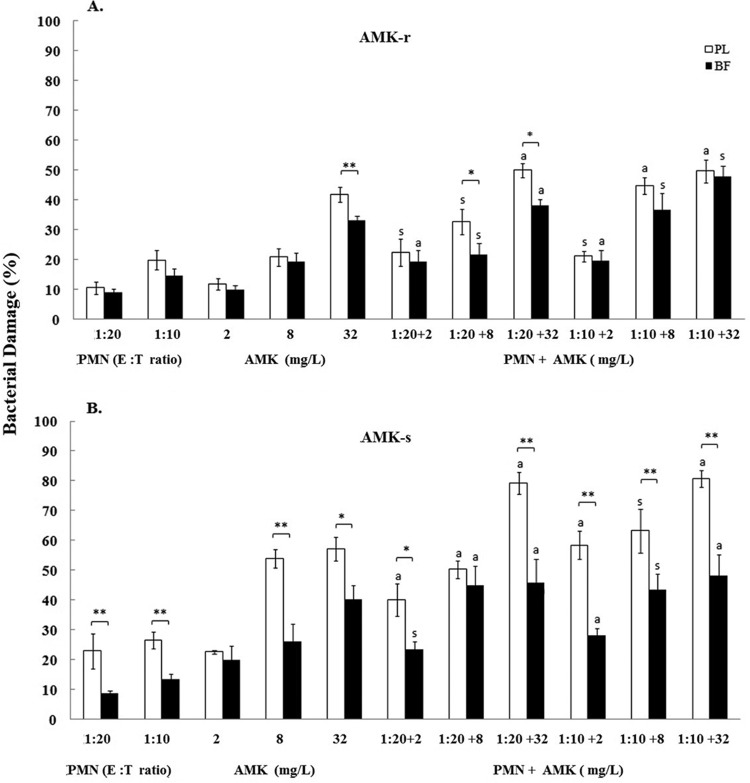
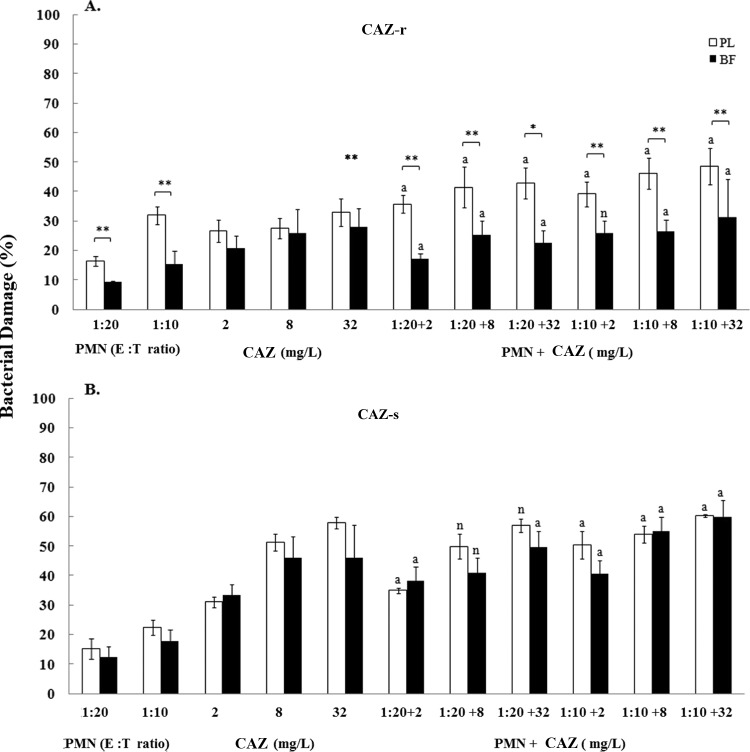
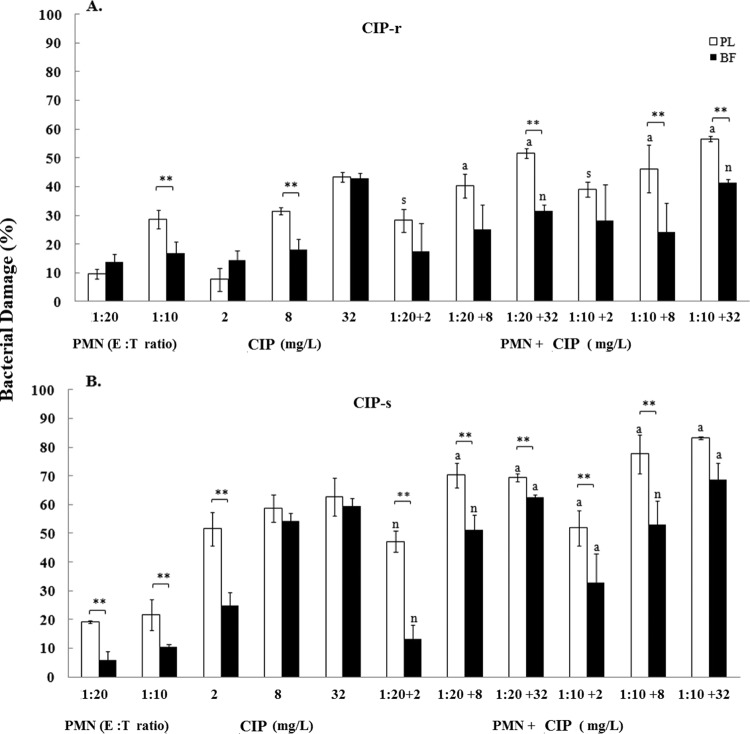
The maximum percentage of biofilm damage caused by AMK and CIP was evidenced at 512 mg/liter against P. aeruginosa isolates and ranged within 92% and 94%. By comparison, the maximum percentages of biofilm damage caused by CAZ were 78% at 128 mg/liter for the CAZr isolate and 97% at 256 mg/liter for the CAZs isolate. Overall, the antibiotics, when used at subinhibitory concentrations, appeared to cause similar damage to biofilms and planktonic cells that ranged between 10% and 62% (Fig. 1, 2, and 3). However, significant (P < 0.01) differences in damage were observed between biofilms and planktonic cells mostly at higher antibiotic concentrations (8 or 32 mg/liter) (Fig. 1A and B, Fig. 2A, and Fig. 3A and B, respectively).
FIG 1.
Bacterial damage of P. aeruginosa amikacin-resistant (AMK-r) (A) and amikacin-suceptible (AMK-s) (B) isolates induced by the coincubation with PMNs and AMK for 24 h. Bacterial damage of planktonic cells (PL) and biofilms (BF) was assessed by XTT assay. Data are presented as means ± SE of the percentage of damage of planktonic cells or biofilms from all experiments with each of the six clinical isolates. Asterisks demonstrate significant differences between planktonic cells and biofilms for the indicated concentrations of PMNs alone, drug alone, or their combination: *, P < 0.05; **, P < 0.01. The lowercase letters show significant differences for the indicated combination of PMNs with AMK: s, synergism; a, additivity.
FIG 2.
Bacterial damage of P. aeruginosa ceftazidime-resistant (CAZ-r) (A) and ceftazidime-susceptible (CAZ-s) (B) isolates induced by the coincubation with PMNs and CAZ for 24 h. Bacterial damage of planktonic cells (PL) and biofilms (BF) was assessed by XTT assay. Data are presented as means ± SE of the percentage of damage of planktonic cells or biofilms from all experiments with each of the six clinical isolates. Asterisks demonstrate significant differences between planktonic cells and biofilms for the indicated concentrations of PMNs alone, drug alone or their combination: *, P < 0.05; **, P < 0.01. The lowercase letters show significant differences for the indicated combination of PMNs with CAZ: a, additivity; n, antagonism.
