Abstract
Although Staphylococcus aureus persistence in osteoblasts, partly as small-colony variants (SCVs), can contribute to bone and joint infection (BJI) relapses, the intracellular activity of antimicrobials is not currently considered in the choice of treatment strategies for BJI. Here, antistaphylococcal antimicrobials were evaluated for their intraosteoblastic activity and their impact on the intracellular emergence of SCVs in an ex vivo osteoblast infection model. Osteoblastic MG63 cells were infected for 2 h with HG001 S. aureus. After killing the remaining extracellular bacteria with lysostaphin, infected cells were incubated for 24 h with antimicrobials at the intraosseous concentrations reached with standard therapeutic doses. Intracellular bacteria and SCVs were then quantified by plating cell lysates. A bactericidal effect was observed with fosfomycin, linezolid, tigecycline, oxacillin, rifampin, ofloxacin, and clindamycin, with reductions in the intracellular inocula of −2.5, −3.1, −3.9, −4.2, −4.9, −4.9, and −5.2 log10 CFU/100,000 cells, respectively (P < 10−4). Conversely, a bacteriostatic effect was observed with ceftaroline and teicoplanin, whereas vancomycin and daptomycin had no significant impact on intracellular bacterial growth. Ofloxacin, daptomycin, and vancomycin significantly limited intracellular SCV emergence. Overall, ofloxacin was the only molecule to combine an excellent intracellular activity while limiting the emergence of SCVs. These data provide a basis for refining the choice of antibiotics to prioritise in the management of BJI, justifying the combination of a fluoroquinolone for its intracellular activity with an anti-biofilm molecule, such as rifampin.
INTRODUCTION
Staphylococcus aureus represents the leading cause of bone and joint infection (BJI) (1, 2). This particular tropism and its ability to cause difficult-to-treat infections lie in the wide panel of staphylococcal virulence factors, which allow host colonization, tissue invasion, and host immune system subversion (3, 4). With regard to BJI, three phenotypic mechanisms provide a bacterial reservoir responsible for staphylococcal BJI chronicity and relapses. First, by promoting immune system and antimicrobial action evasions, biofilm formation has been associated with persistent BJIs, emphasizing the need of infected tissue removal, especially in cases of orthopedic device-associated infections (ODIs) (5–7). Second, implications of the ability of staphylococci to invade and persist within bone cells, and especially osteoblasts, in BJI chronicity has been suggested for years by numerous studies evaluating this mechanism using a few laboratory strains (8–10). We have recently demonstrated this hypothesis among a large collection of clinical BJI isolates of methicillin-susceptible and -resistant S. aureus (11, 12). Finally, bacterial phenotype switching to small-colony variants (SCVs) has been associated with BJI persistence and is enhanced under adverse/stressful growing conditions, such as those for bacteria embedded in biofilms, internalized within host cells, and/or in the presence of antibiotics (13–15).
To date, the choice of antimicrobial therapy for S. aureus BJI relies mainly on in vivo experimental models of BJI or foreign body infections, and is guided by the in vitro antibacterial activity and bone diffusion of antimicrobials (1, 2, 16). Recently, certain pathophysiological mechanisms of BJI have also been taken into consideration. For instance, the use of rifampin is recommended in ODI due to its activity into staphylococcal biofilm (16, 17). Although S. aureus can be internalized into human osteoblasts and persist in bone cells partly as SCVs, which can lead to an intracellular bacterial reservoir responsible for BJI chronicity and relapse, the intracellular activity of antimicrobials is not currently considered in the treatment strategies of BJI. Therefore, we aimed to evaluate the intraosteoblastic activity of the main antimicrobials used for staphylococcal BJI in an in vitro model of osteoblast infection and to assess their impact on the emergence of intracellular SCVs.
MATERIALS AND METHODS
Bacterial strain.
The methicillin-susceptible S. aureus HG001 strain was used for all of the experiments. The MICs of the antimicrobials tested in the cellular model were determined by the Etest method using Mueller-Hinton agar according to the manufacturer's instructions (bioMérieux, Marcy l'Etoile, France) and the recommendations of the French Committee for Antimicrobial Susceptibility Testing (CA-SFM).
MG63 osteoblastic cell culture.
All cell culture reagents were obtained from Gibco (Paisley, United Kingdom). The human osteoblastic cell line MG63 (CRL-1427) (18), purchased from LGC standard (USA), was routinely cultured in a humidified incubator at 37°C in a 5% CO2 atmosphere in a growth medium (CGM) consisting of Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, 25 mM HEPES, and 2 mM l-glutamine with or without 100 U of penicillin/ml and 100 μg of streptomycin/ml (CGM with antibiotic). The cells were passaged once a week and used up to passage 20 after thawing. Prior to assays, osteoblasts were seeded at 40,000 cells per well into 48-well tissue culture plates (Falcon, Le Pont de Claix, France) in 500 μl of growth medium with antibiotics and cultured for 24 h until achieving 70 to 80% confluence.
Bacterial suspension standardization and osteoblast infection.
Prior to the assays, the S. aureus HG001 strain was subcultivated on Columbia agar supplemented with 5% sheep blood (COS; bioMérieux) at 37°C for 24 h. Three colonies were then used to inoculate 5 ml of brain heart infusion (tube, 15 by 130 mm; BHI; AES, Bruz, France) incubated overnight at 37°C. The suspensions were washed and resuspended in CGM at a concentration corresponding to a multiplicity of infection of 100 by using a previously established clone-specific regression formula correlating the bacterial density (CFU/ml) with the optical density at 600 nm (OD600): CFU/ml = (7 × 108 × OD600) – (3 × 107) (data not shown). Normalized bacterial suspensions were then sonicated for 10 min at 100% (Bactsonic; LaboModern, Paris, France) to minimize clumping and added to the bone cell culture wells. After incubation for 30 min at 4°C to allow sedimentation of the bacteria while blocking internalization, all of the cultures were simultaneously transferred to 37°C to synchronize the beginning of the internalization step. After 2 h, the cell cultures were washed twice with 500 μl of DMEM, followed by incubation for 1 h with growth medium supplemented with 10 μg of lysostaphin (Sigma-Aldrich, Saint-Quentin Fallavier, France)/ml to kill the remaining extracellular staphylococcal cells.
