Abstract
Oritavancin is a recently approved lipoglycopeptide antimicrobial agent with activity against Gram-positive pathogens. Its extended serum elimination half-life and concentration-dependent killing enable single-dose treatment of acute bacterial skin and skin structure infections. At the time of regulatory approval, new agents, including oritavancin, are not offered in the most widely used susceptibility testing devices and therefore may require application of surrogate testing using a related antimicrobial to infer susceptibility. To evaluate vancomycin as a predictive susceptibility marker for oritavancin, 26,993 recent Gram-positive organisms from U.S. and European hospitals were tested using reference MIC methods. Organisms included Staphylococcus aureus, coagulase-negative staphylococci (CoNS), beta-hemolytic streptococci (BHS), viridans group streptococci (VGS), and enterococci (ENT). These five major pathogen groups were analyzed by comparing results with FDA-approved susceptible breakpoints for both drugs, as well as those suggested by epidemiological cutoff values and supported by pharmacokinetic/pharmacodynamic analyses. Vancomycin susceptibility was highly accurate (98.1 to 100.0%) as a surrogate for oritavancin susceptibility among the indicated pathogen species. Furthermore, direct MIC comparisons showed high oritavancin potencies, with vancomycin/oritavancin MIC90 results of 1/0.06, 2/0.06, 0.5/0.12,1/0.06, and >16/0.06 μg/ml for S. aureus, CoNS, BHS, VGS, and ENT, respectively. In conclusion, vancomycin demonstrated acceptable accuracy as a surrogate marker for predicting oritavancin susceptibility when tested against the indicated pathogens. In contrast, 93.3% of vancomycin-nonsusceptible enterococci had oritavancin MIC values of ≤0.12 μg/ml, indicating a poor predictive value of vancomycin for oritavancin resistance against these organisms. Until commercial oritavancin susceptibility testing devices are readily available, isolates that when tested show vancomycin susceptibility can be inferred to be susceptible to oritavancin by using FDA-approved breakpoints.
INTRODUCTION
Newly approved (by the U.S. Food and Drug Administration [FDA] or European Medicines Agency [EMA]) antimicrobial agents rarely have validated commercial susceptibility testing products/systems available at the time of commercialization. In fact, the recent histories of such products demonstrate delays of years, even for those drugs possessing qualities that would favorably impact patient care. To support susceptibility assessments of these approved antimicrobial agents for treatment of indicated infections, clinical microbiology laboratories have resorted to “surrogate” susceptibility testing of a similar (class representative) currently tested agent to predict susceptibility of organisms to the new antimicrobial agent (1–6). This option has been successfully applied to several antimicrobial classes since the first standardization of susceptibility testing methods (7, 8).
Oritavancin, formerly LY333328 (9, 10), is a recently approved lipoglycopeptide with broad-spectrum activity against Gram-positive pathogens, including some strains nonsusceptible to vancomycin (11) (see Table 1, below). Initial global surveillance study results (11) across 12 countries demonstrated potent oritavancin activity in vitro against staphylococci (including methicillin-resistant Staphylococcus aureus strains [MRSA]), Streptococcus pneumoniae, and enterococci (including vancomycin-resistant enterococci [VRE]), a level of activity clarified by the subsequent understanding that a surfactant (polysorbate 80) was required in testing media for accurate MIC determinations in the plastic trays used for the broth microdilution MIC method (12, 13). Furthermore, the concentration-dependent bactericidal activity of oritavancin is based on the two sites of oritavancin action (the cell wall and membrane) and has led to pharmacokinetic/pharmacodynamic (PK/PD) investigations suggesting an optimal single 1,200-mg dosing regimen for acute bacterial skin and skin structure infections (ABSSSI) (14–17). This single dose of oritavancin was demonstrated to be noninferior to a vancomycin regimen administered twice daily for 7 to 10 days for treatment of adults with ABSSSI (18, 19).
TABLE 1.