FIG 3.
Bacterial damage of P. aeruginosa ciprofloxacin-resistant (CIP-r) (A) and ciprofloxacin-susceptible (CIP-s) (B) isolates induced by the coincubation with PMNs and CIP for 24 h. Bacterial damage of planktonic cells (PL) and biofilms (BF) was assessed by XTT assay. Data are presented as means ± SE of the percentage of damage of planktonic cells or biofilms from all experiments with each of the six clinical isolates. Asterisks demonstrate significant differences between planktonic cells and biofilms for the indicated concentrations of PMNs alone, drug alone, or their combination (**, P < 0.01). The lowercase letters show significant differences for the indicated combination of PMNs with CIP: s, synergism; a, additivity; n, antagonism.
PMN activity on P. aeruginosa biofilms and planktonic cells.
Overall, the activity of PMNs against P. aeruginosa isolates was limited. With the exception of AMKr and CAZs isolates, the damage induced by PMNs against mature biofilms was significantly less than that induced against their corresponding planktonic cells: from 9% ± 1.4% versus 11% ± 2.1% for the AMKr isolate (1:20; not significant [NS]) (Fig. 1A) to 13.4% ± 1.8% versus 26.5% ± 2.9% for the AMKs isolate (1:10; P < 0.01) (Fig. 1B), from 9.3% ± 0.5% versus 16.5% ± 1.6% for the CAZr isolate (1:20; P < 0.01) (Fig. 2A) to 18% ± 4.1% versus 22% ± 2.5% for the CAZs isolate (1:10; NS) (Fig. 2B), and from 14% ± 2.6% versus 10% ± 1.6% for the CIPr isolate (1:20; NS) (Fig. 3A) to 10% ± 1.1% versus 22% ± 5.4% for the CIPs isolate (1:10; P < 0.01) (Fig. 3B). In general, for both resistant and susceptible isolates, the biofilm damage effected by PMNs ranged from 9% to 14% at a 1:20 E:T ratio and from 10% to 18% at a 1:10 E:T ratio (Fig. 1A to 3B). By comparison, the damage effected by PMNs against planktonic cells ranged from 11% to 23% at the 1:20 ratio and from 20% to 32% at the 1:10 ratio (Fig. 1 to 3).