Antimicrobial intracellular activity and impact on intracellular SCV selection.
After killing the extracellular bacteria with lysostaphin, the infected cells were washed twice with DMEM and then incubated for 24 h with growth medium containing the tested antimicrobials at three concentrations. The “bone concentration” (Cbone) corresponded to the intraosseous concentrations reached in humans when using standard therapeutic dosages and was determined after a pharmacologic literature review (19, 20). These molecules were also used at minimal (Cmin = Cbone/3) and maximal (Cmax = Cbone × 3) concentrations to assess the existence of potential dose effects (Table 1). For each condition, lysostaphin at 10 μg/ml was also added to the growth medium to kill the bacteria released upon host cell lysis, thus preventing these bacteria from reinfecting new host cells.
TABLE 1.
Antimicrobial susceptibility of S. aureus HG001 and antimicrobial concentrationsa
| Antimicrobial | MIC (mg/liter) | Usual plasmatic concn (mg/liter) | Usual bone/plasma ratio | Concn (mg/liter)b |
||
|---|---|---|---|---|---|---|
| Cmin | Cbone | Cmax | ||||
| Beta-lactams | ||||||
| Oxacillin | 0.094 | 50 | 0.17 | 3.33 | 10 | 30 |
| Ceftaroline | 0.19 | 20 | 0.19 | 1.33 | 4 | 12 |
| Clindamycin | 0.032 | 4−14 | 0.35 | 1.33 | 4 | 12 |
| Fosfomycin | 2 | 4−14 | 0.35 | 1.33 | 4 | 12 |
| Glyco/lipopeptides | ||||||
| Vancomycin | 1.5 | 20–40 | 0.21 | 2 | 6 | 18 |
| Teicoplanin | 1.5 | 10–70 | 0.21 | 1 | 3 | 9 |
| Daptomycin | 0.19 | 4–11 | 0.24 | 1.7 | 5 | 15 |
| Linezolid | 1 | 20 | 0.4 | 2.67 | 8 | 24 |
| Ofloxacin | 0.5 | 5 | 0.5 | 0.67 | 2 | 6 |
| Rifampin | 0.004 | 10–30 | 0.27 | 2 | 6 | 18 |
| Tigecycline | 0.125 | 0.2–1.5 | 0.35 | 0.1 | 0.3 | 0.9 |
MICs were determined by using the standard diffusion method (Etest). Usual plasmatic and bone/plasma concentration ratios were determined after a literature review, especially from the review of Landersdorfer et al. (19).
Cbone, bone concentration; Cmin, minimal concentration; Cmax, maximal concentration.
After 24 h of incubation, the osteoblasts were washed twice with DMEM and subsequently lysed by a 10-min incubation with sterile water. Cell lysates were sonicated to minimize clumping, and dilutions of these lysates were spiral-plated in duplicate on COS using a WASP automated plater (AES Chemunex, Bruz, France). After overnight incubation at 37°C, the plates were photographed, and the wild-type and SCV colonies were enumerated. A commonly used operational definition of SCVs based on colony size states that colonies with a size less than one-fifth of that of the wild-type strain can be considered SCVs (14). Previous reports were based on visual inspection of cultures by an operator. To eliminate operator dependency, SCV quantification was performed using an automated process in which a high-resolution picture of each plate was taken and analyzed by means of the image analysis software ImageJ (W. S. Rasband, National Institutes of Health, Bethesda, MD) (21), with a customized macro involving color thresholding, awatershed algorithm, and particle analysis, in order to extract the distribution of colony areas. The wild-type colony area was defined as the median area of all colonies (because the median is robust to outliers, the presence of SCVs did not influence significantly this measure), and SCVs were defined, according to the usual operational definition, as colonies with an area less than one-fifth of the median area.
Of note, antimicrobial-induced cytotoxicity was assessed by quantifying lactate dehydrogenase (LDH) release (resulting from damaged cells) in the cell culture supernatant of osteoblasts incubated with each tested antimicrobial at Cmax using a colorimetric method (Dimension Vista automated clinical chemistry analyser; Siemens Healthcare Diagnostics, Tarrytown, NY).
Statistical analysis.
For every concentration, each antimicrobial agent was evaluated in triplicate in three independent experiments. The results are presented as means with the 95% confidence intervals (CI) of the nine measure points available for each condition. To standardize the results, intracellular inocula were normalized for 100,000 osteoblasts and expressed as changes observed in the number of intracellular CFU (Δlog CFU) at 24 h compared to untreated cells using the Mann-Whitney U-test. Intracellular SCVs were expressed using the ratio of the number of SVC colonies among antimicrobial agent-treated cells compared to untreated osteoblasts. The existence of a dose effect was assessed by linear regression between the three used concentrations. A P value of <0.05 was considered significant. All analyses were performed using GraphPad Prism (v5.03; GraphPad Software, San Diego, CA).
RESULTS
Susceptibility studies.
The MICs, as determined by the Etest method, showed that S. aureus HG001 was fully susceptible to all of the antistaphylococcal molecules tested in the present study (Table 1).
Antimicrobial agent-induced cellular toxicity.
The data obtained by the LDH release assay demonstrated that the antimicrobials used at Cmax (and consequently at Cmin and Cbone) had no impact on LDH concentration in the supernatant and thus were not responsible for cell death.
Intracellular action.
The intracellular effect of the antimicrobials was expressed by the inoculum change between the initial and 24 h inocula (Δlog CFU) and compared to untreated cells. Of note, the mean initial inoculum was 1.8 × 106 CFU/100,000 osteoblasts (95% CI [1.4 × 106 to 2.1 × 106]).
