Abstract
Ceftazidime is a beta-lactam compound that exerts a time-dependent bactericidal effect. Numerous arguments are in favor of continuous administration of ceftazidime, both for reasons of clinical efficacy and to preserve bacteriological mutation. We report a prospective, single-center, parallel-group, randomized, controlled trial comparing two modes of administration of ceftazidime, namely, continuous administration (loading dose of 20 mg/kg of body weight followed by 60 mg/kg/day) versus intermittent administration (20 mg/kg over 30 min every 8 h) in 34 patients with ventilator-associated pneumonia due to Gram-negative bacilli. The study was performed over 48 h with 13 and 18 assessments of serum ceftazidime in the continuous-infusion group (group A) and the intermittent-fusion group (group B), respectively. Bronchoalveolar lavage (BAL) was performed at steady state in both groups at 44 h to determine ceftazidime levels in the epithelial lining fluid. We chose a predefined threshold of 20 mg/liter for serum concentrations of ceftazidime because of ecological conditions in our center. The median time above 20 mg/liter (T>20 mg) was 100% in group A versus 46% in group B. In group A, 14/17 patients had 100% T>20 mg, versus only 1/17 patients in group B. In the epithelial lining fluid, the median concentration of ceftazidime was 12 mg/liter in group A versus 6 mg/liter in group B. A threshold of 8 mg/liter in the epithelial lining fluid was achieved twice as often in group A as in group B. This study of ceftazidime concentrations in the epithelial lining fluid indicates that continuous infusion presents advantages in terms of pharmacodynamics and predictable efficacy in patients presenting ventilator-associated pneumonia.
INTRODUCTION
Ceftazidime is a third-generation cephalosporin that is frequently used in the treatment of ventilator-associated pneumonia (VAP) because of its efficacy against Pseudomonas aeruginosa. Ceftazidime is a beta-lactam compound that exerts a time-dependent bactericidal effect. The pharmacodynamic property that predicts better clinical efficacy in vitro is the time during which the tissue concentration of the antibiotic is greater than the MIC of the organism (1, 2). Critically ill patients with severe sepsis present wide intra- and interindividual variations in volume of distribution, thus altering the pharmacokinetics of the antibiotic (2–4). A number of elements plead in favor of continuous administration of ceftazidime, both for reasons of clinical efficacy and to preserve bacteriological mutation (2, 5). In view of local ecological conditions in our center (MIC of ceftazidime for Pseudomonas aeruginosa of <4 mg/liter), we aimed to achieve a minimum serum concentration of 20 mg/liter.
The primary objective of this study was to show that continuous infusion of ceftazidime is superior to intermittent infusion, as assessed by the concentration of ceftazidime in the epithelial lining fluid (ELF), in patients with VAP due to Gram-negative bacilli. The secondary objective was to show that continuous infusion is superior to intermittent infusion of ceftazidime in terms of the length of time during which the serum concentration of ceftazidime is maintained above a predefined threshold.
MATERIALS AND METHODS
Study design.
We designed a single-center, controlled randomized trial in two parallel groups comparing two modes of administration of ceftazidime, namely, continuous and intermittent administration, according to a standard therapeutic management approach consisting of 3 injections per 24 h, with weight-adjusted doses, in patients undergoing mechanical ventilation and presenting hypoxemic pneumonia of nosocomial original due to Gram-negative bacilli. All patients who met the inclusion criteria (age of >18 years and confirmed diagnosis of VAP due to Gram-negative bacilli) and who did not present any noninclusion criteria (weight of >110 kg, being pregnant or breastfeeding, known allergy to beta-lactam antibiotics, renal failure with creatinine clearance of <60 ml/min as calculated by the Cockcroft-Gault formula, known history of pulmonary fibrosis, or concurrent participation in another clinical trial) were consecutively included in our study. Patients were randomly allocated to a treatment group. Randomization was performed in blocks of 6 patients. The study was performed in accordance with French legislation for human research, with the Declaration of Helsinki, and with good clinical practice. The study was approved by the Committee for the Protection of Persons Participating in Human Research (Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale) of the Champagne-Ardennes region (France), authorization number 050230.