Damage of P. aeruginosa isolates caused by the combined treatment with PMNs and antimicrobial agents. (i) Combined effect of PMNs with AMK.
The simultaneous treatment of AMKr biofilms and planktonic cells with the combination of PMNs and AMK exhibited several synergistic effects (biofilms, 1:20 with 2 mg/liter and 1:10 with 8 to 32 mg/liter; planktonic cells, 1:20 with 2 mg/liter and 8 mg/liter and 1:10 with 8 mg/liter; P < 0.01) (Fig. 1). Likewise, the effects of PMNs combined with AMK against biofilms (1:20 and 1:10 with 8 mg/liter) or planktonic cells (1:10 with 2 mg/liter) of the AMKs isolate showed synergism (P < 0.01) (Fig. 1B). Additivity was observed against biofilms and planktonic cells of AMKr and AMKs isolates (P < 0.01) (Fig. 1A and B), whereas no antagonistic interactions were shown for AMK.
(ii) Combined effect of PMNs with CAZ.
CAZ showed additive interactions with most combinational treatments against both cell forms of P. aeruginosa CAZr and CAZs isolates (P < 0.01) (Fig. 2A and B). However, under biofilm conditions, the combination of CAZ (2 and 8 mg/liter) with PMNs (1:20) was antagonistic for CAZr and CAZs isolates (Fig. 2A and B). Similarly, for planktonic cells, the combinations of CAZ (8 and 32 mg/liter) with PMNs (1:20) showed antagonism for the corresponding susceptible isolates (Fig. 2B).
(iii) Combined effect of PMNs with CIP.
Synergistic effects were observed when planktonic cells of the CIPr isolate were treated with the combination of PMNs (1:20 and 1:10) with CIP (2 mg/liter; P < 0.01) (Fig. 3A). CIP demonstrated additive effects with PMNs against planktonic cells (P < 0.01) (Fig. 3A and B), whereas biofilms were resistant to most combinational treatments. Additionally, for both cell forms antagonistic interactions were observed. Under biofilm conditions, the combination of CIP (32 mg/liter for the CIPr isolate and 2 to 8 mg/liter for the CIPs isolate) with PMNs (1:20 and 1:10 for the CIPr and CIPs isolates) was antagonistic (Fig. 3A and B). Under planktonic conditions, the combination treatment of CIP (2 mg/liter) with PMNs (1:20) showed antagonism for the susceptible isolate (Fig. 3B). Taken together, statistically significant antagonism leading to bacterial growth was shown for selective combinations of CAZ and CIP with PMNs but not for AMK.
The results of the combined studies showed that all combinations of AMK with PMNs produced either synergism or additivity in damaging biofilms and planktonic cells of the corresponding isolates. In contrast, 83% and 58% of CAZ and CIP combinations, respectively, showed synergistic or additive effects in damaging cells of P. aeruginosa isolates. The maximum biofilm damage of the combinations tested was observed with PMNs at a 1:10 E:T ratio combined with 32 mg/liter of the following antibiotics: 48% for AMK against AMKr and AMKs isolates (Fig. 1A and B); 31% and 60% for CAZ against CAZr and CAZs isolates, respectively (Fig. 2A and B); and 41% and 69% for CIP against CIPr and CIPs isolates, respectively (Fig. 3A and B). Of the three resistant P. aeruginosa isolates studied, a high level of resistance was exhibited by biofilms and planktonic cells of AMKr and CAZr isolates to corresponding antibiotics (128 mg/liter), followed by the CIPr isolate to CIP (64 and 32 mg/liter, respectively).
DISCUSSION
In this study, we demonstrated that all combinations of AMK with PMNs resulted in synergistic or additive effects against both planktonic cells and biofilms of P. aeruginosa isolates compared to each component alone. More than 75% of CAZ combinations exhibited additivity against biofilms of P. aeruginosa isolates, whereas CIP was mostly either antagonistic or not interactive with PMNs against biofilms of P. aeruginosa. To the best of our knowledge, this is the first report on the simultaneous interactions of antipseudomonal antibiotics with human PMNs against resistant and susceptible P. aeruginosa isolates collected from sputa of CF patients.