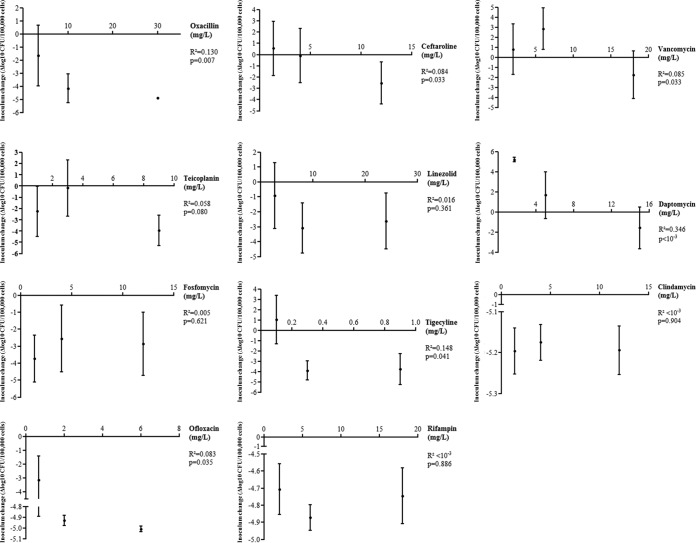
At the bone concentration, vancomycin and daptomycin were not able to significantly prevent the intracellular growth observed in untreated cells (+3.2 log10 CFU/100,000 cells; 95% CI [+2.1 to +4.3]), highlighted by an intracellular inoculum increase of +2.9 (95% CI [+0.8 to +4.9]; P = 0.830) and +1.7 (95% CI [−0.7 to +4.0]) log10 CFU/100,000 cells (P = 0.070), respectively. Compared to untreated cells, an intracellular bacteriostatic effect was observed with ceftaroline and teicoplanin, which was estimated at −0.1 (95% CI [−2.5 to +2.3]; P = 0.002) and −0.2 (95% CI [−2.7 to +2.3]; P = 0.001) log10 CFU/100,000 osteoblasts, respectively. At the bone concentration, a significant bactericidal effect was observed with fosfomycin (−2.5 log10 CFU/100,000 cells; 95% CI [−0.6 to −4.5]; P < 10−4), linezolid (−3.1 log10 CFU/100,000 cells; 95% CI [−2.9 to −4.8]; P < 10−4), tigecycline (−3.9 log10 CFU/100,000 cells; 95% CI [−2.9 to −4.8]; P < 10−4), oxacillin (−4.2 log10 CFU/100,000 cells; 95% CI [−3.1 to −5.3]; P < 10−4), rifampin (−4.9 log10 CFU/100,000 cells; 95% CI [−4.8 to −4.9]; P < 10−4), ofloxacin (−4.9 log10 CFU/100,000 cells; 95% CI [−4.9 to −5.0]; P < 10−4), and clindamycin (−5.2 log10 CFU/100,000 cells; 95% CI [−5.1 to −5.2]; P < 10−4) (Fig. 1A and Table 2).
FIG 1.
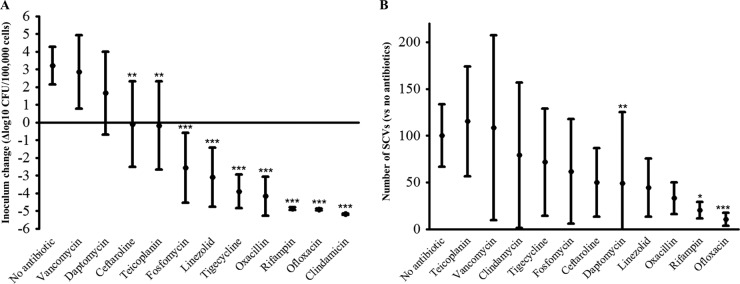
Intraosteoblastic inoculum change and intracellular proportion of small-colony variants in the presence of the main antistaphylococcal molecules at the usual bone concentration. The change in the number of intracellular CFU (Δlog CFU; means and 95% CI) at 2 h, starting from an initial intracellular inoculum of 1.8 × 106 (95% CI =1.4 × 106 to 2.1 × 106) for 100,000 osteoblasts, was compared to untreated cells (Mann-Whitney U-test). CI, confidence interval; SCV, small-colony variants; **, P < 0.01; ***, P < 0.00.
TABLE 2.