Comparative in vitro potencies of oritavancin and vancomycin against 26,993 Gram-positive pathogens isolated in the United States and Europe, 2011 to 2013
| Pathogen (no. tested) and antimicrobial | Cumulative % inhibited at MIC (μg/ml) of: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | |
| S. aureus (17,717) | ||||||||||
| Oritavancin | 23.7 | 64.8 | 91.3a | 98.8 | >99.9 | 100.0 | ||||
| Vancomycin | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 20.7 | 98.5 | 100.0 | ||
| CoNS (2,073) | ||||||||||
| Oritavancin | 36.2 | 66.0 | 95.0 | 99.8 | 100.0 | |||||
| Vancomycin | 0.0 | 0.0 | 0.0 | 0.1 | 0.4 | 11.6 | 52.3 | 99.7 | 100.0 | |
| BHS (2,357) | ||||||||||
| Oritavancin | 44.7 | 72.8 | 84.8 | 92.7 | 98.1 | >99.9 | 100.0 | |||
| Vancomycin | 0.0 | 0.0 | 0.0 | 0.1 | 45.5 | 99.8 | 100.0 | |||
| VGS (1,248) | ||||||||||
| Oritavancin | 75.5 | 86.0 | 94.6 | 99.3 | 100.0 | |||||
| Vancomycin | 0.0 | 0.0 | 0.0 | 0.7 | 10.4 | 86.4 | 100.0 | |||
| Enterococci (3,598)b | ||||||||||
| Oritavancin | 66.4 | 85.3 | 94.6 | 98.4 | 99.7 | 100.0 | ||||
| Vancomycin | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 5.8 | 59.6 | 77.9 | 78.9 | 79.3 |
Underlined values are MIC90s. The MIC90 for vancomycin among the tested enterococci was >16 μg/ml.
Includes E. faecalis (n = 2,217) and E. faecium (n = 1,381) isolates. MIC50s were 0.015 and 1 μg/ml for oritavancin and vancomycin, respectively, for 746 VRE isolates (MIC, >16 μg/ml), including 696 E. faecium isolates and 50 E. faecalis isolates.
As oritavancin emerges into clinical practice, the potential for immediate use of a surrogate agent (vancomycin) for susceptibility testing appears to be a prudent consideration. In our study, the results of reference MIC testing of oritavancin and vancomycin against a recent collection (from 2011 to 2013) of Gram-positive pathogens from the United States and Europe are presented. Analysis of a vancomycin surrogate susceptibility categorization to predict oritavancin susceptibility/activity at recently determined FDA MIC breakpoint levels (≤0.12 or ≤0.25 μg/ml) are presented along with corresponding accuracy rates previously reported (19).
(These results were presented in part at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy in Denver, CO, 10 to 13 September 2013 [20].)
MATERIALS AND METHODS
Bacterial strains.
All Gram-positive organisms tested in the SENTRY Antimicrobial Surveillance Program (i.e., U.S. and Europe isolates) against oritavancin and vancomycin in 2011 to 2013 were used for cross-susceptibility analysis. This included 26,993 strains: Staphylococcus aureus (17,717 strains, nearly 50% MRSA), coagulase-negative staphylococci (CoNS; 2,073 strains), beta-hemolytic streptococci (BHS; 2,357 strains), viridans group streptococci (VGS; 1,248 strains), and enterococci (Enterococcus faecalis and Enterococcus faecium; 3,598 strains; 746 were VRE). Results reported by Vidaillac et al. (21) were also included for oritavancin and vancomycin when testing vancomycin-intermediate S. aureus (VISA) and vancomycin-resistant S. aureus (VRSA).
Susceptibility testing and analysis.
All organisms were tested by the reference broth microdilution method of the Clinical and Laboratory Standards Institute (CLSI) (7, 8) with appropriate medium supplementation with 2.5 to 5.0% lysed horse blood for testing fastidious streptococci. These tests were performed in validated broth microdilution panels produced by Thermo Fisher Scientific (Cleveland, Ohio, USA), and quality assurance was confirmed by using the following quality control (QC) organisms: S. aureus ATCC 29213, E. faecalis ATCC 29212, and S. pneumoniae ATCC 49619 (8). All QC results were within specified ranges.
Analysis followed the general intermethod comparison guidelines provided in CLSI documents (7, 22) and previously applied to other agents active against Gram-positive pathogens and in the same antimicrobial class (2, 4). Interpretations for oritavancin focused on the use of a single surrogate agent (vancomycin) to predict concurrent susceptibility while minimizing false-positive (susceptibility) errors. Comparisons used published breakpoint criteria (8) or recently approved oritavancin breakpoints (19) for susceptibility at either ≤0.12 or ≤0.25 μg/ml, consistent with PK/PD data and clinical outcomes, but without assignment of an intermediate category (see Table 2, below). No other surrogate was considered, in an attempt to include in the analysis only an agent chemically similar and having a comparable, wide Gram-positive spectrum of activity; however, note that oritavancin would still remain active in vitro against many strains of VRE, as summarized in recent publications (23, 24), but not against VISA or VRSA strains (21).