Patients.
Patients were recruited in the mixed intensive care unit (ICU) of the university teaching hospital (Hôpital Robert Debré) of Reims (France). Patients with clinical symptoms suggestive of VAP underwent bronchoalveolar lavage (BAL). When BAL revealed the presence of Gram-negative bacilli, eligible patients were included in the study once they had provided written informed consent.
After inclusion, patients were randomized to one of the two treatment arms, as follows. Patients in group A were treated by continuous administration of ceftazidime, given as an intravenous (i.v.) bolus of 20 mg/kg over 30 min, followed by 60 mg/kg/day as a continuous infusion. Patients in group B were treated by intermittent administration, i.e., 20 mg/kg ceftazidime over 30 min, administered every 8 h. Patients in both groups also received tobramycin at a dose of 5 mg/kg i.v. over 30 min, once daily.
Data collection.
Age, sex, weight, creatinine clearance, and SAPS (simplified acute physiology score) II were collected. Pharmacokinetic and pharmacodynamic properties of ceftazidime were also collected: T>20 mg, fraction of time, during the first 48 h of treatment, during which serum concentrations of ceftazidime remained above the 20-mg/liter threshold; AUC0–48, area under the concentration-time curve calculated over 48 h; CELF, concentration of ceftazidime in the epithelial lining fluid; Cmin, minimum ceftazidime concentration; Ceq, steady-state ceftazidime concentration; Cmax, maximum ceftazidime concentration; and V, volume of distribution.
Endpoint assessment.
The mean MIC of ceftazidime for P. aeruginosa in our department was estimated at 2 mg/liter. The target threshold value for ceftazidime in the ELF was thus fixed at 8 mg/liter, with a corresponding serum threshold of 20 mg/liter.
The primary endpoint was average concentration of ceftazidime in the ELF at 44 h. The half-life of ceftazidime is approximately 2 h. With continuous infusion, steady state is achieved after 5 half-lives (2, 6), i.e., approximately 10 h. By intermittent infusion, boluses were administered every 8 h, and thus, the 44th hour represents the median time between the 5th and 6th injection. Therefore, we estimated that assessment of ceftazidime concentrations at 44 h would guarantee measurement at steady state in both groups.
The secondary endpoint was the fraction of time, during the first 48 h of treatment, during which serum concentrations of ceftazidime remained above the 20-mg/liter threshold.
Pharmacological methods for ceftazidime measurement.
Bronchoalveolar lavage was performed using a flexible fiberscope by injecting 60 ml of sterile saline solution (0.9%). The first sample recovered was disposed of, and the second sample, which was of varying quantity (around 6 ml), was separated into 2 aliquots for assessment of urea and ceftazidime and sent immediately to the laboratory for analysis. All blood tests were performed in 5-ml dry tubes and dispatched to the Pharmacology & Toxicology Laboratory of the Reims University teaching hospital for analysis. After centrifugation, samples were frozen at −20°C and stored for later analysis. Ceftazidime concentrations in the serum and BAL fluid samples were analyzed after a simple deproteinization step, using reverse-phase high-performance liquid chromatography, coupled with UV detection at 258 nm.
Epithelial lining fluid and BAL fluid.
We analyzed penetration of ceftazidime into the pulmonary tissue by determining the concentration of ceftazidime in the ELF. After deproteinization, ceftazidime concentrations in the serum and ELF were determined by reverse-phase high performance liquid chromatography, coupled with UV detection. Briefly, 500 μl of methanol was added to 100 μl of the fluid sample (serum or ELF). The mixture was shaken for 1 min on a vortex mixer and then centrifuged for 4 min at 10,000 rpm. Then, 20 μl of the surfactant was injected into the chromatography system. The analytical column was reverse phase, and the mobile phase consisted of a mixture of isocratic acetonitrile-phosphate buffer adjusted to pH 3 (13/87 [vol/vol]), delivered at a flow rate of 1.2 ml/min with UV detection at 258 nm. With these analysis conditions, the time required for analysis of each sample is less than 6 min, the quantification limit is 1 mg/liter, and the linear range is from 1 to 200 mg/liter. The accuracy and repeatability of the method were <5% for the range of concentrations studied (5 mg/liter and 50 mg/liter).