Summary of the antimicrobial impact on intracellular global inoculum and SCVsa
| Antimicrobial agent and parameter | Minimal concn |
Bone concn |
Maximal concn |
Dose effect |
||||
|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) | P | Mean (95% CI) | P | Mean (95% CI) | P | R2 | P | |
| Oxacillin | ||||||||
| Δlog CFU | −1.7 (−4.0 to +0.7) | <10−3 | −4.2 (−5.3 to −3.1) | <10−3 | −4.9 (−5.0 to −4.8) | <10−3 | 0.130 | 0.007 |
| SCVs (%) | 34.5 (16.1 to 52.9) | 0.041 | 33.2 (16.3 to 50.0) | 0.053 | 7.8 (1.0 to 14.6) | <10−3 | 0.136 | 0.007 |
| Ceftaroline | ||||||||
| Δlog CFU | +0.5 (−1.9 to +3.0) | 0.006 | −0.1 (−2.5 to +2.3) | 0.002 | −2.6 (−4.4 to −0.7) | <10−3 | 0.084 | 0.033 |
| SCVs (%) | 96.3 (−29.1 to 221.7) | 0.082 | 50.0 (13.1 to 86.8) | 0.071 | 82.9 (−36.4 to 202.1) | 0.044 | <10−3 | 0.992 |
| Vancomycin | ||||||||
| Δlog CFU | +0.8 (−1.7 to +3.3) | 0.333 | +2.9 (+0.8 to +4.9) | 0.830 | −1.7 (−4.1 to 0.6) | 0.002 | 0.085 | 0.033 |
| SCVs (%) | 44.4 (−8.2 to 97.1) | 0.072 | 108.5 (9.4 to 207.6) | 0.800 | 53.5 (3.6 to 103.4) | 0.185 | 0.001 | 0.824 |
| Teicoplanin | ||||||||
| Δlog CFU | −2.3 (−4.5 to −0.0) | <10−3 | −0.2 (−2.7 to +2.3) | 0.001 | −3.9 (−5.3 to −2.6) | <10−3 | 0.058 | 0.080 |
| SCVs (%) | 134.6 (5.2 to 263.9) | 0.978 | 115.3 (56.5 to 174.2) | 0.374 | 117.2 (24.2 to 210.1) | 0.844 | 0.001 | 0.831 |
| Linezolid | ||||||||
| Δlog CFU | −0.9 (−3.1 to +1.3) | <10−3 | −3.1 (−4.8 to −1.4) | <10−3 | −3.1 (−4.8 to −1.4) | <10−3 | 0.016 | 0.361 |
| SCVs (%) | 30.1 (16.3 to 43.9) | 0.025 | 44.6 (13.5 to 75.8) | 0.087 | 35.8 (6.9 to 64.6) | 0.023 | <10−3 | 0.924 |
| Daptomycin | ||||||||
| Δlog CFU | +5.2 (+5.0 to +5.4) | 0.078 | +1.7 (−0.7 to +4.0) | 0.070 | −1.6 (−3.7 to +0.5) | <10−3 | 0.346 | <10−3 |
| SCVs (%) | 65.1 (2.3 to 127.9) | 0.052 | 49.1 (−26.9 to 125.1) | 0.004 | 83.3 (−7.9 to 174.5) | 0.057 | 0.005 | 0.622 |
| Fosfomycin | ||||||||
| Δlog CFU | −3.7 (−5.1 to −2.3) | <10−3 | −2.6 (−4.5 to −0.6) | <10−3 | −2.9 (−4.7 to −1.0) | <10−3 | 0.005 | 0.621 |
| SCVs (%) | 52.5 (−13.1 to 118.1) | 0.015 | 61.7 (5.7 to 117.6) | 0.057 | 5.3 (−2.4 to 12.9) | <10−3 | 0.051 | 0.100 |
| Tigecycline | ||||||||
| Δlog CFU | −3.7 (−5.1 to −2.3) | 0.005 | −3.9 (−4.8 to −2.9) | <10−3 | −3.8 (−4.7 to −1.0) | <10−3 | 0.148 | 0.041 |
| SCVs (%) | 77.1 (22.1 to 132.2) | 0.354 | 71.7 (14.2 to 129.1) | 0.128 | 91.9 (−45.2 to 229.0) | 0.015 | 0.002 | 0.765 |
| Clindamycin | ||||||||
| Δlog CFU | −5.2 (−5.2 to −5.1) | <10−3 | −5.2 (−5.2 to −5.1) | <10−3 | −5.2 (−5.3 to −5.1) | <10−3 | <10−3 | 0.904 |
| SCVs (%) | 59.7 (13.2 to 106.2) | 0.425 | 79.1 (1.3 to 157.0) | 0.415 | 88.9 (16.3 to 161.5) | 0.786 | 0.011 | 0.549 |
| Ofloxacin | ||||||||
| Δlog CFU | −3.2 (−4.9 to −1.4) | <10−3 | −4.9 (−5.0 to −4.9) | <10−3 | −5.0 (−5.0 to −5.0) | <10−3 | 0.083 | 0.035 |
| SCVs (%) | 16.4 (3.3 to 29.6) | 0.003 | 10.4 (3.4 to 17.3) | 0.001 | 12.4 (4.1 to 20.6) | 0.001 | 0.003 | 0.709 |
| Rifampin | ||||||||
| Δlog CFU | −4.7 (−4.9 to −4.6) | <10−3 | −4.9 (−4.9 to −4.8) | <10−3 | −4.7 (−4.9 to −4.6) | <10−3 | <10−3 | 0.886 |
| SCVs (%) | 27.1 (12.5 to 41.8) | 0.021 | 20.3 (11.6 to 29.0) | 0.011 | 18.4 (7.7 to 29.2) | 0.010 | 0.018 | 0.339 |
The reduction of intracellular inoculum (Δlog CFU) represents the decrease in intracellular bacteria after 24 h compared to untreated cells by Mann-Whitney U-test. SCVs represent the number of colonies with an area inferior to 5-fold less of the median area measured for all colonies on each plate and are expressed relative to untreated cells. The existence of a dose effect was assessed by linear regression between the three used concentrations.
At the minimal concentration, only rifampin (−4.7 log10 CFU/100,000 cells; 95% CI [−4.9 to −4.6]; P < 10−4), clindamycin (−5.2 log10 CFU/100,000 cells; 95% CI [−5.2 to −5.1]; P < 10−4), ofloxacin (−3.2 log10 CFU/100,000 cells; 95% CI [−4.9 to −1.4]; P < 10−3), and fosfomycin (−3.7 log10 CFU/100,000 cells; 95% CI [−5.1 to −2.3]; P < 10−4) were bactericidal. At the maximal concentration, all antibiotics were bactericidal, with the exception of vancomycin and daptomycin (Fig. 2 and Table 2). The combined analysis of the three concentrations used for each antimicrobial revealed a significant dose effect for oxacillin, ceftaroline, vancomycin, daptomycin, tigecycline, and ofloxacin (Fig. 2 and Table 2).
FIG 2.
Dose effect of the main antistaphylococcal molecules on intraosteoblastic inoculum. The results are expressed by the change in the number of intracellular CFU (Δlog CFU; means and 95% CI) at 2 h, starting from an initial intracellular inoculum of 1.8 × 106 (95% CI = 1.4 × 106 to 2.1 × 106) for 100,000 osteoblasts. The dose effect was assessed using linear regression between the three used concentrations. CI, confidence interval; *, P < 0.05.