TABLE 2.
Direct comparisons of oritavancin and vancomycin reference MICs for 19 Gram-positive pathogens in ABSSSI, 2011 to 2013
| Pathogen (no. tested)a | Vancomycin MIC (μg/ml) (susceptibility category)b | Oritavancin MIC (μg/ml) |
Surrogate accuracy (%) | ||||
|---|---|---|---|---|---|---|---|
| ≤0.06 | 0.12c | 0.25d | 0.5 | 1 | |||
| S. aureus (17,717) | 4 (I) | 0 | 0 | 0 | 0 | 0 | 98.8 |
| 2 (S) | 195 | 55 | 10 | 0 | 0 | ||
| 1 (S) | 12,474 | 1,130 | 189 | 1 | 0 | ||
| ≤0.5 (S) | 3,501 | 147 | 15 | 0 | 0 | ||
| BHS (2,357)e | 1 (S) | 1 | 3 | 0 | 1 | 0 | 98.1 |
| 0.5 (S) | 1,080 | 69 | 46 | 21 | 1 | ||
| ≤0.25 (S) | 920 | 113 | 80 | 22 | 0 | ||
| S. anginosus group (368)f | 1 (S) | 128 | 0 | 0 | 0 | 0 | 100.0 |
| 0.5 (S) | 226 | 0 | 0 | 0 | 0 | ||
| ≤0.25 (S) | 14 | 0 | 0 | 0 | 0 | ||
| E. faecalis (2,164), vancomycin susceptible | 4 (S) | 26 | 1 | 0 | 0 | 0 | 99.7 |
| 2 (S) | 620 | 9 | 1 | 0 | 0 | ||
| ≤1 (S) | 1,454 | 47 | 6 | 0 | 0 | ||
Only the indicated species were tabulated (19).
Susceptibility categories: I, intermediate; S, susceptible.
The susceptibility breakpoint for staphylococci (S. aureus) and enterococci (E. faecalis) (19).
The susceptibility breakpoint for beta-hemolytic streptococci (S. pyogenes, S. agalactiae, and S. dysgalactiae) and the S. anginosus group (19).
Beta-hemolytic streptococci included only S. pyogenes, S. agalactiae, and S. dysgalactiae.
Includes S. anginosus, S. constellatus, and S. intermedius.
RESULTS AND DISCUSSION
Comparative in vitro activities of oritavancin and vancomycin.
Table 1 presents the cumulative percentages of isolates from five pathogen groups inhibited by increasing concentrations of oritavancin and vancomycin. Among 17,717 S. aureus isolates, vancomycin (MIC90, 1 μg/ml) inhibited all strains at ≤2 μg/ml. Oritavancin was 16-fold more active than vancomycin, with a MIC90 of 0.06 μg/ml against S. aureus. Oritavancin was also 4-fold (BHS group) to >128-fold more active than vancomycin against other analyzed Gram-positive species, with the greatest potency difference recorded against the enterococci (MIC90 values of 0.06 μg/ml for oritavancin versus >8 μg/ml for vancomycin). All 26,993 tested surveillance strains had oritavancin MIC values of ≤1 μg/ml, and >99.9% of staphylococci had oritavancin MICs of ≤0.25 μg/ml (Table 1). These findings confirm and update data described in several previous publications (14, 23–26).
Surrogate testing of staphylococci.
Table 2 summarizes the ability of FDA-published oritavancin susceptibility breakpoints to be predicted by the vancomycin MIC for testing the indicated species (19). Using the ≤2-μg/ml vancomycin susceptibility breakpoint for S. aureus, the predicted oritavancin susceptibility rate was 98.8% for strains having oritavancin MICs of ≤0.12 μg/ml (FDA breakpoint for S. aureus) (19). The epidemiological cutoff value (ECOFF) for oritavancin was calculated at ≤0.12 μg/ml (27). Among the 2,073 CoNS strains tested (Table 3), vancomycin susceptibility results (≤4 μg/ml) predicted oritavancin susceptibility at ≤0.12 and ≤0.25 μg/ml with 99.8 and 100.0% accuracy, respectively. Whereas this high level of accuracy suggests that vancomycin susceptibility is predictive of oritavancin activity against these organisms, there is no FDA-established breakpoint for oritavancin against CoNS.