Of note, the concentrations shown to have an interactive effect in our study (2 to 32 mg/liter) (Fig. 1 to 3) are below or around the ranges of concentrations achieved by intravenous (i.v.) administration to and especially by inhalation of these antipseudomonal agents by CF or non-CF patients. For example, after i.v. infusion of 300 mg/m2 AMK to healthy adults, the peak concentration was 52.4 mg/liter (25). In CF patients, doses of oral CIP ranging from 500 to 1,000 mg yielded peak serum concentrations of between 2.8 and 4.6 mg/liter (26). In addition, i.v. dosing of 200 mg CIP twice a day (b.i.d.) resulted in a peak level in serum of 4.9 ± 2.9 mg/liter and a level in bronchial mucosa of 21.6 ± 5.6 mg/liter (27). AMK at 30 mg/kg body weight/day i.v. once daily has given peak serum concentrations of 116 ± 37 mg/liter and sputum concentrations of 5.9 ± 2.7 mg/liter, whereas, ceftazidime at 200 mg/kg/day as a continuous infusion has given steady-state serum levels of 56 ± 23 mg/liter (28). On the other hand, during inhalation of antibiotics high concentrations are achieved in respiratory tract alveolar macrophages and epithelial lining fluid (ELF). For example, during administration of nebulized AMK at 400 mg b.i.d. to mechanically ventilated patients, a median concentration of 976 mg/liter of ELF was achieved (29). In addition, when liposomal CIP was given intranasally to rats with pneumonia, 387 ± 11 mg/liter was achieved in alveolar macrophages and 55 ± 3 mg/liter in ELF, whereas when CIP alone was administered by the same route, the respective concentrations were 44 ± 3 and 20 ± 1 mg/liter (30).
Microorganisms in the form of biofilms are generally resistant to antimicrobial agents. The same is true for P. aeruginosa. The intrinsic resistance of the biofilm lifestyle to antipseudomonal chemotherapy may be due to several factors, including the secretion of extracellular matrix that compromises antibiotic penetration, neutralizes antimicrobial agents through metal chelation, or limits oxygen and nutrient availability (18, 31).
In the antibiotic-pathogen-host interaction, antibacterial agents may affect pathogen viability either directly or indirectly by passive diffusion or modulation of the phagocytes' antibacterial responses. Subinhibitory concentrations of certain antibiotics have been shown to alter the cell wall morphology and the expression of structural and soluble virulence factors in such a manner that the pathogen becomes more susceptible to the action of phagocytes (26, 27). In the context of the triple interaction, phagocyte-mediated alterations of pathogen metabolism or cell structure may lead to increased or decreased susceptibility of the pathogen to the antibacterial effect of the antibiotic (32, 33).
As demonstrated in this study, after 24 h of incubation, PMNs exhibited minimal damage against biofilms of P. aeruginosa isolates. Moreover, PMN-induced damage was E:T ratio dependent. Our results are consistent with previous reports that the presence of PMNs triggers biofilms to release toxic components that compromise the phagocytic activity of PMNs, resulting in failure of immune cells to protect against bacterial biofilms (9, 10, 34). Nevertheless, several reports have shown that after PMNs come in contact with biofilm cells, they may not exhibit significant motility but are able to mount a respiratory burst, degranulate, and engage in phagocytosis (8, 35, 36).
A previous study of AMK's killing capacity toward planktonic P. aeruginosa cells has reported that subinhibitory (1/4× MIC and 1/2× MIC) concentrations of aminoglycosides decrease growth of P. aeruginosa (37). In addition to their inhibitory effect on protein synthesis, aminoglycosides can disturb the normal structure of P. aeruginosa lipid bilayer, displacing anionic lipopolysaccharides by means of their electropositivity. In this way, these cells are killed by a combination of aberrant protein production and cell lysis (38, 39). In our study, AMK showed concentration-dependent killing for planktonic and biofilm-grown P. aeruginosa cells. That the combination of AMK with PMNs produced synergistic or additive effects suggests that both AMK and PMNs contributed to such an effect. Although the relative contributions of each component cannot be inferred, the pro-oxidant activity of AMK has been demonstrated in time-dependent cellular experiments in which low concentrations of the drug caused enhanced hydrogen peroxide release by human PMNs (40). Nevertheless, the maximum biofilm damage induced by the combined action of amikacin (32 mg/liter) with PMNs (1:10) was not higher than 48%, confirming the resistant phenotype of P. aeruginosa biofilms. However, an additional point that is a contributing factor in resilience of P. aeruginosa biofilms is the low permeability of aminoglycosides through the mucoid exopolysaccharide matrix (41, 42). The high concentrations of aerosolized aminoglycoside in treatment of P. aeruginosa infections in CF patients may overcome this low impermeability.