Dosing antibiotics at the level of the ELF is a validated technique for the measurement of antibiotic concentrations in the pulmonary extracellular spaces (6). To evaluate pulmonary penetration of ceftazidime, BAL was performed at 44 h after the start of drug administration. The concentration of ceftazidime in the ELF was obtained by measuring ceftazidime in the BAL liquid, and the ratio of serum to BAL fluid urea. At steady state, the concentration of urea in the ELF is the same as that in the serum. The ratio of serum urea concentration to BAL fluid urea concentration thus represents the dilution factor for BAL fluid that allows estimation of the ceftazidime concentration in the ELF as [ceftazidime]BAL × ([urea]serum/[urea]BAL) (1), where [urea]serum and [urea]BAL represent, respectively, the concentrations of urea in the serum and BAL fluid.
Analysis methods. (i) Pharmacokinetic analysis.
Samples were taken over the first 48 h as follows. In group A, 13 blood samples to measure ceftazidime concentrations were taken, at 0, 15, 30, and 45 min and at 1, 2, 4, 8, 16, 24, 32, 40, and 48 h. In group B, 18 blood samples to measure ceftazidime concentrations were taken, at 0, 15, 30, and 45 min and at 1, 2, 4, 8, 9, 16, 17, 24, 25, 32, 33, 40, 41, and 44 h. In both groups, BAL was performed to obtain a sample of ELF at 44 h after treatment initiation.
For all patients, the course of serum ceftazidime concentrations over time is presented on a semilogarithmic scale.
In group B, individual pharmacokinetic profiles were estimated using simulation based on 7 samples obtained after the first ceftazidime injection. After 8 h, only 2 blood tests were performed per 8-h period (one immediately before administration of the next bolus of ceftazidime and another 1 h after injection). Thus, in total, 18 blood tests for ceftazidime measurement were performed in group B.
Thus, in group A, individual pharmacokinetic profiles were modeled based on the 9 blood tests performed during the first 16 h, making it possible to observe the achievement of steady state. For the remaining 22 h, the number of blood tests was limited to 5. Thus, only 13 blood tests in total were performed to measure serum ceftazidime in group A.
A two-compartment pharmacokinetic model used to establish individual profiles of ceftazidime concentration in the study population. Analysis was performed by nonlinear regression using WinNonlin software, version 4.1 (Pharsight/Certara, Saint Louis, MO, USA). The pharmacokinetic parameters retained in the analysis were volume of distribution at steady state (V) and the area under the curve calculated over 48 h (AUC0–48). The ratio of AUC to MIC was calculated for each patient with Pseudomonas aeruginosa infection.
(ii) Statistical analysis.
Quantitative variables are described as medians and 5th and 95th percentiles, due to their nonnormal distributions. Qualitative variables are expressed as n (percentage).
The Wilcoxon test was used to compare quantitative variables, and Fisher's exact test was used for qualitative variables. A P value of <0.05 was considered statistically significant. All analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Study population.
In total, 34 patients were included, 17 in each group. Table 1 presents the baseline characteristics of the two groups. There were no significant differences between groups in terms of age, sex, weight, creatinine clearance, and SAPS II. Among the 34 patients included, 5 (14.7%) died in the ICU (3 in group A and 2 in group B).
TABLE 1.