TABLE 3.
Vancomycin test result accuracy for prediction of oritavancin susceptibilitya
| Pathogen or species group (no. tested) | Surrogate accuracy for breakpoint (μg/ml) of: |
|
|---|---|---|
| ≤0.12 | ≤0.25 | |
| S. aureus (17,717) | 98.8b | >99.9 |
| CoNS (2,073) | 99.8 | 100.0 |
| S. epidermidis (1,177) | 100.0 | 100.0 |
| S. haemolyticus (182) | 98.4 | 100.0 |
| S. lugdunensis (138) | 100.0 | 100.0 |
| Enterococci (2,840)c | 99.7 | 100.0 |
| E. faecalis (2,164)a | 99.7 | 100.0 |
| E. faecium (676) | 100.0 | 100.0 |
| Beta-hemolytic streptococci (2,357) | NAd | 98.1 |
| S. pyogenes (1,209) | NA | 97.8 |
| S. agalactiae (1,016) | 96.4 | 98.5 |
| S. dysgalactiae (132) | NA | 97.7 |
| Viridans group streptococci (1,248) | 99.3 | 100.0 |
| S. anginosus group (368) | 100.0 | 100.0 |
| S. mitis group (406) | 99.8 | 100.0 |
Based on two breakpoint concentrations (≤0.12 and ≤0.25 μg/ml) when tested against 26,993 Gram-positive pathogens isolated during 2011 to 2013.
Underlined results indicate the accuracy at the FDA breakpoint for the indicated species (19). For BHS, data were tabulated only the three indicated species.
Data were tabulated only for vancomycin-susceptible strains.
NA, not acceptable; accuracy was <95.0%.
Figure 1 shows a scattergram of oritavancin and vancomycin MIC values among the 17,717 S. aureus surveillance isolates and also the results of testing 60 VISA and 10 VRSA isolates (21). S. aureus strains that were vancomycin nonsusceptible (NS; MIC, ≥4 μg/ml) had oritavancin MIC values ranging from 0.12 to 4 μg/ml, with 98.6% of isolates NS at ≥0.25 μg/ml (MIC50/MIC90, 0.5/2 μg/ml). As noted above, 98.8% of vancomycin-susceptible strains were also inhibited by oritavancin at ≤0.12 μg/ml (FDA breakpoint for susceptibility) (19)
FIG 1.
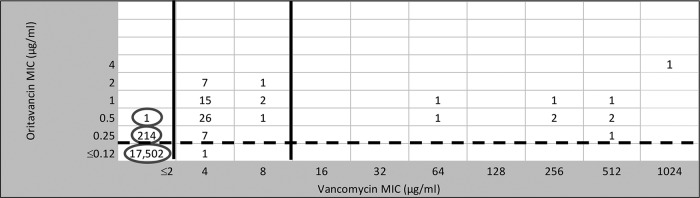
Scattergram comparing 17,717 S. aureus surveillance isolates (obtained between 2011 and 2013) tested against oritavancin and vancomycin (note the circled results). Also, data for 60 VISA (vancomycin MICs of 4 or 8 μg/ml) and 10 VRSA (MICs of 64 to 1,024 μg/ml) isolates are shown (originally reported in reference 21). Solid vertical lines, CLSI breakpoints; broken horizontal lines, FDA breakpoints (for vancomycin and oritavancin, respectively) (8, 19).
Surrogate testing of streptococci.
BHS and VGS had oritavancin MIC90s of 0.12 and 0.06 μg/ml, respectively (Table 1). For BHS, vancomycin surrogate accuracy was 98.1% (Tables 2 and 3) for the FDA-approved oritavancin breakpoint of ≤0.25 μg/ml. Use of the vancomycin susceptibility surrogate among VGS similarly produced excellent predictive accuracy for oritavancin susceptibility, ranging from 99.3% at ≤0.12 μg/ml (ECOFF) to 100.0% at ≤0.25 μg/ml (19) (Table 3).