Several antibiotics with different chemical structures and mechanisms of action have the capacity to activate or repress a great number of genes, including those involved in virulence and metabolic processes, when P. aeruginosa cells are exposed to low concentrations of these antibiotics; such a capacity is distinct from their known inhibitory activity (43). Subinhibitory levels of antibiotics may even enhance biofilm formation, but this does not pertain to this study, as each antibiotic was added after mature biofilm was formed. Investigations have shown that subinhibitory concentrations of CAZ, apart from blocking cell wall synthesis, exhibit strong quorum-sensing inhibitory activity that leads to decreased production of several virulence factors. CAZ inhibits the production of protease, elastase, chitinase, and rhamnolipids released by P. aeruginosa to protect biofilms against the action of antibiotics and the oxidative metabolites of PMNs (44–46). Furthermore, Labro et al. showed that subinhibitory concentrations of CAZ induced higher PMN oxidative burst against P. aeruginosa than untreated bacteria, and this was due to alterations in bacterial structure (47). Findings from previous investigations could partially explain the additive interactions we observed by most CAZ concentrations in combination with PMNs against biofilm and planktonic cells of P. aeruginosa isolates.
In this study, CIP was found to interact synergistically or additively with both E:T ratios of PMNs against planktonic cells of P. aeruginosa isolates. The significant interactions we observed between CIP and PMNs are consistent with earlier reports showing that quinolones and in particular CIP kill P. aeruginosa either directly, by disrupting the regulatory mechanisms that control cell morphology and production of certain virulence factors (14, 48), or indirectly, by entering PMNs and killing the bacteria intracellularly (49). However, biofilm-grown cells have shown variable results in this study and other reports: although we observed a concentration-dependent effect for P. aeruginosa biofilm cells exposed to CIP, the majority of the combinations of CIP and PMNs produced either nonsignificant or antagonistic interactions. Contradicting results on CIP activity against biofilms of P. aeruginosa have been reported by two studies on the secretion of proteases by these bacteria exposed to CIP. Oldak et al. (50) found that secretion of proteases continued even after exposure to CIP for 4 days, supporting bacterial growth through the supply of nutrients, whereas Skindersoe et al. (45) reported that CIP decrease the expression of several virulence factors, including proteases, in P. aeruginosa with the concomitant decrease in bacterial growth. Furthermore, a recent study showed that CIP induced the production and release of hydroxyl radicals by P. aeruginosa biofilms, which may be a contributing factor to the killing of cells embedded in biofilm (51). Although the exact mechanisms for the observed antagonism cannot be concluded from our study, we hypothesize that the oxidative burst of PMNs as they come in contact with bacteria may interfere with the release of hydroxyl radicals induced by CIP or with other functions of the antibiotic, causing an antagonistic effect or nonsignificant interaction to take place. As ciprofloxacin acts on topoisomerase and DNA gyrase of replicating bacteria, the lower replication rate of P. aeruginosa within biofilms also may contribute to the lack of synergistic or additive interaction.
In conclusion, subinhibitory concentrations of AMK combined with PMNs significantly damage biofilms of P. aeruginosa resistant and susceptible strains, while the combination of ceftazidime with polymorphonuclear leukocytes follows in order of efficacy. However, CIP interacts either antagonistically with PMNs or shows an insignificant result for most of the combined treatments against the corresponding biofilm cells. Further animal studies are needed to extend these in vitro findings, test the impact of biofilm production by P. aeruginosa on AMK susceptibility, and explore host-antibiotic interactions in vivo.
ACKNOWLEDGMENTS
The authors list the following potential conflicts of interest. E.R. has received research grant support from Pfizer, Gilead, Enzon, Schering, and Wyeth, has served as consultant to Schering, Gilead, Astellas Gilead, Cephalon, and Pfizer, and has been in the speakers' bureau of Wyeth, Schering, Merck, Aventis, Astellas. T.J.W. is a Scholar of the Henry Schueler Foundation, and a Scholar of Pediatric Infectious Diseases of the Sharp Family Foundation; he receives support from the Save Our Sick Kids Foundation, as well as research grants for experimental and clinical antimicrobial pharmacotherapeutics from Astellas, Cubist, Novartis, Merck, ContraFect, and Pfizer, and has served as consultant to Astellas, ContraFect, Drais, iCo, Novartis, Pfizer, Methylgene, SigmaTau, and Trius. The remaining authors have no relevant disclosures.