Baseline characteristics in the two study groups
| Characteristic | Value for groupa |
|
|---|---|---|
| Continuous infusion (n = 17) | Intermittent infusion (n = 17) | |
| Age (yr) | 70 (44–82) | 61 (31–79) |
| No. (%) of men/women | 13 (76.5)/4 (23.5) | 14 (82.4)/3 (17.6) |
| Wt (kg) | 72 (51–104) | 75 (45–107) |
| Creatinine clearance | 101 (80–144) | 115 (86–137) |
| SAPS II | 43 (18–83) | 41 (17–70) |
Values are medians (5th and 95th percentiles) for all data except no. of men/women. The differences between values for the two groups were not significant for any characteristic.
Microbiological findings.
Among the 34 episodes of VAP, 8 (23.5%) were due to multiple pathogens. Infection was predominantly caused by P. aeruginosa (67.6% of organisms identified). The median MIC of ceftazidime for P. aeruginosa was 1.5 mg/liter, with a range of 0.5 to 8.0 mg/liter (by agar culture).
Pharmacological findings.
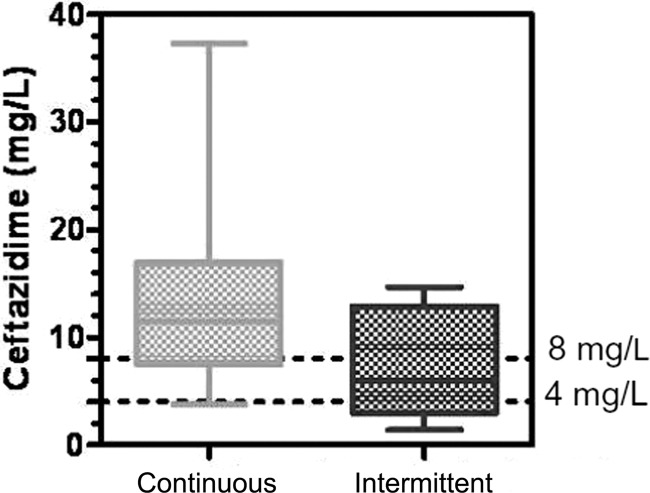
For each patient included, serum ceftazidime concentrations were modeled by computer based on the values obtained from the different blood tests. The individual profiles were averaged for each group, yielding two summary curves of the kinetics of serum ceftazidime concentrations (Fig. 1 and 2). The spread of ceftazidime concentrations in the ELF (CELF) for each group is shown in Fig. 3.
FIG 1.
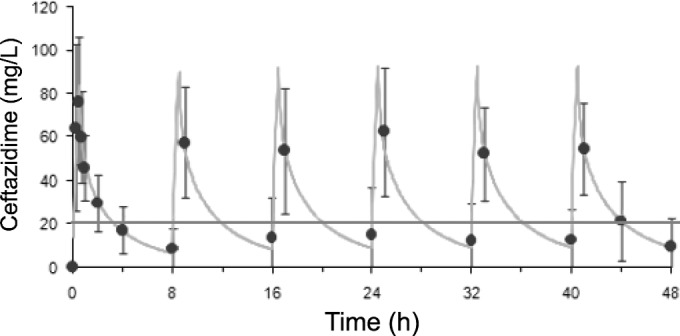
Course of serum concentrations of ceftazidime over time with intermittent administration. Each point represents the mean ± standard deviation for each blood sample. The curve represents the mean of the computer-assisted modeling.
FIG 2.
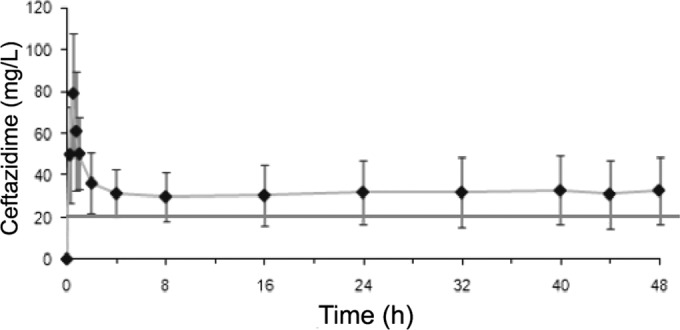
Course of serum concentrations of ceftazidime over time with continuous perfusion. Each point represents the mean ± standard deviation for each blood sample. The curve represents the mean of the computer-assisted modeling.
