Abstract
Preliminary enthusiasm over the encouraging spectrum and in vitro activities of siderophore conjugates, such as MB-1, was recently tempered by unexpected variability in in vivo efficacy. The need for these conjugates to compete for iron with endogenously produced siderophores has exposed a significant liability for this novel antibacterial strategy. Here, we have exploited dependence on efflux for siderophore secretion in Pseudomonas aeruginosa and provide evidence that efflux inhibition may circumvent this in vivo-relevant resistance liability.
TEXT
The extent and severity of antibiotic resistance in Gram-negative pathogens require the development of innovative therapeutic interventions that not only circumvent existing clinically relevant resistance mechanisms but will also result in limited spontaneous resistance emergence. Although standard in vitro methodologies exist for predicting resistance development, translational deficiencies that can stem from their lack of in vivo physiological relevance are becoming increasingly apparent. We previously described the siderophore-monobactam conjugate MB-1 (Fig. 1 [1]), which has broad-spectrum in vitro activities against multidrug-resistant (MDR) Gram-negative pathogens, including Pseudomonas aeruginosa, when tested using standard methods established by the Clinical and Laboratory Standards Institute (CLSI) (1). Unfortunately, we have also demonstrated that despite these encouraging in vitro activities, the efficacy of MB-1 in a neutropenic murine infection model was highly variable and not directly predictable from traditional in vitro activity assays (2). Instead, the development of a modified in vitro resistance frequency assay, which relies on the utilization of more in vivo-relevant iron conditions, has proven to be more predictive of the efficacy of MB-1 in vivo. Additionally, this assay has demonstrated a role for endogenous siderophores, such as pyoverdine, in mediating the adaptive cellular response that enables P. aeruginosa to transiently resist MB-1 activity (2). While these results suggest that the use of MB-1 as monotherapy is not a viable option, we have speculated about alternative strategies that may mitigate this adaptive response and resurrect MB-1 as a legitimate antibacterial agent. Among these potentiation-type approaches, we considered the idea that inhibiting endogenous siderophore functionality should circumvent the adaptation phenotype mediated by the competition for iron by native siderophore systems. Siderophore secretion into the extracellular milieu has been shown to be dependent on functional efflux pumps in Gram-negative bacteria (3–5). In the case of P. aeruginosa, the secretion of newly synthesized pyoverdine is accomplished by the ABC transporter-type efflux system encoded by pvdR, pvdT, and opmQ (6). Additionally, this system is responsible for pyoverdine recycling after successive rounds of iron acquisition and delivery (7).
FIG 1.
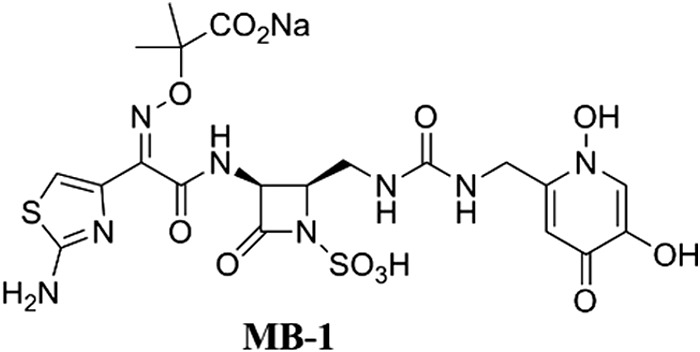
Chemical structure of the MB-1 siderophore-monobactam conjugate.
To prove that inhibiting the activity of this pump can potentiate the activity of MB-1, we generated a pvdRT-opmQ deletion in P. aeruginosa strain JJ4-36, which is a clinical isolate that has demonstrated a significant propensity for adaptation to MB-1. This mutant was constructed by replacing the C terminus of PvdR (amino acids 68 to 691), all of PvdT, and the majority of OpmQ (amino acids 1 to 450) with a gentamicin resistance cassette from pPS856 (8), subcloning into pEX100T (9), and delivering it to recipient cells via triparental mating as described previously (10). This derivative (heretofore referred to as ΔRTQ), along with the parental strain and isogenic pyoverdine biosynthesis-deficient pvdA deletion mutant, was tested in the MB-1 adaptation assay using methods described previously (2). Briefly, Luria-Bertani cultures of each P. aeruginosa strain were grown to mid-log phase, and ∼1 × 107 cells were spread onto chelexed, dialyzed, Mueller-Hinton broth (CDMHB) (solidified with 1.5% agarose) plates containing increasing concentrations of MB-1 and incubated at 37°C for 40 h. While the wild-type JJ4-36 strain showed substantial growth at MB-1 concentrations as high as 32 μg/ml, each of the mutants was limited to growth of few, if any, colonies on the CDMHB plate with 0.25 μg/ml MB-1 (Table 1, row 1).
