Abstract
Burkholderia mallei, the causative agent of glanders, is a CDC Tier 1 Select Agent for which there is no preventive vaccine and antibiotic therapy is difficult. In this study, we show that a combination of vaccination using killed cellular vaccine and therapy using moxifloxacin, azithromycin, or sulfamethoxazole-trimethoprim can protect BALB/c mice from lethal infection even when given 5 days after infectious challenge. Vaccination only, or antibiotic therapy only, was not efficacious. Although antibiotics evaluated experimentally can protect when given before or 1 day after challenge, this time course is not realistic in the cases of natural infection or biological attack, when the patient seeks treatment after symptoms develop or after a biological attack has been confirmed and the agent has been identified. Antibiotics can be efficacious after a prolonged interval between exposure and treatment, but only if the animals were previously vaccinated.
INTRODUCTION
Glanders, caused by the etiological agent Burkholderia mallei, is a very severe disease in humans, characterized by extreme pain, prostration, fever, and abscesses that can be found primarily in the spleen and liver but also in any organ and tissue throughout the body (1, 2). Although glanders was originally described over 2,000 years ago, effective countermeasures have not been sufficiently developed to provide protection for those at risk. The lack of suitable medical countermeasures, high infectivity by aerosol exposure, severe clinical symptoms, and a high rate of lethal infections in untreated cases resulted in classifying this microorganism as a CDC Tier 1 Select Agent (3).
Although poorly delineated, glanders disease symptoms can be acute (a more fulminant and rapidly fatal clinical course, including fever, malaise, myalgia, fatigue, inflammation, and swelling of the face and limbs and the development of painful nodules involving the face, arms, and legs [2, 4]) or chronic (multiple subcutaneous abscesses, enlarged lymph nodes, nodules which may ulcerate in respiratory and alimentary mucosa, clinical history of remission and exacerbation, necrotic foci in bones, and nodules in the viscera [5]). In the absence of effective treatment, glanders is almost always fatal. Localized infection is unusual.
Since cases of human glanders are rare, the compiled knowledge of this disease occurred mostly before the antibiotic era. Most human cases resulted from contact with infected equids, although the most recent cases were laboratory acquired (6, 7). Organisms usually enter the body through abrasions, through mucous membranes, or by inhalation. The incubation period is variable, ranging from less than a day to several weeks (1, 7). The infection will eventually become systemic.
In vitro susceptibility testing has shown that B. mallei is sensitive to a variety of antibiotics, including sulfonamides, aminoglycosides, ciprofloxacin, novobiocin, several tetracyclines, imipenem, and ceftazidime (8–10). Limited information exists regarding effective antibiotic treatments. The most-studied cases of human glanders followed laboratory exposures. Sulfonamides were used successfully in the first six U.S. laboratory-acquired infections (7). The most recent case had disseminated disease and developed abscesses of the spleen and liver. This patient was gravely ill, requiring ventilator assistance before improving on a prolonged course of several antibiotics, including imipenem and doxycycline (6). A 6-month course of doxycycline and azithromycin followed, and the patient recovered completely.
There is no evidence for immunity against glanders by virtue of previous infection (11). No glanders vaccine candidates described to date have provided consistent sterilizing immunity in animal studies, but some candidates were shown to extend the time to death after infectious challenge (12–14). Studies in my laboratory have also shown that killed cellular glanders vaccines can protect a majority of mice for at least 3 weeks after aerosol challenge, but mice typically remain infected (data not shown). Amemiya et al. found that nonviable B. mallei failed to protect mice from a parenteral live challenge (15). They examined heat-killed B. mallei, irradiation-inactivated B. mallei, and an irradiation-inactivated B. mallei capsule mutant in the BALB/c model of glanders and found a mixed T-cell helper 1 (Th1)- and Th2-like immune response to all of the nonviable cell preparations. It was suggested that nonviable B. mallei cell preparations did not protect mice in the study because of the induction of a mixed cytokine response and increased IgG1 versus IgG2a subclass response.
In this study, I combined 2 imperfect countermeasures against glanders (antibiotic therapy and vaccination) and show that this strategy can provide protection from acute lethal disease and also has the potential of eliminating residual infection after challenge.
MATERIALS AND METHODS
Experimental animals.