REFERENCES
- 1.Driscoll JA, Brody SL, Kollef MH. 2007. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs 67:351–368. doi: 10.2165/00003495-200767030-00003. [DOI] [PubMed] [Google Scholar]
- 2.Gjodsbol K, Christensen JJ, Karlsmark T, Jorgensen B, Klein BM, Krogfelt KA. 2006. Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J 3:225–231. doi: 10.1111/j.1742-481X.2006.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyczak JB, Cannon CL, Pier GB. 2002. Lung infections associated with cystic fibrosis. Clin Microbiol Rev 15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foweraker J. 2009. Recent advances in the microbiology of respiratory tract infection in cystic fibrosis. Br Med Bull 89:93–110. doi: 10.1093/bmb/ldn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morita Y, Tomida J, Kawamura Y. 2014. Responses of Pseudomonas aeruginosa to antimicrobials. Front Microbiol 4:422. doi: 10.3389/fmicb.2013.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen PO, Givskov M, Bjarnsholt T, Moser C. 2010. The immune system vs. Pseudomonas aeruginosa biofilms. FEMS Immunol Med Microbiol 59:292–305. doi: 10.1111/j.1574-695X.2010.00706.x. [DOI] [PubMed] [Google Scholar]
- 7.Williams BJ, Dehnbostel J, Blackwell TS. 2010. Pseudomonas aeruginosa: host defence in lung diseases. Respirology 15:1037–1056. doi: 10.1111/j.1440-1843.2010.01819.x. [DOI] [PubMed] [Google Scholar]
- 8.Jesaitis AJ, Franklin MJ, Berglund D, Sasaki M, Lord CI, Bleazard JB, Duffy JE, Beyenal H, Lewandowski Z. 2003. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J Immunol 171:4329–4339. doi: 10.4049/jimmunol.171.8.4329. [DOI] [PubMed] [Google Scholar]
- 9.Bjarnsholt T, Jensen PO, Burmolle M, Hentzer M, Haagensen JA, Hougen HP, Calum H, Madsen KG, Moser C, Molin S, Hoiby N, Givskov M. 2005. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology 151:373–383. doi: 10.1099/mic.0.27463-0. [DOI] [PubMed] [Google Scholar]
- 10.Alhede M, Bjarnsholt T, Jensen PO, Phipps RK, Moser C, Christophersen L, Christensen LD, van Gennip M, Parsek M, Hoiby N, Rasmussen TB, Givskov M. 2009. Pseudomonas aeruginosa recognizes and responds aggressively to the presence of polymorphonuclear leukocytes. Microbiology 155:3500–3508. doi: 10.1099/mic.0.031443-0. [DOI] [PubMed] [Google Scholar]
- 11.Moskowitz SM, Foster JM, Emerson JC, Gibson RL, Burns JL. 2005. Use of Pseudomonas biofilm susceptibilities to assign simulated antibiotic regimens for cystic fibrosis airway infection. J Antimicrob Chemother 56:879–886. doi: 10.1093/jac/dki338. [DOI] [PubMed] [Google Scholar]
- 12.Fricks-Lima J, Hendrickson CM, Allgaier M, Zhuo H, Wiener-Kronish JP, Lynch SV, Yang K. 2011. Differences in biofilm formation and antimicrobial resistance of Pseudomonas aeruginosa isolated from airways of mechanically ventilated patients and cystic fibrosis patients. Int J Antimicrob Agents 37:309–315. doi: 10.1016/j.ijantimicag.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poole K. 2011. Pseudomonas aeruginosa: resistance to the max. Front Microbiol 2:65. doi: 10.3389/fmicb.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonstein SA, Burnham JC. 1993. Effect of low concentrations of quinolone antibiotics on bacterial virulence mechanisms. Diagn Microbiol Infect Dis 16:277–289. doi: 10.1016/0732-8893(93)90078-L. [DOI] [PubMed] [Google Scholar]
- 15.Wozniak DJ, Keyser R. 2004. Effects of subinhibitory concentrations of macrolide antibiotics on Pseudomonas aeruginosa. Chest 125:62S–69S. doi: 10.1378/chest.125.2_suppl.62S. [DOI] [PubMed] [Google Scholar]
- 16.Fonseca AP, Extremina C, Fonseca AF, Sousa JC. 2004. Effect of subinhibitory concentration of piperacillin/tazobactam on Pseudomonas aeruginosa. J Med Microbiol 53:903–910. doi: 10.1099/jmm.0.45637-0. [DOI] [PubMed] [Google Scholar]