TABLE 1.
Adaptive resistance to MB-1 by P. aeruginosa strain JJ4-36 wild-type and isogenic pyoverdine biosynthesis and secretion mutants
| CDMHB plate addition | Highest MB-1 concn (μg/ml) with growth in: |
||
|---|---|---|---|
| WT JJ4-36 | JJ4-36 ΔpvdA | JJ4-36 ΔRTQ | |
| Unsupplemented | 32 | <0.25 | 0.25 |
| 1% WT CMa | NTb | 16 | 4 |
| 1% ΔpvdA CM | NT | 0.25 | 0.25 |
CM, conditioned medium (defined as cell-free spent medium harvested from wild-type [WT] or pyoverdine-deficient [ΔpvdA] P. aeruginosa strain JJ4-36 grown overnight in CDMHB at 37°C).
NT, not tested.
One potential interpretation of these results is that the PvdRT-OpmQ system was responsible for directly effluxing MB-1 from the cell rather than mediating resistance indirectly through the transport of pyoverdine. To test this, the adaptation assay was performed by supplementing CDMHB with conditioned media from either wild-type strain JJ4-36 or the isogenic ΔpvdA mutant. Conditioned media, described as the cell-free supernatants from CDMHB cultures grown overnight, were previously demonstrated to restore the growth of ΔpvdA cells in the presence of MB-1 if they were prepared from pyoverdine-producing strains, whereas conditioned media prepared from pyoverdine-deficient mutants did not provide this restoration (2), demonstrating the importance of this endogenous siderophore in mediating the adaptive response. Here, if the PvdRT-OpmQ system is responsible for the physical removal of MB-1, then supplementation with wild-type JJ4-36-conditioned medium should not promote the growth of JJ4-36 ΔRTQ cells on plates containing MB-1. As shown in Table 1 (compare rows 2 and 3), when 1% conditioned medium from wild-type JJ4-36 was added to CDMHB plates, these mutant cells were capable of growing in the presence of up to 4 μg/ml MB-1 (i.e., the presence of pyoverdine reduced MB-1 activity). In contrast, plates that were not supplemented, or were supplemented with 1% conditioned medium from JJ4-36 ΔpvdA, did not have any detectable growth after 40 h of incubation. These data suggest that this efflux pump does not mediate MB-1 resistance through the active efflux of this siderophore conjugate.
These preliminary results demonstrate the importance of this particular efflux pump in mediating adaptation to MB-1, prompting the hypothesis that an efflux pump inhibitor (EPI) that functionally inhibits the PvdRT-OpmQ system would alleviate this liability for MB-1 efficacy. A variety of compounds that have demonstrated activity against either resistance-nodulation-division (RND) pumps or ABC transporter-type systems were tested. Specifically, phenylalanine-arginine β-naphthylamide (PAβN) (11), 1-(1-naphthylmethyl)-piperazine (NMP) (12), reserpine (13), probenecid (14), verapamil (15), and gemfibrozil (16) were all tested for their abilities to potentiate MB-1 activity in the adaptation assay. Using concentrations that were previously shown to affect the activities of various efflux pumps, improvements in MB-1 activity were not observed when it was combined with PAβN, NMP, probenecid, verapamil, or gemfibrozil. The combination of MB-1 with 25 μg/ml reserpine, however, showed at least an 8-fold reduction in the emergence of adaptively resistant colonies (Table 2). Lowering the reserpine concentration to 6.25 μg/ml decreased the degree of MB-1 potentiation, although a clear beneficial effect was still detected. Attempts to increase the reserpine concentration to 100 μg/ml did not yield any significant improvements relative to those seen when 25 μg/ml was used, although this may have been due to apparent solubility issues at this elevated concentration. In the absence of MB-1, however, none of the EPIs tested at the indicated concentrations had any detectable effect on P. aeruginosa growth in this assay (data not shown).
TABLE 2.