Specific-pathogen-free female BALB/c mice (10 mice per group) (Charles River-NCI, Frederick, MD), weighing between 20 and 25 g and approximately 6 to 8 weeks old, were housed in transparent plastic cages with microisolator tops in a biosafety level 3 facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International. The mice were given commercial rodent feed and tap water ad libitum. In conducting research with animals, the investigators adhered to the Guide for the Care and Use of Laboratory Animals (16). The USAMRIID IACUC approved all animal experiments described in this paper.
Microorganisms.
Burkholderia mallei (ATCC 23344; China 7 strain) was obtained from the American Type Culture Collection (ATCC, Manassas, VA). Prior to animal challenge, this strain was serially passaged three times in hamsters and designated GB15.1-3. In some experiments, GB15.1-3 isolated from a human case of glanders (designated strain FMH23344) was used as a challenge agent (6). B. mallei was propagated in glycerol (4%) tryptone broth (GTB; Difco) and on glycerol (4%) tryptone agar (GTA).
Experimental design.
Mice (10 per group) were vaccinated intraperitoneally (i.p.) with 100 μg of heat-killed (80°C for 3 h) Burkholderia pseudomallei strain 1026b. Four weeks later, mice were boosted with the same dose and route of candidate vaccine. Six weeks after the boost, mice were challenged with B. mallei by small-particle aerosol (see below).
After the index death, mice were treated with moxifloxacin (Moxi), azithromycin (Az), or sulfamethoxazole-trimethoprim (ST). Moxifloxacin therapy (16 mg/kg of body weight; every 12 h [Q12h]; i.p.) was initiated on day 3 or day 5 after challenge and administered for 3, 5, or 10 days. Azithromycin therapy was initiated on day 3 or day 5 after challenge (15 mg/kg; Q12h; i.p.) and administered for 5 or 10 days. Sulfamethoxazole (200-mg/kg)-trimethoprim (40-mg/kg; Q12h; i.p.) therapy was initiated on day 5 after challenge and administered for 5 or 10 days. Antibiotic dosing was based on appropriate pharmacokinetic (PK) values. Survival of individual mice within groups was monitored for at least 21 days after challenge.
Aerosol exposure.
Mice were exposed in a whole-body chamber to an aerosol generated with a Collison nebulizer (approximately 1-μm-diameter particle size) (17). Prior to use in challenge experiments, 25 ml of GTB was inoculated with 20 μl of B. mallei stock culture and incubated overnight in a shaking incubator (200 rpm) at 37°C. The concentration of microorganisms in the overnight bacterial culture was estimated using absorbance at 660 nm and a standard curve. The culture concentration was confirmed using plate counts. The bacterial culture was then diluted to the appropriate concentration predetermined to deliver the approximate desired challenge dose. After the aerosol exposure, actual doses were then calculated after determining the concentration of bacteria in all-glass impingers (determined using plate counts) and the respiratory volume and respiratory rate in mice of similar age and weight. Challenge doses ranged from 1 to 5 50% lethal doses (LD50). One LD50 is approximately 1,000 CFU of B. mallei when delivered by small-particle aerosol to BALB/c mice.
Presence of bacteria in spleens.
In order to estimate whether vaccinated, challenged, and treated survivors harbored residual bacterial infection, mice surviving the 21-day observation period were euthanized with CO2, and the presence of bacteria in the spleens was determined. Spleens were excised and dissociated in 5 ml of Hanks balanced salt solution (HBSS), and 0.1 ml of cell suspension was plated in triplicate on GTA, or spleens were cut longitudinally with a sterile scissors and “stamped” on the surface of GTA 3 times. Plates were incubated at 37°C for 72 h, and spleens were scored as “infected” or “not infected” based on bacterial growth. Typically, spleens with no obvious signs of abscess were dissociated and plated on GTA, while spleens with abscess were stamped on GTA to confirm the presence of viable bacteria. The sensitivity of the plating method for bacterial detection was approximately 50 microorganisms per spleen and greater.
Statistical analysis.
In order to determine whether differences in survival between untreated (HBSS inoculation) and treated (vaccine and/or antibiotic therapy) groups were significant after challenge, P values were calculated using the log rank test (Prism 6; GraphPad software).
RESULTS
Mice treated with azithromycin.