- 17.Alhajlan M, Alhariri M, Omri A. 2013. Efficacy and safety of liposomal clarithromycin and its effect on Pseudomonas aeruginosa virulence factors. Antimicrob Agents Chemother 57:2694–2704. doi: 10.1128/AAC.00235-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walters MC III, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. 2003. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother 47:317–323. doi: 10.1128/AAC.47.1.317-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing; 17th informational supplement. CLSI document M100-S17 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 20.Caiazza NC, Merritt JH, Brothers KM, O'Toole GA. 2007. Inverse regulation of biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J Bacteriol 189:3603–3612. doi: 10.1128/JB.01685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peeters E, Nelis HJ, Coenye T. 2008. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods 72:157–165. doi: 10.1016/j.mimet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Roilides E, Uhlig K, Venzon D, Pizzo PA, Walsh TJ. 1993. Enhancement of oxidative response and damage caused by human neutrophils to Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect Immun 61:1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez LM, Alvarez BL, Codony F, Fittipaldi M, Adrados B, Penuela G, Morato J. 2010. A new microtitre plate screening method for evaluating the viability of aerobic respiring bacteria in high surface biofilms. Lett Appl Microbiol 51:331–337. doi: 10.1111/j.1472-765X.2010.02902.x. [DOI] [PubMed] [Google Scholar]
- 24.Chatzimoschou A, Katragkou A, Simitsopoulou M, Antachopoulos C, Georgiadou E, Walsh TJ, Roilides E. 2011. Activities of triazole-echinocandin combinations against Candida species in biofilms and as planktonic cells. Antimicrob Agents Chemother 55:1968–1974. doi: 10.1128/AAC.00959-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bodey GP, Valdivieso M, Feld R, Rodriguez V. 1974. Pharmacology of amikacin in humans. Antimicrob Agents Chemother 5:508–512. doi: 10.1128/AAC.5.5.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldfarb J, Wormser GP, Inchiosa MA Jr, Guideri G, Diaz M, Gandhi R, Goltzman C, Mascia AV. 1986. Single-dose pharmacokinetics of oral ciprofloxacin in patients with cystic fibrosis. J Clin Pharmacol 26:222–226. doi: 10.1002/j.1552-4604.1986.tb02938.x. [DOI] [PubMed] [Google Scholar]
- 27.Fabre D, Bressolle F, Gomeni R, Arich C, Lemesle F, Beziau H, Galtier M. 1991. Steady-state pharmacokinetics of ciprofloxacin in plasma from patients with nosocomial pneumonia: penetration of the bronchial mucosa. Antimicrob Agents Chemother 35:3521–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byl B, Baran D, Jacobs F, Herschuelz A, Thys JP. 2001. Serum pharmacokinetics and sputum penetration of amikacin 30 mg/kg once daily and of ceftazidime 200 mg/kg/day as a continuous infusion in cystic fibrosis patients. J Antimicrob Chemother 48:325–327. doi: 10.1093/jac/48.2.325. [DOI] [PubMed] [Google Scholar]
- 29.Luyt CE, Clavel M, Guntupalli K, Johannigman J, Kennedy JI, Wood C, Corkery K, Gribben D, Chastre J. 2009. Pharmacokinetics and lung delivery of PDDS-aerosolized amikacin (NKTR-061) in intubated and mechanically ventilated patients with nosocomial pneumonia. Crit Care 13:R200. doi: 10.1186/cc8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chono S, Tanino T, Seki T, Morimoto K. 2008. Efficient drug delivery to alveolar macrophages and lung epithelial lining fluid following pulmonary administration of liposomal ciprofloxacin in rats with pneumonia and estimation of its antibacterial effects. Drug Dev Ind Pharm 34:1090–1096. doi: 10.1080/03639040801958421. [DOI] [PubMed] [Google Scholar]
- 31.Mulcahy H, Charron-Mazenod L, Lewenza S. 2008. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog 4:e1000213. doi: 10.1371/journal.ppat.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tauber SC, Nau R. 2008. Immunomodulatory properties of antibiotics. Curr Mol Pharmacol 1:68–79. doi: 10.2174/1874467210801010068. [DOI] [PubMed] [Google Scholar]
- 33.Labro MT. 2012. Immunomodulatory effects of antimicrobial agents. Part I. Antibacterial and antiviral agents. Expert Rev Anti Infect Ther 10:319–340. doi: 10.1586/eri.12.11. [DOI] [PubMed] [Google Scholar]
- 34.van Gennip M, Christensen LD, Alhede M, Qvortrup K, Jensen PO, Hoiby N, Givskov M, Bjarnsholt T. 2012. Interactions between polymorphonuclear leukocytes and Pseudomonas aeruginosa biofilms on silicone implants in vivo. Infect Immun 80:2601–2607. doi: 10.1128/IAI.06215-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen ET, Kharazmi A, Lam K, Costerton JW, Hoiby N. 1990. Human polymorphonuclear leukocyte response to Pseudomonas aeruginosa grown in biofilms. Infect Immun 58:2383–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolpen M, Hansen CR, Bjarnsholt T, Moser C, Christensen LD, van Gennip M, Ciofu O, Mandsberg L, Kharazmi A, Doring G, Givskov M, Hoiby N, Jensen PO. 2010. Polymorphonuclear leucocytes consume oxygen in sputum from chronic Pseudomonas aeruginosa pneumonia in cystic fibrosis. Thorax 65:57–62. doi: 10.1136/thx.2009.114512. [DOI] [PubMed] [Google Scholar]