FIG 3.
Ceftazidime concentrations in the epithelial lining fluid.
With regard to the primary endpoint, the median concentration of ceftazidime in the ELF was 12 mg/liter in group A versus 6 mg/liter in group B (P < 0.08) (Table 2). Regarding the secondary endpoint, the fraction of time during which the serum concentration of ceftazidime remained above the threshold of 20 mg/liter was 100% (range, 3 to 100) in group A versus 46% (range, 16 to 100) in group B (P < 0.003). There was wide interindividual variation in ceftazidime concentrations in the serum and in the ELF in both groups (Fig. 3). However, no significant difference was observed between groups in terms of AUC over 48 h or volume of distribution at steady state (V).
TABLE 2.
Pharmacokinetic and pharmacodynamic properties of ceftazidime
| Propertya | Value for groupb |
Pc | |
|---|---|---|---|
| Continuous infusion (n = 17) | Intermittent infusion (n = 17) | ||
| T>20 mg (%) | 100 (3–100) | 46 (16–100) | 0.003 |
| AUC0–48 (mg · h/liter) | 1,348 (972–3,200) | 1,361 (566–3,969) | NS |
| CELF (44 h) (mg/liter) | 12 (1–40) | 6 (0–28) | 0.08 |
| Pulmonary penetration (%) | 42 (5–62) | 44 (0–97) | NS |
| Cmin (mg/liter) | ND | 6 (0.7–33) | |
| Ceq (mg/liter) | 27 (13–82) | ND | |
| Cmax (mg/liter) | ND | 95 (46–177) | |
| V (liters/kg) | 0.4 (0.1–0.8) | 0.3 (0.2–0.8) | NS |
T>20 mg, fraction of time, during the first 48 h of treatment, during which serum concentrations of ceftazidime remained above the 20-mg/liter threshold; AUC0–48, area under the curve calculated over 48 h; CELF, concentration of ceftazidime in the epithelial lining fluid; Cmin, minimum ceftazidime concentration; Ceq, steady-state ceftazidime concentration; Cmax, maximum ceftazidime concentration; V, volume of distribution.
Values are medians (5th and 95th percentiles). ND, not determined.
NS, not significant.
In patients with confirmed infection by P. aeruginosa (11 in group A and 9 in group B), the AUC/MIC ratio was 670 (144 to 2,133) and 891 (378 to 3,082) for groups A and B, respectively; the difference was not significant (P = 0.52). Among these patients, the median MICs were 1.75 mg/liter (0.75 to 8) and 1.50 mg/liter (0.5 to 2) for groups A and B, respectively, and again, the difference was not significant (P = 0.10).
DISCUSSION
This pharmacological study investigated ceftazidime concentrations at two levels, namely, in the serum and in the ELF, and made it possible to attain the initial objective. The method used in this study is similar to that used in other works. For example, Benko et al. (7) drew 18 blood samples on days 2 and 4 from each group in their prospective, randomized, crossover study of 12 critically ill patients with infections with suspected Gram-negative organisms, while Lipman et al. (8) investigated continuous versus intermittent infusion in a randomized trial of 18 patients in critical care with 11 samples in each group. Our findings confirm the pharmacodynamic advantage of continuous infusion, in terms of serum concentrations of ceftazidime, as reflected by the proportion of time during which the serum concentration of ceftazidime remained above the threshold of 20 mg/liter (T>20 mg). Several other authors have reported similar findings (9–13). Contrary to the observations of Benko et al. (7), the median AUC over 48 h did not differ significantly between modes of administration in our study. However, Benko et al. used a crossover design, which reduces the impact of interindividual variability.
The profile of the kinetics of serum ceftazidime concentrations in our study was in line with pharmacokinetic models. During continuous administration, the ceftazidime concentration stabilizes quickly at a steady-state concentration. Intermittent administration gives rise to variations in serum concentrations that may in turn cause therapy to be inefficacious or select resistant strains (14).