Impact of antistaphylococcal antibiotics on intracellular SCV emergence.
There was no SCV in the challenge inocula. After 24 h, the number of SCVs in untreated cells was 13,514/100,000 osteoblasts (95% CI [6,448 to 20,580]), corresponding to 4.1% (95% CI [2.6 to 5.7]) of all colonies. Our data showed that this number did not increase, regardless of the antibiotic and concentration tested.
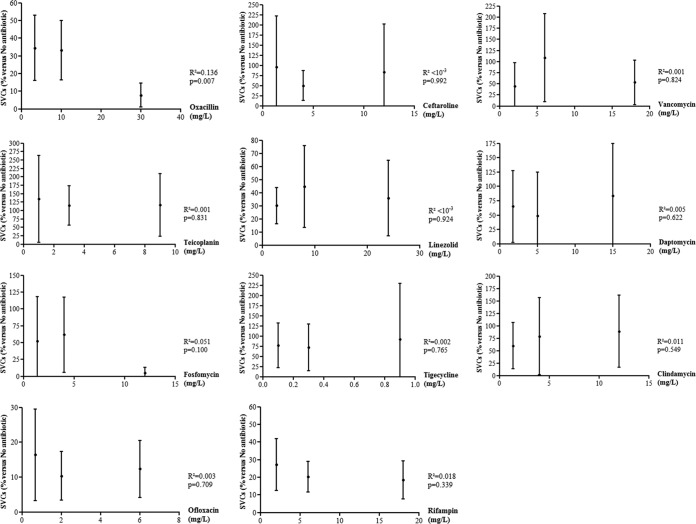
At the bone concentration, the number of SCVs was significantly decreased in the osteoblasts treated with ofloxacin, rifampin, and daptomycin, with a reduction of −79.7% (95% CI [-88.4 to −71.0]; P = 0.001), −89.7% (95% CI [−96.6 to −82.7]; P = 0.011), and −50.9% (95% CI [−25.1 to +126.9]; P = 0.004), respectively, compared to untreated cells (Fig. 1B and Table 2).
In addition, oxacillin (−92.2%; 95% CI [−85.4; −99.0]; P < 10−3), ceftaroline (−17.1; 95% CI [−102.1 to +136.4]; P = 0.044), linezolid (−64.2; 95%CI [−93.1 to −35.4]; P = 0.023), fosfomycin (−94.7; 95% CI [−87.1 to +102.4]; P < 10−3), and tigecycline (−8.1; 95% CI [−129.0 to +145.2]; P = 0.015) reduced the proportion of intracellular SCVs but only at the maximum concentration. A slight but significant dose effect was observed only with oxacillin (Fig. 3 and Table 2). Other differences were not statistically significant.
FIG 3.
Dose effect of the main antistaphylococcal molecules on the intraosteoblastic emergence of small-colony variants. The change in the number of intracellular CFU (Δlog CFU; means and 95% CI) at 2 h, starting from an initial intracellular inoculum of 1.8 × 106 (95% CI = 1.4 × 106 to 2.1 × 106) for 100,000 osteoblasts, was compared to untreated cells (Mann-Whitney U-test). The dose effect was assessed using linear regression between the three used concentrations. CI, confidence interval; SCV, small-colony variants; *, P < 0.05.
DISCUSSION
Considering that intraosteoblastic S. aureus constitutes a bacterial reservoir leading to chronicity and relapse, targeting intracellular bacteria might be a major therapeutic issue in the antimicrobial therapy for BJI and should to be taken into account (11, 12). Compared to previous evaluations of the intracellular activity of antimicrobials, the present study was specifically adapted to BJI by the following: (i) the use of a human osteoblast infection model, thereby allowing a better estimation of the situation encountered in BJI, as opposed to using monocyte-macrophage cells, as previously described (22, 23), and (ii) the evaluation of the antimicrobial intraosseous concentrations reached in humans when using standard therapeutic doses and not plasmatic (or higher) concentrations, which do not correspond to the therapeutic tissue reality (19). Even if the distribution of antibiotics in the different parts of bones and joints (i.e., cortical and medullar bone tissue, joint fluid, synovial …) is likely heterogeneous, our model tried to closely fit with actually known pharmacologic conditions.
At the bone concentration, vancomycin and daptomycin did not result in a significant reduction in the intracellular inoculum, and ceftaroline and teicoplanin appeared only bacteriostatic. Conversely, the other tested antimicrobials achieved a significant intracellular bactericidal effect: rifampin, ofloxacin, and clindamycin were the most active molecules. These results must be analyzed with regard to the relative intracellular distribution of bacteria and antimicrobials and the physicochemical parameters of intracellular compartments. After the internalization step, S. aureus is processed in a phagolysosome, which is characterized by a low pH, and certain S. aureus strains are able to escape from the phagosome. The proportion of bacteria released into the cytoplasm depends on the time from infection (ranging from 10 to 30% at 2 h to 60 to 80% after 8 h) but also on various virulence factors, including delta- and beta-toxins and phenol-soluble modulins (24). Within this context, the low activity of vancomycin on intracellular staphylococci can consequently lie in its slow uptake and modest cellular accumulation compared to teicoplanin, a more lipophilic glycopeptide, which shows a more extensive and faster accumulation (25, 26). Conversely, the good intracellular activity of clindamycin, fluoroquinolones, and rifampin can be explained by their well-known rapid accumulation in eukaryotic cells (27–29). However, fluoroquinolones are mainly located in the cytosol, whereas clindamycin and rifampin are distributed both in the cytosol and phagosomes, likely allowing these molecules to target all intracellular S. aureus cells (30). Moreover, the local environment, and especially the acidic pH, can be unfavorable to the activity of some antibiotics. For instance, such conditions only slightly affect fluoroquinolones and clindamycin, with the retention of their good intracellular activity (30). Interestingly, some studies have even shown that methicillin-resistant S. aureus strains recover their susceptibility to β-lactam antibiotics when they are phagocytised by eukaryotic cells due to the acidic pH in phagolysosomes (31, 32). Indeed, penicillin-binding protein (PBP) 2a, a low β-lactam-affinity PBP encoded by the mecA gene and conferring resistance to penicillin, can be acetylated by β-lactams due to a pH-induced conformational change (33). This mechanism can possibly be involved in the increased intracellular activity of β-lactams against methicillin-resistant S. aureus due to a similar conformational modification of natural PBPs.