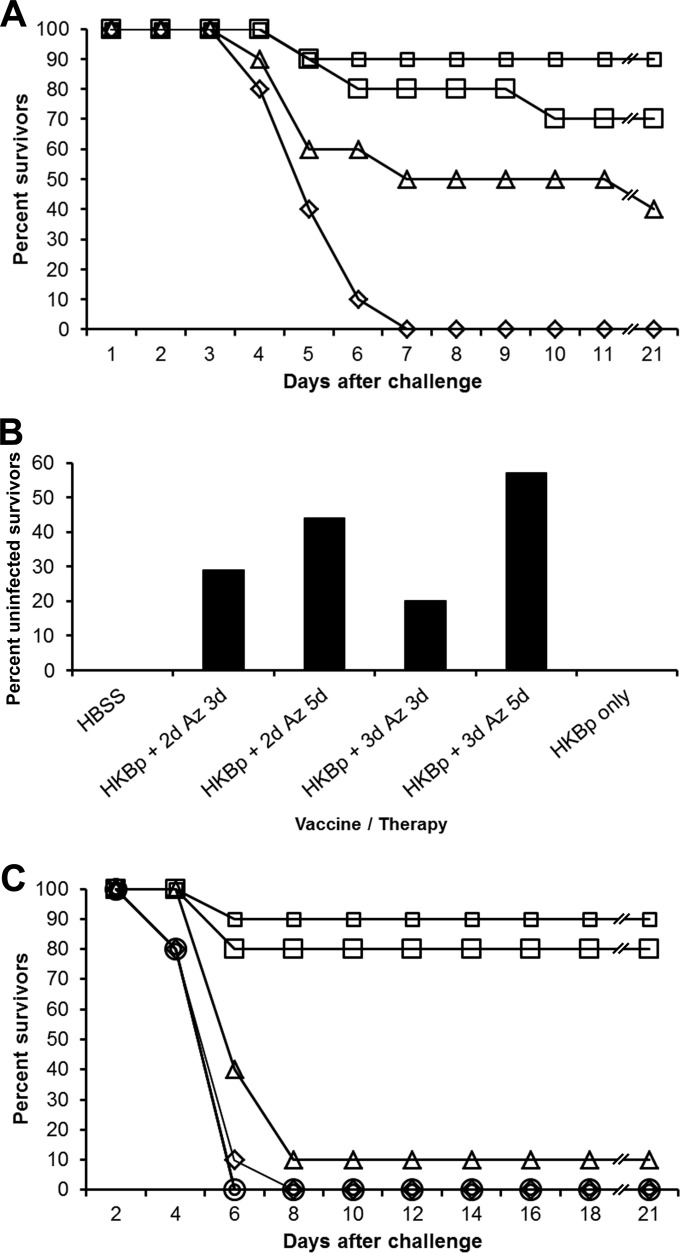
Azithromycin was successfully used to treat a case of human glanders and was the first antibiotic that we tested (18). In this trial, vaccinated mice were challenged by aerosol with approximately 5 LD50 of B. mallei China 7. Antibiotic therapy was initiated on the 4th day after challenge (the day of the index death) and continued for 3 or 5 days. Ninety percent of the mice given azithromycin for 3 days survived, whereas 70% of mice treated for 5 days survived (Fig. 1A). Forty percent of the mice receiving only vaccine survived through the 21-day observation period, but all the unvaccinated and untreated mice died. Survival rates for the groups given vaccine and azithromycin were significantly higher (P < 0.01) than that for the group given HBSS. Vaccine given without therapy also aided survival (P < 0.05).
FIG 1.
Azithromycin therapy. (A) Azithromycin efficacy in previously immunized mice. Mice were given HBSS (diamonds) or immunized with glanders vaccine (HKBp) (triangles) followed by B. mallei aerosol challenge (ATCC 23344; 5 LD50). Azithromycin therapy (Az) was initiated 4 days after challenge for durations of 3 (small squares) or 5 (large squares) days. Line breaks (//) indicate changes in the scale of the x axis. (B) Lack of residual infection after azithromycin therapy. Mice were given HBSS or immunized with glanders vaccine (HKBp) followed by B. mallei aerosol challenge (ATCC 23344; 3 LD50). Azithromycin therapy (Az) of immunized mice was initiated 2 (+ 2d) or 3 (+ 3d) days after challenge for durations of 3 or 5 days. (C) Azithromycin efficacy in immunized and unimmunized mice. Mice were given HBSS (diamonds) or immunized with glanders vaccine (HKBp) (triangles) followed by B. mallei aerosol challenge (ATCC 23344; 3 LD50). Azithromycin therapy (Az) was initiated 5 days after challenge for durations of 5 days (unimmunized mice, small circles; immunized mice, small squares) or 10 days (unimmunized mice, large circles; immunized mice, large squares). Note that line breaks (//) indicate changes in the scale of the x axis.