Determination of MB-1 potentiation capabilities by various EPIs in the CDMHB adaptation assay
| Compound (μg/ml) | Highest MB-1 concn (μg/ml) with growth in: |
|||
|---|---|---|---|---|
| WTa JJ4-36 |
JJ4-36 ΔRTQMB-1 | |||
| MB-1 | Aztreonam | Ciprofloxacin | ||
| No combination | ≥32 | 32 | 2 | 0.25 |
| PAβN (50) | ≥32 | 8 | 0.5 | NTb |
| NMP (100) | ≥32 | 32 | 1 | NT |
| Reserpine (6.25) | 8 | NT | NT | NT |
| Reserpine (25) | 4 | 32 | 2 | 0.25 |
| Probenecid (712.5) | ≥32 | NT | NT | NT |
| Verapamil (50) | ≥32 | NT | NT | NT |
| Gemfibrozil (50) | ≥32 | NT | NT | NT |
WT, wild type.
NT, not tested.
Although reserpine alone was not found to cause any growth inhibition in this assay, the possibility that it was acting synergistically with MB-1 by affecting a distinct cellular target was assessed in two separate assays. First, the adaptation assay was repeated using JJ4-36 ΔRTQ cells and MB-1 concentrations lower than those used initially (0.03 to 0.5 μg/ml). This was done in the presence or absence of 25 μg/ml reserpine. After 40 h of incubation, the growth patterns of reserpine-treated plates and that of untreated plates were indistinguishable (Table 2), suggesting that reserpine was not killing cells through an alternative mechanism. The second approach involved conducting the adaptation assay with other antibacterial agents, again in combination with reserpine or alone, using wild-type JJ4-36 cells. Aztreonam was tested due to its structural similarity to MB-1 and conserved mode of action, and ciprofloxacin was included to measure reserpine potentiation effects using a different class of antibiotic. Table 2 shows that in each case, the degree of JJ4-36 growth was unaffected by the inclusion of 25 μg/ml reserpine, suggesting that its ability to potentiate MB-1 was specifically caused by PvdRT-OpmQ inhibition.
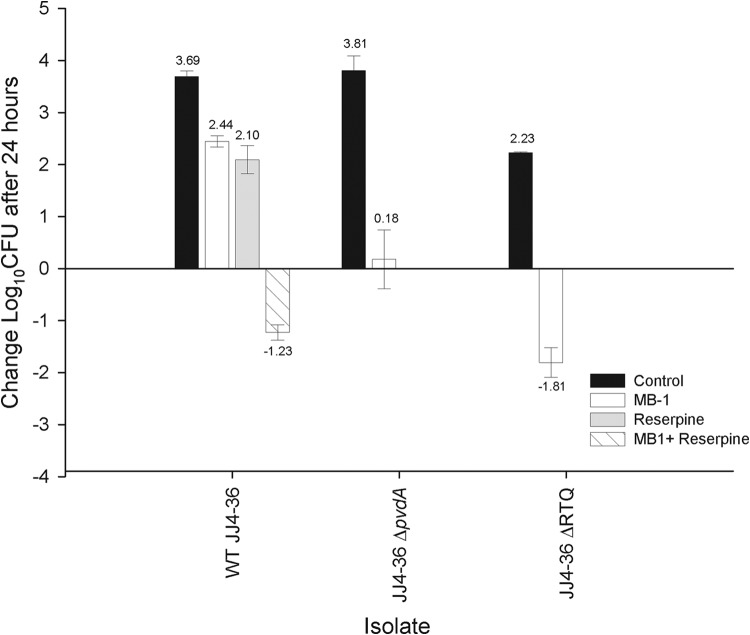
To determine the translatability of the in vitro results generated with this combination, the efficacy of MB-1 against wild-type strain JJ4-36 was tested in the presence and absence of reserpine using an MB-1 humanized dosing regimen, which required multiple doses to maintain free-drug concentrations at 4 μg/ml in a neutropenic mouse model of thigh infection described previously (2). The JJ4-36 ΔRTQ mutant was also included to confirm the hypersusceptibility witnessed with it in the in vitro adaptation assay. MB-1 susceptibility of the ΔRTQ mutant was confirmed, as evidenced by the recoverable number of mutant cells (4.25-log lower relative to that of wild-type strain JJ4-36 after 24 h of infection; Fig. 2, white bars). Interestingly, MB-1 treatment against this mutant showed a further 1.99-log decrease in bacterial density compared with the JJ4-36 ΔpvdA mutant, a phenotype which may be explained by fitness costs associated with efflux deficiency, as suggested by the 1.58-log reduction in bacterial burden recovered from the corresponding control groups (Fig. 2). A 3.67-log decrease in wild-type JJ4-36 bacterial burden was also observed when MB-1 treatment was combined with 30 mg/kg reserpine (relative to MB-1 monotherapy), despite the fact that treatment with each of these compounds individually had a considerably weaker impact on bacterial growth after 24 h (Fig. 2). It should be noted that only a single dose of reserpine was administered during the course of this study, and the resulting exposure of this regimen was not evaluated. With a more thorough understanding of reserpine pharmacokinetics and safety, it is possible that additional doses would have further increased the amount of bacterial killing.