To our knowledge, only two studies previously assessed the intraosteoblastic activity of some antimicrobials. Kreis et al. recently obtained results concordant with ours using tigecycline and rifampin but with tigecycline concentrations 30-fold higher than therapeutic bone concentrations (34). Ellington et al. also suggested an excellent intraosteoblastic activity of rifampin, clindamycin, and macrolides, but these compounds were used at MICs and not at bone concentrations (22). Other studies were performed using monocyte or macrophage cell lines, showing that the intracellular activity of the antistaphylococcal depends on the exposure time and the extracellular concentration of the molecules tested, which emphasizes the importance of using therapeutic bone concentrations (35). As in the present work, these studies have also highlighted a superiority of fluoroquinolones on internalized bacteria and the inefficiency of vancomycin in macrophages (35–37). Finally, animal models have also been used to evaluate the intracellular activity of antistaphylococcal molecules. In a mouse model of staphylococcal peritonitis, Sandberg et al. classified the intracellular activity of antimicrobials as follows: dicloxacillin > rifampin > gentamicin (38). However, a peritonitis model does not match the tissue reality of BJI, for which antibiotic diffusion problems is a major concern. In another mouse model of foreign-device infection, Murillo et al. confirmed the superiority of fluoroquinolones over β-lactam antibiotics in the eradication of intracellular S. aureus (39).
The interpretation of the results obtained for SCVs is more complex, since the observed results after 24 h of treatment is a balance between the emergence of these phenotypic variants, which can be promoted by the stress induced by the tested antibiotics themselves, and the efficacy of these same antibiotics on the SCVs. A previous study has shown that most antibiotics were able to kill intracellular SCVs, depending on the concentration used (40). Using a therapeutic concentration, we showed that among intracellularly active antimicrobials, only ofloxacin was able to limit the intracellular emergence of SCVs. Finally, an accurate definition of SCV, involving not only colony size but also metabolism markers, for example, is lacking and may have helped to more precisely describe these variants.
Some limitations of our study must be addressed. First, only one S. aureus reference strain was tested, which may represent a limitation to the extrapolation of our results to different clinical isolates. Moreover, it would have been interesting to assess the intracellular concentrations of antibiotics, which was not technically feasible in our laboratory. Nevertheless, as mentioned above, only a subcellular pharmacodynamics analysis would have been relevant, taking into account both the intracellular location of bacteria and antimicrobials. Thus, the binding of each antimicrobial to culture medium proteins was not considered and may have impacted the available amount of antibiotic for intracellular diffusion. However, this parameter is rarely taken into account in bone diffusion studies in the literature. Consequently, the choice of concentrations used in our study is most likely the most relevant according to current pharmacological knowledge. Finally, antimicrobials were added after a 2-h period of cell infection. However, Ellington et al. showed that the intracellular antibiotic activity decreased when staphylococci persisted for 12 h intracellularly before treatment, likely due to a change in the bacterial cell wall (22). In addition, the short duration of treatment (24 h) can explain why antibiotics with a slow bactericidal effect, such as vancomycin, showed no significant activity in our model. A similar study using various infection and treatment periods could be interesting to confirm these results under our experimental conditions. Similarly, the use of such models will be very interesting regarding the evaluation of SCV, since their emergence can be impacted by the duration of intracellular persistence (14) and likely by the length of contact with antibiotics.
In conclusion, our results provide the first assessment of the intraosteoblastic activity of a large panel of antimicrobial used in BJI, as evaluated at therapeutic bone concentrations. Ofloxacin exhibited the best therapeutic pattern, with an excellent intracellular activity while limiting the emergence of SCVs. These data provide a basis for refining the choice of antibiotics to prioritise in the management of difficult-to-treat S. aureus BJI. For instance, our results justify and promote the combination a fluoroquinolone due to its good intracellular activity with an already well-known anti-biofilm molecule, such as rifampin.
ACKNOWLEDGMENTS
The Lyon Bone and Joint Infection Study Group includes physicians (Florence Ader, François Biron, André Boibieux, Anissa Bouaziz, Evelyne Braun, Christian Chidiac, Fatiha Daoud, Tristan Ferry, Judith Karsenty, Johanna Lippman, Patrick Miailhes, Thomas Perpoint, Dominique Peyramond, Marie-Paule Vallat, and Florent Valour), surgeons (Cédric Barrey, Pierre Breton, Fabien Boucher, Romain Desmarchelier, Michel-Henry Fessy, Olivier Guyen, Christophe Lienhart, Sébastien Lustig, Alain-Ali Mojallal, Philippe Neyret, Franck Trouillet, Gualter Vaz, and Antony Viste), microbiologists (Frédéric Laurent, Jean-Philippe Rasigade, and François Vandenesch), specialists in nuclear medicine (Emmanuel Deshayes, Francesco Giammarile, Marc Janier, and Isabelle Morelec), PK/PD specialists (Marie-Claude Gagnieu, Sylvain Goutelle, and Michel Tod), and a clinical research assistant (Eugénie Mabrut).