The originality of this work lies in the fact that we studied the diffusion of ceftazidime to the ELF by two different modes of administration and showed a borderline significant result in favor of continuous infusion. The assessment of ceftazidime concentrations in the ELF was performed at 44 h after initiation of therapy in order to ensure that the continuous-infusion group had achieved steady state and to place it between the two last injections in the intermittent infusion group. It would probably have been useful to evaluate an additional measure of kinetics by testing another sample, but the ethics committee refused permission to perform any additional fiberscopic examinations. In the literature, only one study has compared continuous versus intermittent administration of ceftazidime in terms of tissue uptake at the site of action (15). In that study, ceftazidime concentrations in the serum and peritoneal exudate were compared after continuous versus intermittent infusion in 12 critically ill patients with peritonitis, and those authors concluded that continuous administration was superior in the context of severe intra-abdominal infection. One study in a porcine model of pneumonia due to P. aeruginosa compared lung deposition of i.v. ceftazidime administered either continuously or intermittently using postmortem biopsies, and those authors found that ceftazidime concentrations in the pulmonary tissue were higher after continuous administration (16).
Boselli et al. (6, 9) also studied ceftazidime concentrations in serum and ELF after administration of a continuous infusion at a dose of 4 g/day in 15 ICU patients with VAP. Steady-state ceftazidime concentration in the epithelial lining fluid was 8.2 ± 4.8 mg/liter, and mean penetration of ceftazidime into the ELF was 20.6 ± 8.9%, values that are comparable to those observed in our study.
In the subgroup of confirmed Pseudomonas aeruginosa infections, the ratio of the AUC over 48 h to the MIC, which reflects overall exposure to the antibiotic, was not significantly different between groups in our study, despite the use of weight-adjusted doses. However, the ratio of AUC to MIC has been evaluated mainly as a predictive factor for efficacy with fluoroquinolones (2, 17). Our failure to observe any significant difference in this parameter could also be explained by the lack of power of our study.
Conclusion.
Several theoretical elements plead in favor of continuous administration of ceftazidime, in terms of both clinical efficacy and preventing the appearance of resistant strains. However, in terms of mortality, there appears to be no difference between the two modes of administration. Evaluation of serum concentrations of ceftazidime confirms that continuous infusion achieves better pharmacodynamic results, with a stable concentration in the serum over the treatment period. With regard to drug concentration at the target treatment site, namely, the epithelial lining fluid, our study also suggests that continuous administration of ceftazidime presents a pharmacodynamic advantage in terms of predictable efficacy in patients with ventilator-associated pneumonia.
ACKNOWLEDGMENTS
We thank Guillaume Hoizey for the determination of samples. We also thank Fiona Ecarnot (EA3920, University Hospital Besancon, France) for translation and editorial assistance.