Since BALB/c mice challenged with B. mallei tend to remain persistently infected, we were interested in knowing whether mice surviving the 21-day observation period were infected. In a study similar to that described above, mice were vaccinated and challenged with 3 LD50 of B. mallei and azithromycin therapy was initiated 2 or 3 days after aerosol challenge for a duration of 3 or 5 days. Mice were euthanized 64 days after challenge. We found that all spleens from surviving control mice (n = 3) and from all vaccinated mice (n = 2) harbored B. mallei (Fig. 1B). However, 29, 44, 20, and 57% of vaccinated mice given azithromycin 2 days after challenge for a duration of 3 (n = 7) or 5 days (n = 9) and vaccinated mice given azithromycin 3 days after challenge for a duration of 3 (n = 5) or 5 days (n = 7), respectively, had no detectable B. mallei in the spleens. Because of the sensitivity of our assay, we were unable to detect bacteria in spleens if fewer than 50 microorganisms were present. Therefore, we were unable to determine whether spleens were actually sterile (no microorganisms present) and did not test whether microorganisms were present in other tissues.
In order to assess whether increased survival in the vaccinated and treated mice was due solely to azithromycin, we conducted a similar study and included groups of unvaccinated mice that received azithromycin. Mice received vaccine only, azithromycin only, or vaccine and azithromycin. Mice were challenged with 3 LD50 of B. mallei beginning 5 days after aerosol challenge for a duration of 5 or 10 days. The index death occurred 3 days after challenge. We did not observe greater than 10% survival in control mice, mice given only vaccine, or mice given only azithromycin for 5 or 10 days after challenge (Fig. 1C). In contrast, at least 80% of the vaccinated and treated mice survived (P < 0.0001). Eighteen weeks after challenge, 67% of surviving vaccinated mice treated with azithromycin for 5 days (n = 6) and 71% of surviving vaccinated mice treated for 10 days (n = 7) had undetectable B. mallei in spleens (data not shown).
Mice treated with moxifloxacin.
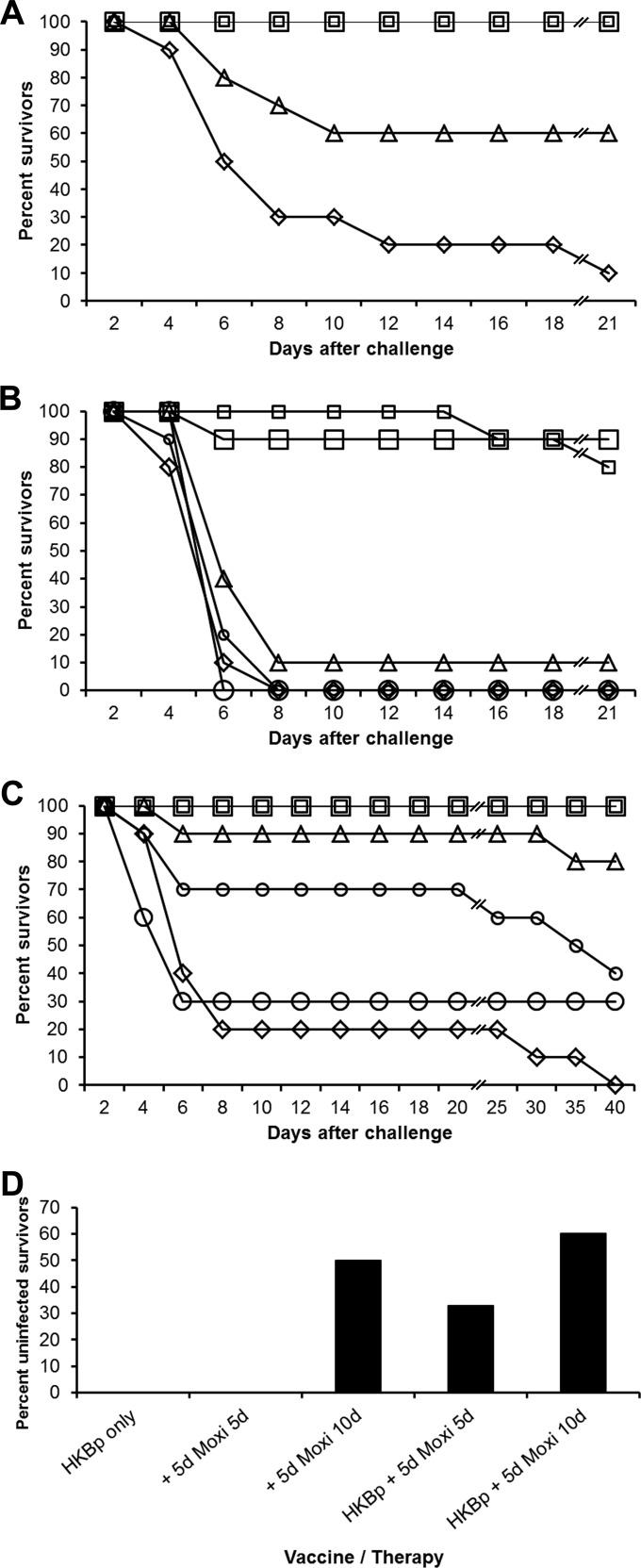