This study was supported by the French Ministry of Health, the French Ministry of Education, the Institut National de la Santé et de la Recherche Médicale (INSERM), the Groupement Inter-Regional à la Recherche Clinique et à l'Inovation (GIRCI-D50829 [J.P.R.]), and bioMérieux (F.V.). The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
REFERENCES
- 1.Lew DP, Waldvogel FA. 2004. Osteomyelitis Lancet 364:369–379. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N Engl J Med 351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 3.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 4.Ferry T, Perpoint T, Vandenesch F, Etienne J. 2005. Virulence determinants in Staphylococcus aureus and their involvement in clinical syndromes. Curr Infect Dis Rep 7:420–428. doi: 10.1007/s11908-005-0043-8. [DOI] [PubMed] [Google Scholar]
- 5.Brady RA, Leid JG, Calhoun JH, Costerton JW, Shirtliff ME. 2008. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol Med Microbiol 52:13–22. doi: 10.1111/j.1574-695X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- 6.Hall-Stoodley L, Stoodley P. 2009. Evolving concepts in biofilm infections. Cell Microbiol 11:1034–1043. doi: 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez CJ Jr, Mende K, Beckius ML, Akers KS, Romano DR, Wenke JC, Murray CK. 2013. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect Dis 13:47. doi: 10.1186/1471-2334-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosse MJ, Gruber HE, Ramp WK. 2005. Internalization of bacteria by osteoblasts in a patient with recurrent, long-term osteomyelitis: a case report. J Bone Joint Surg Am 87:1343–1347. [DOI] [PubMed] [Google Scholar]
- 9.Ellington JK, Reilly SS, Ramp WK, Smeltzer MS, Kellam JF, Hudson MC. 1999. Mechanisms of Staphylococcus aureus invasion of cultured osteoblasts. Microb Pathog 26:317–323. doi: 10.1006/mpat.1999.0272. [DOI] [PubMed] [Google Scholar]
- 10.Hudson MC, Ramp WK, Nicholson NC, Williams AS, Nousiainen MT. 1995. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb Pathog 19:409–419. doi: 10.1006/mpat.1995.0075. [DOI] [PubMed] [Google Scholar]
- 11.Rasigade JP, Trouillet-Assant S, Ferry T, Diep BA, Sapin A, Lhoste Y, Ranfaing J, Badiou C, Benito Y, Bes M, Couzon F, Tigaud S, Lina G, Etienne J, Vandenesch F, Laurent F. 2013. PSMs of hypervirulent Staphylococcus aureus act as intracellular toxins that kill infected osteoblasts. PLoS One 8:e63176. doi: 10.1371/journal.pone.0063176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valour F, Rasigade JP, Trouillet-Assant S, Gagnaire J, Bouaziz A, Karsenty J, Lacour C, Bes M, Lustig S, Bénet T, Chidiac C, Etienne J, Vandenesch F, Ferry T, Laurent F, Lyon BJI Study Group . Delta-toxin production deficiency in Staphylococcus aureus: a diagnostic marker of bone and joint infection chronicity linked with osteoblast invasion and biofilm formation. Clin Microbiol Infect, in press. [DOI] [PubMed] [Google Scholar]
- 13.Proctor RA, van Langevelde P, Kristjansson M, Maslow JN, Arbeit RD. 1995. Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin Infect Dis 20:95–102. doi: 10.1093/clinids/20.1.95. [DOI] [PubMed] [Google Scholar]
- 14.Tuchscherr L, Medina E, Hussain M, Volker W, Heitmann V, Niemann S, Holzinger D, Roth J, Proctor RA, Becker K, Peters G, Loffler B. 2011. Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol Med 3:129–141. doi: 10.1002/emmm.201000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Von Eiff C, Peters G, Becker K. 2006. The small colony variant (SCV) concept – the role of staphylococcal SCVs in persistent infections. Injury 37(Suppl 2):S26–S33. doi: 10.1016/j.injury.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. 2013. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 17.Saginur R, Stdenis M, Ferris W, Aaron SD, Chan F, Lee C, Ramotar K. 2006. Multiple combination bactericidal testing of staphylococcal biofilms from implant-associated infections. Antimicrob Agents Chemother 50:55–61. doi: 10.1128/AAC.50.1.55-61.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pautke C, Schieker M, Tischer T, Kolk A, Neth P, Mutschler W, Milz S. 2004. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res 24:3743–3748. [PubMed] [Google Scholar]
- 19.Landersdorfer CB, Bulitta JB, Kinzig M, Holzgrabe U, Sorgel F. 2009. Penetration of antibacterials into bone: pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin Pharmacokinet 48:89–124. doi: 10.2165/00003088-200948020-00002. [DOI] [PubMed] [Google Scholar]
- 20.Montange D, Berthier F, Leclerc G, Serre A, Jeunet L, Berard M, Muret P, Vettoretti L, Leroy J, Hoen B, Chirouze C. 2014. Penetration of daptomycin into bone and synovial fluid in joint replacement. Antimicrob Agents Chemother 58:3991–3996. doi: 10.1128/AAC.02344-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai Z, Chattopadhyay N, Liu WJ, Chan C, Pignol JP, Reilly M. 2011. Optimized digital counting colonies of clonogenic assays using ImageJ software and customized macros: comparison with manual counting. Radiat Biol 87:1135–1146. doi: 10.3109/09553002.2011.622033. [DOI] [PubMed] [Google Scholar]
- 22.Ellington JK, Harris M, Hudson MC, Vishin S, Webb LX, Sherertz R. 2006. Intracellular Staphylococcus aureus and antibiotic resistance: implications for treatment of staphylococcal osteomyelitis. J Orthop Res 24:87–93. doi: 10.1002/jor.20003. [DOI] [PubMed] [Google Scholar]