FIG 2.
In vivo efficacy of MB-1, either alone or in combination, against wild-type (WT) P. aeruginosa strain JJ4-36 and its isogenic pyoverdine biosynthesis (pvdA) or secretion (RTQ) mutant in a neutropenic mouse model of thigh infection. Numbers above each data point represent the mean change in numbers of CFU after 24-h relative to 0-h control animals. Efficacy was determined using, where appropriate, Student's t test or analysis of variance followed by a Tukey test for multiple comparisons. Each treatment was statistically different than the control (P < 0.001); for wild-type strain JJ4-36, combined treatment with MB-1 and reserpine was also statistically better (P > 0.001) than reserpine or MB-1 monotherapy, which were not statistically different from one another (P = 0.06).
In an effort to definitively demonstrate reserpine-mediated inhibition of pyoverdine secretion, wild-type JJ4-36 cells were grown in CDMHB, and supernatant samples were collected to assess pyoverdine secretion over time. Unfortunately, absorbance measurements of reserpine-treated cultures at 405 nm, which is a routinely used method for detecting pyoverdine production in P. aeruginosa cultures, did not demonstrate a significant difference from that in mock-treated control samples (data not shown). In addition, even though the reserpine combination with MB-1 in the adaptation assay resulted in a reduction in the concentration of MB-1 in which wild-type JJ4-36 cells might grow (Table 2), those colonies that did emerge on plates containing lower MB-1 concentrations still produced the typical fluorescent-green halo that has been shown to represent pyoverdine production (2). Taken together, these data prevent reserpine from being classified as a bona fide inhibitor of the PvdRT-OpmQ ABC transporter in P. aeruginosa. Based on the in vitro and in vivo results with the ΔRTQ mutant, however, it still seems feasible that specific inhibition of this efflux pump imparts the necessary effects to potentiate the activity of siderophore conjugates, such as MB-1.
In this report, we provide early insight into an innovative approach to circumventing adaptation-mediated resistance to MB-1 and demonstrate significant improvements in in vivo efficacy for MB-1 when combined with the EPI reserpine. This plant alkaloid has been described to function as an EPI in various Gram-positive pathogens, such as Staphylococcus aureus (13, 17) and Streptococcus pneumoniae (18), aiding in the restoration of antibiotic susceptibility among MDR strains of these clinically prevalent bacteria. Our data suggest that reserpine potentiates MB-1 activity, in vitro and in vivo, although the exact mechanism(s) behind this potentiation is still unclear. From data generated previously, we proposed a model in which a subpopulation of P. aeruginosa cells that hyperproduced pyoverdine may provide adaptive protection to neighboring cells by enabling them to acquire iron more rapidly than MB-1 bactericidal activity can be achieved (2). With that in mind, we speculate that reserpine is acting to prevent pyoverdine hyperproduction and the downstream protective effect for nearby cells, even though it does not appear to affect normal pyoverdine production in the absence of MB-1. As a result, this would limit the amount of adaptive resistance observed in the absence of this EPI, although this hypothesis requires additional investigation to confirm or refute it. Also, while reserpine itself may not be an acceptable combination partner for safety and tolerability reasons, we are encouraged that the efforts described here provide mechanistic proof of concept for this novel resistance mitigation strategy.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 8th edition. CLSI document M07-A8 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 2.Tomaras AP, Crandon JL, McPherson CJ, Banevicius MA, Finegan SM, Irvine RL, Brown MF, O'Donnell JP, Nicolau DP. 2013. Adaptation-based resistance to siderophore-conjugated antibacterial agents by Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:4197–4207. doi: 10.1128/AAC.00629-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furrer JL, Sanders DN, Hook-Barnard IG, McIntosh MA. 2002. Export of the siderophore enterobactin in Escherichia coli: involvement of a 43 kDa membrane exporter. Mol Microbiol 44:1225–1234. doi: 10.1046/j.1365-2958.2002.02885.x. [DOI] [PubMed] [Google Scholar]