When vaccinated mice were treated with moxifloxacin beginning 3 days after challenge with 1.5 LD50 of B. mallei for a duration of 3 or 5 days, all vaccinated and treated mice survived (P < 0.0001), whereas 90% of the control mice and 40% of the mice given vaccine only (P < 0.05) died (Fig. 2A). To investigate whether moxifloxacin alone can facilitate survival after delayed-onset treatment, a similar experiment was conducted but some groups of mice were given only moxifloxacin. Vaccinated mice were challenged (3 LD50 of B. mallei), and treatment was initiated 5 days after challenge (index death on day 3). Ten percent or less of the control mice, mice given vaccine only, or unvaccinated mice given moxifloxacin survived until the end of the observation period (Fig. 2B). At least 80% of the vaccinated mice that were treated with moxifloxacin survived (P < 0.0001), whereas no protection (P > 0.05) was provided to unvaccinated mice given moxifloxacin.
FIG 2.
Moxifloxacin therapy. (A) Moxifloxacin efficacy in previously immunized mice. Mice were given HBSS (diamonds) or immunized with glanders vaccine (HKBp) (triangles) followed by B. mallei aerosol challenge (ATCC 23344; 1.5 LD50). Immunized mice were given moxifloxacin therapy (Moxi) beginning 3 days after challenge for durations of 3 (small squares) or 5 (large squares) days. Note that line breaks (//) indicate changes in the scale of the x axis. (B) Moxifloxacin efficacy in immunized and unimmunized mice. Mice were given HBSS (diamonds) or immunized with glanders vaccine (HKBp) (triangles) followed by B. mallei aerosol challenge (ATCC 23344; 3 LD50). Moxifloxacin therapy (Moxi) was initiated 5 days after challenge for durations of 5 (unimmunized mice, small circles; immunized mice, small squares) or 10 (unimmunized mice, large circles; immunized mice, large squares) days. Line breaks (//) indicate changes in the scale of the x axis. (C) Moxifloxacin efficacy in immunized and unimmunized mice. Mice were given HBSS (diamonds) or immunized with glanders vaccine (HKBp) (triangles) followed by B. mallei aerosol challenge (B. mallei FMH23344; 1 LD50). Moxifloxacin therapy (Moxi) was initiated 5 days after challenge for durations of 5 (unimmunized mice, small circles; immunized mice, small squares) or 10 (unimmunized mice, large circles; immunized mice, large squares) days. Note that line breaks (//) indicate changes in the scale of the x axis. (D) Lack of residual infection after moxifloxacin therapy. Mice were given HBSS or immunized with glanders vaccine (HKBp) followed by B. mallei aerosol challenge (B. mallei FMH23344; 1 LD50). Moxifloxacin therapy (Moxi) was initiated 5 days (+ 5d) after challenge for durations of 5 or 10 days.