- 37.Zhanel GG, Karlowsky JA, Hoban DJ, Davidson RJ. 1991. Antimicrobial activity of subinhibitory concentrations of aminoglycosides against Pseudomonas aeruginosa as determined by the killing-curve method and the postantibiotic effect. Chemotherapy 37:114–121. doi: 10.1159/000238842. [DOI] [PubMed] [Google Scholar]
- 38.Walker SG, Beveridge TJ. 1988. Amikacin disrupts the cell envelope of Pseudomonas aeruginosa ATCC 9027. Can J Microbiol 34:12–18. doi: 10.1139/m88-003. [DOI] [PubMed] [Google Scholar]
- 39.Kadurugamuwa JL, Beveridge TJ. 1997. Natural release of virulence factors in membrane vesicles by Pseudomonas aeruginosa and the effect of aminoglycoside antibiotics on their release. J Antimicrob Chemother 40:615–621. doi: 10.1093/jac/40.5.615. [DOI] [PubMed] [Google Scholar]
- 40.Gressier B, Brunet C, Dine T, Luyckx M, Ballester L, Cazin M, Cazin JC. 1998. In vitro activity of aminoglycosides on the respiratory burst response in human polymorphonuclear neutrophils. Methods Find Exp Clin Pharmacol 20:819–824. doi: 10.1358/mf.1998.20.10.487532. [DOI] [PubMed] [Google Scholar]
- 41.Shigeta M, Tanaka G, Komatsuzawa H, Sugai M, Suginaka H, Usui T. 1997. Permeation of antimicrobial agents through Pseudomonas aeruginosa biofilms: a simple method. Chemotherapy 43:340–345. doi: 10.1159/000239587. [DOI] [PubMed] [Google Scholar]
- 42.Abdi-Ali A, Mohammadi-Mehr M, Agha Alaei Y. 2006. Bactericidal activity of various antibiotics against biofilm-producing Pseudomonas aeruginosa. Int J Antimicrob Agents 27:196–200. doi: 10.1016/j.ijantimicag.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Goh EB, Yim G, Tsui W, McClure J, Surette MG, Davies J. 2002. Transcriptional modulation of bacterial gene expression by subinhibitory concentrations of antibiotics. Proc Natl Acad Sci U S A 99:17025–17030. doi: 10.1073/pnas.252607699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garske LA, Beatson SA, Leech AJ, Walsh SL, Bell SC. 2004. Sub-inhibitory concentrations of ceftazidime and tobramycin reduce the quorum sensing signals of Pseudomonas aeruginosa. Pathology 36:571–575. doi: 10.1080/00313020400011300. [DOI] [PubMed] [Google Scholar]
- 45.Skindersoe ME, Alhede M, Phipps R, Yang L, Jensen PO, Rasmussen TB, Bjarnsholt T, Tolker-Nielsen T, Hoiby N, Givskov M. 2008. Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother 52:3648–3663. doi: 10.1128/AAC.01230-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jensen PO, Bjarnsholt T, Phipps R, Rasmussen TB, Calum H, Christoffersen L, Moser C, Williams P, Pressler T, Givskov M, Hoiby N. 2007. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology 153:1329–1338. doi: 10.1099/mic.0.2006/003863-0. [DOI] [PubMed] [Google Scholar]
- 47.Labro MT, el Benna J. 1990. Comparison of cefodizime with various cephalosporins for their indirect effect on the human neutrophil oxidative burst in vitro. J Antimicrob Chemother 26(Suppl C):49–57. doi: 10.1093/jac/26.suppl_C.49. [DOI] [PubMed] [Google Scholar]
- 48.Schlaeffer F, Blaser J, Laxon J, Zinner S. 1990. Enhancement of leucocyte killing of resistant bacteria selected during exposure to aminoglycosides or quinolones. J Antimicrob Chemother 25:941–948. doi: 10.1093/jac/25.6.941. [DOI] [PubMed] [Google Scholar]
- 49.Canton E, Peman J, Cabrera E, Velert M, Orero A, Pastor A, Gobernado M. 1999. Killing of Gram-negative bacteria by ciprofloxacin within both healthy human neutrophils and neutrophils with inactivated O2-dependent bactericidal mechanisms. Chemotherapy 45:268–276. doi: 10.1159/000007196. [DOI] [PubMed] [Google Scholar]
- 50.Oldak E, Trafny EA. 2005. Secretion of proteases by Pseudomonas aeruginosa biofilms exposed to ciprofloxacin. Antimicrob Agents Chemother 49:3281–3288. doi: 10.1128/AAC.49.8.3281-3288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jensen PO, Briales A, Brochmann RP, Wang H, Kragh KN, Kolpen M, Hempel C, Bjarnsholt T, Hoiby N, Ciofu O. 2014. Formation of hydroxyl radicals contributes to the bactericidal activity of ciprofloxacin against Pseudomonas aeruginosa biofilms. Pathog Dis 70:440–443. doi: 10.1111/2049-632X.12120. [DOI] [PubMed] [Google Scholar]