- 4.Allard KA, Viswanathan VK, Cianciotto NP. 2006. lbtA and lbtB are required for production of the Legionella pneumophila siderophore legiobactin. J Bacteriol 188:1351–1363. doi: 10.1128/JB.188.4.1351-1363.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Page WJ, Kwon E, Cornish AS, Tindale AE. 2003. The csbX gene of Azotobacter vinelandii encodes an MFS efflux pump required for catecholate siderophore export. FEMS Microbiol Lett 228:211–216. doi: 10.1016/S0378-1097(03)00753-5. [DOI] [PubMed] [Google Scholar]
- 6.Hannauer M, Yeterian E, Martin LW, Lamont IL, Schalk IJ. 2010. An efflux pump is involved in secretion of newly synthesized siderophore by Pseudomonas aeruginosa. FEBS Lett 584:4751–4755. doi: 10.1016/j.febslet.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 7.Imperi F, Tiburzi F, Visca P. 2009. Molecular basis of pyoverdine siderophore recycling in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 106:20440–20445. doi: 10.1073/pnas.0908760106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86. doi: 10.1016/S0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 9.Schweizer HP, Hoang TT. 1995. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene 158:15–22. doi: 10.1016/0378-1119(95)00055-B. [DOI] [PubMed] [Google Scholar]
- 10.McPherson CJ, Aschenbrenner LM, Lacey BM, Fahnoe KC, Lemmon MM, Finegan SM, Tadakamalla B, O'Donnell JP, Mueller JP, Tomaras AP. 2012. Clinically relevant Gram-negative resistance mechanisms have no effect on the efficacy of MC-1, a novel siderophore-conjugated monocarbam. Antimicrob Agents Chemother 56:6334–6342. doi: 10.1128/AAC.01345-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lomovskaya O, Warren MS, Lee A, Galazzo J, Fronko R, Lee M, Blais J, Cho D, Chamberland S, Renau T, Leger R, Hecker S, Watkins W, Hoshino K, Ishida H, Lee VJ. 2001. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob Agents Chemother 45:105–116. doi: 10.1128/AAC.45.1.105-116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kern WV, Steinke P, Schumacher A, Schuster S, von Baum H, Bohnert JA. 2006. Effect of 1-(1-naphthylmethyl)-piperazine, a novel putative efflux pump inhibitor, on antimicrobial drug susceptibility in clinical isolates of Escherichia coli. J Antimicrob Chemother 57:339–343. doi: 10.1093/jac/dki445. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz FJ, Fluit AC, Luckefahr M, Engler B, Hofmann B, Verhoef J, Heinz HP, Hadding U, Jones ME. 1998. The effect of reserpine, an inhibitor of multidrug efflux pumps, on the in-vitro activities of ciprofloxacin, sparfloxacin and moxifloxacin against clinical isolates of Staphylococcus aureus. J Antimicrob Chemother 42:807–810. doi: 10.1093/jac/42.6.807. [DOI] [PubMed] [Google Scholar]
- 14.Lismond A, Tulkens PM, Mingeot-Leclercq MP, Courvalin P, Van Bambeke F. 2008. Cooperation between prokaryotic (Lde) and eukaryotic (MRP) efflux transporters in J774 macrophages infected with Listeria monocytogenes: studies with ciprofloxacin and moxifloxacin. Antimicrob Agents Chemother 52:3040–3046. doi: 10.1128/AAC.00105-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balganesh M, Dinesh N, Sharma S, Kuruppath S, Nair AV, Sharma U. 2012. Efflux pumps of Mycobacterium tuberculosis play a significant role in antituberculosis activity of potential drug candidates. Antimicrob Agents Chemother 56:2643–2651. doi: 10.1128/AAC.06003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seral C, Carryn S, Tulkens PM, Van Bambeke F. 2003. Influence of P-glycoprotein and MRP efflux pump inhibitors on the intracellular activity of azithromycin and ciprofloxacin in macrophages infected by Listeria monocytogenes or Staphylococcus aureus. J Antimicrob Chemother 51:1167–1173. doi: 10.1093/jac/dkg223. [DOI] [PubMed] [Google Scholar]
- 17.Gibbons S, Udo EE. 2000. The effect of reserpine, a modulator of multidrug efflux pumps, on the in vitro activity of tetracycline against clinical isolates of methicillin resistant Staphylococcus aureus (MRSA) possessing the tet(K) determinant. Phytother Res 14:139–140. doi:. [DOI] [PubMed] [Google Scholar]
- 18.Garvey MI, Piddock LJ. 2008. The efflux pump inhibitor reserpine selects multidrug-resistant Streptococcus pneumoniae strains that overexpress the ABC transporters PatA and PatB. Antimicrob Agents Chemother 52:1677–1685. doi: 10.1128/AAC.01644-07. [DOI] [PMC free article] [PubMed] [Google Scholar]