In another test of the ability of moxifloxacin to extend survival in vaccinated mice, we used the B. mallei challenge strain isolated from a human glanders patient (FMH23344; 1 LD50) and extended the observation period through day 39 because of the relatively low challenge dose. All control mice and 60 to 70% of mice given only moxifloxacin died during the observation period (P > 0.01; Fig. 2C), while 100% of the vaccinated mice given either moxifloxacin regimen survived (P < 0.0001). Eighty percent of the mice given vaccine only survived through the observation period (P < 0.0001). When spleens from survivors were examined for sterility on day 44 to 47 after challenge, at least 50% of the mice treated with moxifloxacin for 10 days (vaccinated [n = 10] and unvaccinated [n = 2]) had no detectable microorganisms in the spleens (Fig. 2D). Thirty-three percent of the vaccinated mice treated with moxifloxacin for 5 days had undetectable levels of bacteria in spleens (n = 9). As mentioned earlier, we were unable to distinguish whether spleens were truly bacterium free, or whether a small undetectable number of residual microorganisms remained. Furthermore, additional tissues and organs were not sampled to determine whether they were infected.
Mice treated with sulfamethoxazole and trimethoprim.
Sulfamethoxazole-trimethoprim was similarly tested for efficacy in vaccinated mice after clinical signs had developed. Mice were given the same challenge strain and dose as those described in the previous experiment. Only 20 and 30% of the unvaccinated mice given sulfamethoxazole-trimethoprim 5 days after challenge for 5 or 10 days, respectively, survived (P > 0.05; Fig. 3A). However, at least 90% of the vaccinated mice survived if they received either regimen of sulfamethoxazole-trimethoprim (P < 0.0001). When spleens from surviving mice were examined for residual microorganisms, only vaccinated mice treated for 10 days yielded sterile spleens in over half of the survivors (5 of 9 mice) (Fig. 3B).
FIG 3.
Sulfamethoxazole-trimethoprim therapy. (A) Sulfamethoxazole-trimethoprim efficacy in immunized and unimmunized mice. Mice were given HBSS (diamonds) or immunized with glanders vaccine (HKBp) (triangles) followed by B. mallei aerosol challenge (B. mallei FMH23344; 1 LD50). Sulfamethoxazole-trimethoprim (ST) therapy was initiated 5 days after challenge for durations of 5 (unimmunized mice, small circles; immunized mice, small squares) or 10 (unimmunized mice, large circles; immunized mice, large squares) days. Line breaks (//) indicate changes in the scale of the x axis. (B) Lack of residual infection after sulfamethoxazole-trimethoprim therapy. Mice were given HBSS or immunized with glanders vaccine (HKBp) followed by B. mallei aerosol challenge (B. mallei FMH23344; 1 LD50). Sulfamethoxazole-trimethoprim (ST) therapy was initiated 5 days (+ 5d) after challenge for durations of 5 or 10 days.
DISCUSSION
Antibiotics have been tested for efficacy against glanders in rodent models by administering them shortly after infectious challenge (10, 19). While such testing may identify antibiotics effective against human disease, this methodology that treats infection before symptoms arise is quite different than what occurs in actual human infection. Most commonly, patients seek treatment after the onset of disease symptoms, which could be days or weeks after infection. Furthermore, in the case of attack with a biological agent, the interval between infection and appropriate treatment delivery could be prolonged due to delayed identification of the biological agent or logistical problems and general confusion inherent in a mass casualty situation. Therefore, antibiotics need to be efficacious when administered after the onset of clinical symptoms and beyond.
While candidate vaccines tested to date against glanders can be somewhat protective in extending the time to death after infection, none have been shown to deliver sterile immunity (12). We previously determined that immunization with heat-killed B. pseudomallei whole cells could protect approximately 50 to 80% of BALB/c female mice from an aerosol challenge using 3 to 5 LD50 of B. mallei (ATCC 23344) for a period of 21 days (data not shown). However, surviving mice would typically remain infected, as determined by B. mallei recovered from the spleens, and surviving mice would die or require euthanasia within 60 days of challenge. Experiments described here were to determine if vaccination and antibiotic therapy were useful in enhancing survival after challenge compared to each treatment alone and whether this “combination therapy” could be useful in reducing residual infection. Because treatment of human diseases generally begins several days after infection, treatment was not initiated until mice exhibited clinical signs and the index death had occurred. Typically that index death occurred in the negative-control group, as vaccination tended to provide protection from death within the first week after challenge.