Surrogate testing of E. faecalis and E. faecium.
Due to the greater in vitro potency and spectrum of activity for oritavancin compared to vancomycin against VRE, the vancomycin surrogate use accuracy calculations can only be applied to vancomycin-susceptible strains (Table 2). As illustrated in Table 1 and Fig. 2, a total of 21.1% of enterococci were nonsusceptible to vancomycin (MIC, ≥8 μg/ml) (8). All vancomycin-nonsusceptible organisms were inhibited by oritavancin at ≤0.5 μg/ml, and 98.4% of all enterococci (Table 1) were inhibited at ≤0.12 μg/ml, the FDA oritavancin breakpoint for vancomycin-susceptible isolates of E. faecalis (19). Nearly all (99.7%) vancomycin-susceptible E. faecalis strains were inhibited by oritavancin at ≤0.12 μg/ml, emphasizing a high degree of accuracy for vancomycin's use as a surrogate susceptibility marker agent (Tables 2 and 3). Among the 2,840 vancomycin-susceptible enterococci (Table 3 and Fig. 1), the vancomycin surrogate utility ranged from 99.7% at an oritavancin breakpoint of ≤0.12 μg/ml (for E. faecalis alone) to 100.0% for E. faecium. Notably, 707 of 758 (93.3%) vancomycin-nonsusceptible enterococci had an oritavancin MIC of ≤0.12 μg/ml, e.g., oritavancin susceptible (Fig. 2).
FIG 2.
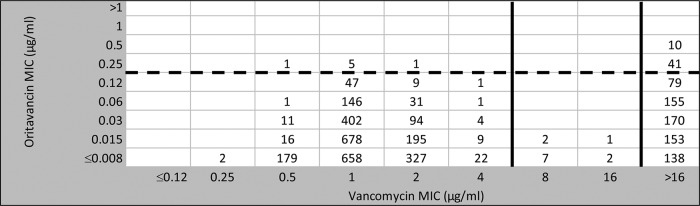
Scattergram comparing 3,598 Enterococcus spp. surveillance isolates (obtained from 2011 to 2013) tested against oritavancin and vancomycin. Breakpoint concentrations for vancomycin (solid vertical lines) were compared to the oritavancin breakpoint (≤0.12 μg/ml for vancomycin-susceptible E. faecalis; broken horizontal line) assigned by the FDA and/or proposed elsewhere based on ECOFF and PK/PD analyses (8, 15–17, 19, 27). A total of 12 and 746 isolates had intermediate susceptibility or resistance to vancomycin, and of those isolates, 93.3% were inhibited by 0.12 μg/ml oritavancin, e.g., they were oritavancin susceptible.
These cross-susceptibility analyses validate the surrogate use of vancomycin results to predict oritavancin activity and should allow the immediate, directed clinical use of this novel lipoglycopeptide agent (14). This antimicrobial susceptibility testing strategy, used effectively for decades with other organism/agent combinations, enables newly approved agents to be represented by other compounds within a class (shared resistance mechanisms) without any compromise to patient care (1–10). The vancomycin surrogate susceptibility testing predictive probabilities based on recently published oritavancin breakpoints (19) for the organism groups studied here (Tables 2 and 3) were as follows: for S. aureus, 98.8% at ≤0.12 μg/ml; for BHS, 98.1% at ≤0.25 μg/ml; for VGS, 100.0% at ≤0.25 μg/ml; for enterococci, 99.7% at ≤0.12 μg/ml. These high rates are considered very acceptable for any of these applied conservative breakpoints that have been qualified via ECOFF data (27) and PK/PD analyses (16) through regulatory (FDA and EUCAST/EMA) processes (19).