REFERENCES
- 1.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 2.Garraffo R, Lavrut T. 2005. Signification clinique des corrélations pharmacocinétique/pharmacodynamie des antibiotiques chez les patients de réanimation. Réanimation 14:264–275. [Google Scholar]
- 3.Bulitta JB, Landersdorfer CB, Hüttner SJ, Drusano GL, Kinzig M, Holzgrabe U, Stephan U, Sörgel F. 2010. Population pharmacokinetic comparison and pharmacodynamic breakpoints of ceftazidime in cystic fibrosis patients and healthy volunteers. Antimicrob Agents Chemother 54:1275–1282. doi: 10.1128/AAC.00936-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gómez CM, Cordingly JJ, Palazzo MG. 1999. Altered pharmacokinetics of ceftazidime in critically ill patients. Antimicrob Agents Chemother 43:1798–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Herendael B, Jeurissen A, Tulkens PM, Vlieghe E, Verbrugghe W, Jorens PG, Ieven M. 2012. Continuous infusion of antibiotics in the critically ill: the new holy grail for beta-lactams and vancomycin? Ann Intensive Care 2:22. doi: 10.1186/2110-5820-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boselli E, Allaouchiche B. 2001. Pulmonary diffusion of antibiotics. Critical analysis of the literature. Ann Fr Anesth Reanim 20:612–630. doi: 10.1016/S0750-7658(01)00439-7. [DOI] [PubMed] [Google Scholar]
- 7.Benko AS, Cappelletty DM, Kruse JA, Rybak MJ. 1996. Continuous infusion versus intermittent administration of ceftazidime in critically ill patients with suspected gram-negative infections. Antimicrob Agents Chemother 40:691–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipman J, Gomersall CD, Gin T, Joynt GM, Young RJ. 1999. Continuous infusion ceftazidime in intensive care: a randomized controlled trial. J Antimicrob Chemother 43:309–311. doi: 10.1093/jac/43.2.309. [DOI] [PubMed] [Google Scholar]
- 9.Boselli E, Breilh D, Rimmelé T, Poupelin JC, Saux MC, Chassard D, Allaouchiche B. 2004. Plasma and lung concentrations of ceftazidime administered in continuous infusion to critically ill patients with severe nosocomial pneumonia. Intensive Care Med 30:989–991. doi: 10.1007/s00134-004-2171-2. [DOI] [PubMed] [Google Scholar]
- 10.Lorente L, Jiménez A, Palmero S, Jiménez JJ, Iribarren JL, Santana M, Martín MM, Mora ML. 2007. Comparison of clinical cure rates in adults with ventilator-associated pneumonia treated with intravenous ceftazidime administered by continuous or intermittent infusion: a retrospective, nonrandomized, open-label, historical chart review. Clin Ther 29:2433–2439. doi: 10.1016/j.clinthera.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Nicolau DP, McNabb J, Lacy MK, Quintiliani R, Nightingale CH. 2001. Continuous versus intermittent administration of ceftazidime in intensive care unit patients with nosocomial pneumonia. Int J Antimicrob Agents 17:497–504. doi: 10.1016/S0924-8579(01)00329-6. [DOI] [PubMed] [Google Scholar]
- 12.Young RJ, Lipman J, Gin T, Gomersall CD, Joynt GM, Oh TE. 1997. Intermittent bolus dosing of ceftazidime in critically ill patients. J Antimicrob Chemother 40:269–273. doi: 10.1093/jac/40.2.269. [DOI] [PubMed] [Google Scholar]
- 13.Teo J, Liew Y, Lee W, Kwa ALH. 2014. Prolonged infusion versus intermittent boluses of β-lactam antibiotics for treatment of acute infections: a meta-analysis. Int J Antimicrob Agents 43:403–411. doi: 10.1016/j.ijantimicag.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Mouton JW, Vinks AA, Punt NC. 1997. Pharmacokinetic-pharmacodynamic modeling of activity of ceftazidime during continuous and intermittent infusion. Antimicrob Agents Chemother 41:733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buijk SLCE, Gyssens IC, Mouton JW, Van Vliet A, Verbrugh HA, Bruining HA. 2002. Pharmacokinetics of ceftazidime in serum and peritoneal exudate during continuous versus intermittent administration to patients with severe intra-abdominal infections. J Antimicrob Chemother 49:121–128. doi: 10.1093/jac/49.1.121. [DOI] [PubMed] [Google Scholar]
- 16.Girardi C, Tonnellier M, Goldstein I, Sartorius A, Wallet F, Rouby JJ, Experimental ICU Study Group . 2006. Lung deposition of continuous and intermittent intravenous ceftazidime in experimental Pseudomonas aeruginosa bronchopneumonia. Intensive Care Med 32:2042–2048. doi: 10.1007/s00134-006-0272-9. [DOI] [PubMed] [Google Scholar]
- 17.Craig WA. 2014. Are blood concentrations enough for establishing pharmacokinetic/pharmacodynamic relationships? Clin Infect Dis 58:1084–1085. doi: 10.1093/cid/ciu055. [DOI] [PubMed] [Google Scholar]