The majority of human glanders cases occurred before the antibiotic era, and the mortality rate was above 90% (20). There have been several cases of human glanders since the 1940s, primarily in laboratory workers, which were successfully treated with antibiotics (6, 7, 21). Six cases were successfully treated with sulfadiazine in 1944 to 1945 (7). In a recent case of laboratory-acquired glanders, the patient received imipenem and doxycycline intravenously for 1 month followed by oral azithromycin and doxycycline for 6 months (6). This treatment regimen was successful, and there was no relapse of disease.
Azithromycin, moxifloxacin, and sulfamethoxazole-trimethoprim were chosen for evaluation in our murine animal model because published reports suggested that they could be useful in treating glanders. In vitro antibiotic testing identified several antibiotics with potential for therapeutic benefit when used in humans (8). In that study, B. mallei strains demonstrated susceptibility to aminoglycosides, macrolides (including azithromycin), quinolones, doxycycline, piperacillin, ceftazidime, and imipenem. While there are no reports demonstrating the therapeutic efficacy of moxifloxacin against glanders in humans, efficacy has been demonstrated in hamsters (22) and in mice challenged by aerosol and treated shortly after infection (S. Demons, unpublished data). Sulfamethoxazole-trimethoprim has been reported to be efficacious against aerosol infection when tested in mice (19). Current recommendations for treating glanders are ceftazidime or meropenem for initial intensive therapy and sulfamethoxazole-trimethoprim or amoxicillin-clavulanic acid for eradication therapy (23).
While azithromycin, moxifloxacin, and sulfamethoxazole-trimethoprim are reported to be efficacious against B. mallei in vitro and when given shortly after challenge before clinical symptoms begin, our testing demonstrated that they delivered only marginal efficacy when administered to clinically ill mice. However, these therapies were efficacious when given to previously vaccinated mice. While the mechanism of this enhanced protection was not investigated, vaccination might stimulate immune responses that restrict bacterial growth, allowing antibiotics to more effectively control infection. Specifically, vaccination could result in the production of gamma interferon, which acts synergistically with antibiotic therapy to inhibit bacterial growth (24). Although we did note residual infection in some mice given combination therapy, optimization of therapeutic and vaccination strategies might result in greater patient survival and lack of residual infection.
ACKNOWLEDGMENTS
Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the U.S. Army.
This research was funded by the Defense Threat Reduction Agency under USAMRIID project number 923678.
REFERENCES
- 1.Robins GD. 1906. A study of chronic glanders in man. Stud R Victoria Hosp 2:1–98. [Google Scholar]
- 2.Steele JH. 1973. The zoonoses: an epidemiologist's viewpoint. Prog Clin Pathol 5:239–286. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2000. Biological and chemical terrorism: strategic plan for preparedness and response. Recommendations of the CDC Strategic Planning Workgroup. MMWR Recomm Rep 49(RR-4):1–14. [PubMed] [Google Scholar]
- 4.Hornick RB. 1982. Diseases due to Pseudomonas mallei and Pseudomonas pseudomallei, p 910–913. In Wedgewood RJ. (ed), Infections in children. Harper & Row, Philadelphia, PA. [Google Scholar]
- 5.Smith GR, Easman CSF. 1990. Bacterial diseases, p 392–397. InParke MT, Collie LH (ed), Principles of bacteriolog, virology and immunity. BC Decker, Philadelphia, PA. [Google Scholar]
- 6.Srinivasan A, Kraus CN, DeShazer D, Becker PM, Dick JD, Spacek L, Bartlett JG, Byrne WR, Thomas DL. 2001. Glanders in a military research microbiologist. N Engl J Med 345:256–258. doi: 10.1056/NEJM200107263450404. [DOI] [PubMed] [Google Scholar]
- 7.Howe C, Miller WR. 1947. Human glanders; report of six cases. Ann Intern Med 26:93–115. doi: 10.7326/0003-4819-26-1-93. [DOI] [PubMed] [Google Scholar]