- 23.Mélard A, Garcia LG, Das D, Rozenberg R, Tulkens PM, Van Bambeke F, Lemaire S. 2013. Activity of ceftaroline against extracellular (broth) and intracellular (THP-1 monocytes) forms of methicillin-resistant Staphylococcus aureus: comparison with vancomycin, linezolid and daptomycin. J Antimicrob Chemother 68:648–658. doi: 10.1093/jac/dks442. [DOI] [PubMed] [Google Scholar]
- 24.Fraunholz M, Sinha B. 2012. Intracellular Staphylococcus aureus: live-in and let die. Front Cell Infect Microbiol 2:43. doi: 10.3389/fcimb.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beauchamp D, Gourde P, Simard M, Bergeron MG. 1992. Subcellular localization of tobramycin and vancomycin given alone and in combination in proximal tubular cells, determined by immunogold labeling. Antimicrob Agents Chemother 36:2204–2210. doi: 10.1128/AAC.36.10.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maderazo EG, Breaux SP, Woronick CL, Quintiliani R, Nightingale CH. 1988. High teicoplanin uptake by human neutrophils. Chemotherapy 34:248–255. doi: 10.1159/000238576. [DOI] [PubMed] [Google Scholar]
- 27.Easmon CS, Crane JP. 1984. Cellular uptake of clindamycin and lincomycin. Br J Exp Pathol 65:725–730. [PMC free article] [PubMed] [Google Scholar]
- 28.García I, Pascual A, Ballesta S, Perea EJ. 2000. Uptake and intracellular activity of ofloxacin isomers in human phagocytic and non-phagocytic cells. Int J Antimicrob Agents 15:201–205. doi: 10.1016/S0924-8579(00)00161-8. [DOI] [PubMed] [Google Scholar]
- 29.Höger PH, Vosbeck K, Seger R, Hitzig WH. 1985. Uptake, intracellular activity, and influence of rifampin on normal function of polymorphonuclear leukocytes. Antimicrob Agents Chemother 28:667–674. doi: 10.1128/AAC.28.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carryn S, Chanteux H, Seral C, Mingeot-Leclercq MP, Van Bambeke F, Tulkens PM. 2003. Intracellular pharmacodynamics of antibiotics. Infect Dis Clin North Am 17:615–634. doi: 10.1016/S0891-5520(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 31.Lemaire S, Olivier A, Van Bambeke F, Tulkens PM, Appelbaum PC, Glupczynski Y. 2008. Restoration of susceptibility of intracellular methicillin-resistant Staphylococcus aureus to beta-lactams: comparison of strains, cells, and antibiotics. Antimicrob Agents Chemother 52:2797–2805. doi: 10.1128/AAC.00123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemaire S, Van Bambeke F, Mingeot-Leclercq M-P, Glupczynski Y, Tulkens PM. 2007. Role of acidic pH in the susceptibility of intraphagocytic methicillin-resistant Staphylococcus aureus strains to meropenem and cloxacillin. Antimicrob Agents Chemother 51:1627–1632. doi: 10.1128/AAC.01192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemaire S, Fuda C, Van Bambeke F, Tulkens PM, Mobashery S. 2008. Restoration of susceptibility of methicillin-resistant Staphylococcus aureus to beta-lactam antibiotics by acidic pH: role of penicillin-binding protein PBP 2a. J Biol Chem 283:12769–12776. doi: 10.1074/jbc.M800079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kreis CA, Raschke MJ, Roßlenbroich SB, Tholema-Hans N, Loffler B, Fuchs T. 2013. Therapy of intracellular Staphylococcus aureus by tigecycline. BMC Infect Dis 13:267. doi: 10.1186/1471-2334-13-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barcia-Macay M, Seral C, Mingeot-Leclercq M-P, Tulkens PM, Van Bambeke F. 2006. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob Agents Chemother 50:841–851. doi: 10.1128/AAC.50.3.841-851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brinch KS, Tulkens PM, Van Bambeke F, Frimodt-Moller N, Hoiby N, Kristensen HH. 2010. Intracellular activity of the peptide antibiotic NZ2114: studies with Staphylococcus aureus and human THP-1 monocytes, and comparison with daptomycin and vancomycin. J Antimicrob Chemother 65:1720–1724. doi: 10.1093/jac/dkq159. [DOI] [PubMed] [Google Scholar]
- 37.Lemaire S, Kosowska-Shick K, Appelbaum PC, Glupczynski Y, Van Bambeke Tulkens PM. 2011. Activity of moxifloxacin against intracellular community-acquired methicillin-resistant Staphylococcus aureus: comparison with clindamycin, linezolid and co-trimoxazole and attempt at defining an intracellular susceptibility breakpoint. J Antimicrob Chemother 66:596–607. doi: 10.1093/jac/dkq478. [DOI] [PubMed] [Google Scholar]
- 38.Sandberg A, Hessler JHR, Skov RL, Blom J, Frimodt-Moller N. 2009. Intracellular activity of antibiotics against Staphylococcus aureus in a mouse peritonitis model. Antimicrob Agents Chemother 53:1874–1883. doi: 10.1128/AAC.01605-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murillo O, Pachón ME, Euba G, Verdaguer R, Carreras M, Cabellos C, Cabo J, Gudiol F, Ariza J. 2009. Intracellular antimicrobial activity appearing as a relevant factor in antibiotic efficacy against an experimental foreign-body infection caused by Staphylococcus aureus. J Antimicrob Chemother 64:1062–1066. doi: 10.1093/jac/dkp326. [DOI] [PubMed] [Google Scholar]
- 40.Garcia LG, Lemaire S, Kahl BC, Kahl BC, Becker K, Proctor RA, Denis O, Tulkens PM, Van Bambeke F. 2012. Pharmacodynamic evaluation of the activity of antibiotics against hemin- and menadione-dependent small-colony variants of Staphylococcus aureus in models of extracellular (broth) and intracellular (THP-1 monocytes) infections. Antimicrob Agents Chemother 56:3700–3711. doi: 10.1128/AAC.00285-12. [DOI] [PMC free article] [PubMed] [Google Scholar]