- 8.Heine HS, England MJ, Waag DM, Byrne WR. 2001. In vitro antibiotic susceptibilities of Burkholderia mallei (causative agent of glanders) determined by broth microdilution and E-test. Antimicrob Agents Chemother 45:2119–2121. doi: 10.1128/AAC.45.7.2119-2121.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenny DJ, Russell P, Rogers D, Eley SM, Titball RW. 1999. In vitro susceptibilities of Burkholderia mallei in comparison to those of other pathogenic Burkholderia spp. Antimicrob Agents Chemother 43:2773–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell P, Eley SM, Ellis J, Green M, Bell DL, Kenny DJ, Titball RW. 2000. Comparison of efficacy of ciprofloxacin and doxycycline against experimental melioidosis and glanders. J Antimicrob Chemother 45:813–818. doi: 10.1093/jac/45.6.813. [DOI] [PubMed] [Google Scholar]
- 11.Kovalev GK. 1971. Glanders (review). Zh Mikrobiol Epidemiol Immunobiol 48:63–70. [PubMed] [Google Scholar]
- 12.Silva EB, Dow SW. 2013. Development of Burkholderia mallei and pseudomallei vaccines. Front Cell Infect Microbiol 3:10. doi: 10.3389/fcimb.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarkar-Tyson M, Smither SJ, Harding SV, Atkins TP, Titball RW. 2009. Protective efficacy of heat-inactivated B. thailandensis, B. mallei or B pseudomallei against experimental melioidosis and glanders. Vaccine 27:4447–4451. doi: 10.1016/j.vaccine.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 14.Whitlock GC, Lukaszewski RA, Judy BM, Paessler S, Torres AG, Estes DM. 2008. Host immunity in the protective response to vaccination with heat-killed Burkholderia mallei. BMC Immunol 9:55. doi: 10.1186/1471-2172-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amemiya K, Bush GV, DeShazer D, Waag DM. 2002. Nonviable Burkholderia mallei induces a mixed Th1- and Th2-like cytokine response in BALB/c mice. Infect Immun 70:2319–2325. doi: 10.1128/IAI.70.5.2319-2325.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 17.Roy CJ, Hale M, Hartings JM, Pitt L, Duniho S. 2003. Impact of inhalation exposure modality and particle size on the respiratory deposition of ricin in BALB/c mice. Inhal Toxicol 15:619–638. doi: 10.1080/713857401. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. 2000. Laboratory-acquired human glanders—Maryland, May 2000. MMWR Morb Mortal Wkly Rep 49:532–535. [PubMed] [Google Scholar]
- 19.Barnes KB, Steward J, Thwaite JE, Lever MS, Davies CH, Armstrong SJ, Laws TR, Roughley N, Harding SV, Atkins TP, Simpson AJ, Atkins HS. 2013. Trimethoprim/sulfamethoxazole (co-trimoxazole) prophylaxis is effective against acute murine inhalational melioidosis and glanders. Int J Antimicrob Agents 41:552–557. doi: 10.1016/j.ijantimicag.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Howe C. 1950. Glanders, p 185–202. In Christian H. (ed), The Oxford medicine. Oxford University Press, New York, NY. [Google Scholar]
- 21.Womack CR, Wells EB. 1949. Co-existent chronic glanders and multiple cystic osseous tuberculosis treated with streptomycin. Am J Med 6:267–271. doi: 10.1016/0002-9343(49)90022-4. [DOI] [PubMed] [Google Scholar]
- 22.Bondareva TA, Borisevich IV, Kalininskii VB, Bondarev VP, Fomenkov OO. 2009. Study of efficacy of modern fluoroquinolones against agent of glanders in experiments in vivo. Zh Mikrobiol Epidemiol Immunobiol 2009(3):10–13. [PubMed] [Google Scholar]
- 23.Lipsitz R, Garges S, Aurigemma R, Baccam P, Blaney DD, Cheng AC, Currie BJ, Dance D, Gee JE, Larsen J, Limmathurotsakul D, Morrow MG, Norton R, O'Mara E, Peacock SJ, Pesik N, Rogers LP, Schweizer HP, Steinmetz I, Tan G, Tan P, Wiersinga WJ, Wuthiekanun V, Smith TL. 2012. Workshop on treatment of and postexposure prophylaxis for Burkholderia pseudomallei and B. mallei infection, 2010. Emerg Infect Dis 18(12):e2. doi: 10.3201/eid1812.120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Propst KL, Troyer RM, Kellihan LM, Schweizer HP, Dow SW. 2010. Immunotherapy markedly increases the effectiveness of antimicrobial therapy for treatment of Burkholderia pseudomallei infection. Antimicrob Agents Chemother 54:1785–1792. doi: 10.1128/AAC.01513-09. [DOI] [PMC free article] [PubMed] [Google Scholar]