The in vitro potency and activity spectrum characteristics of oritavancin (14, 23–26), combined with a novel single-dosing strategy (15–17), may represent a potential therapeutic option not previously available for the treatment of ABSSSI (18). The present lack of in vitro susceptibility testing devices for oritavancin, owing to suboptimal disk/agar diffusion tests, is compounded by projected delays in availability of automated antimicrobial susceptibility testing devices containing oritavancin. These limitations can be initially overcome by the application of a validated surrogate susceptibility marker testing strategy (7, 8, 12, 13), as demonstrated here with vancomycin. To this end, oritavancin susceptibility could be inferred with a high degree of confidence when the tested strain is vancomycin susceptible based on the currently utilized laboratory susceptibility testing method and when both peptides are influenced by common resistance mechanisms. One limitation of this approach is that oritavancin maintains in vitro activity against many strains of VRE (Fig. 1) (14, 23, 24); however, it should be noted that among enterococci, only vancomycin-susceptible isolates of E. faecalis are indicated pathogens (Table 2) in the oritavancin FDA prescribing information (19). Furthermore, S. aureus NS to vancomycin usually have oritavancin MIC results of ≥0.25 μg/ml (21). Overall, the surrogate testing approach for oritavancin may be opportune, but we strongly urge further studies to develop accurate commercial susceptibility tests for direct assessment of oritavancin activity in the clinical microbiology laboratory.
ACKNOWLEDGMENTS
R.N.J., R.E.M., and J.D.T. are employees or subcontractors of JMI Laboratories, which coordinates an oritavancin international surveillance program for The Medicines Company. G.M. and F.F.A. are employees of The Medicines Company. JMI Laboratories receives grant funding from various other pharmaceutical/diagnostics industry sources for in vitro evaluations/surveillance of glycopeptide-like agents that could be impacted by these analyses.
R.N.J. is the guarantor for the data. We also acknowledge the following JMI Laboratories employees for support via analysis and manuscript preparation: M. Janechek, D. Farrell, R. Flamm, H. Sader, K. Hass, A. Fuhrmeister, and J. Streit.
REFERENCES
- 1.Barry AL, Jones RN. 1987. Cross susceptibility and absence of cross resistance to cefotetan and cefoxitin. J Clin Microbiol 25:1570–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones RN, Flamm RK, Sader HS, Stilwell MG. 2013. Interim susceptibility testing for ceftaroline, a new MRSA-active cephalosporin: selecting potent surrogate β-lactam markers to predict ceftaroline activity against clinically indicated species. Diagn Microbiol Infect Dis 75:89–93. doi: 10.1016/j.diagmicrobio.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Jones RN, Pfaller MA. 2001. Can antimicrobial susceptibility testing results for ciprofloxacin or levofloxacin predict susceptibility to a newer fluoroquinolone, gatifloxacin? Report from The SENTRY Antimicrobial Surveillance Program (1997–99). Diagn Microbiol Infect Dis 39:237–243. http://dx.doi.org/10.1016/S0732-8893(01)00229-2. [DOI] [PubMed] [Google Scholar]
- 4.Jones RN, Sader HS, Fritsche TR, Hogan PA, Sheehan DJ. 2006. Selection of a surrogate agent (vancomycin or teicoplanin) for initial susceptibility testing of dalbavancin: results from an international antimicrobial surveillance program. J Clin Microbiol 44:2622–2625. doi: 10.1128/JCM.00576-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones RN, Sader HS, Fritsche TR, Janechek MJ. 2007. Selection of a surrogate beta-lactam testing agent for initial susceptibility testing of doripenem, a new carbapenem. Diagn Microbiol Infect Dis 59:467–472. doi: 10.1016/j.diagmicrobio.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Jones RN, Zurenko GE. 1993. Prediction of bacterial susceptibility to cefpodoxime by using the ceftriaxone minimum inhibitory concentration result. Diagn Microbiol Infect Dis 17:313–316. doi: 10.1016/0732-8893(93)90041-5. [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2012. M07-A9. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2014. M100-S24. Performance standards for antimicrobial susceptibility testing: 24th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 9.Nicas TI, Mullen DL, Flokowitsch JE, Preston DA, Snyder NJ, Zweifel MJ, Wilkie SC, Rodriguez MJ, Thompson RC, Cooper RD. 1996. Semisynthetic glycopeptide antibiotics derived from LY264826 active against vancomycin-resistant enterococci. Antimicrob Agents Chemother 40:2194–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones RN, Barrett MS, Erwin ME. 1997. In vitro activity and spectrum of LY333328, a novel glycopeptide derivative. Antimicrob Agents Chemother 41:488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeckel ML, Preston DA, Allen BS. 2000. In vitro activities of LY333328 and comparative agents against nosocomial gram-positive pathogens collected in a 1997 global surveillance study. Antimicrob Agents Chemother 44:1370–1374. doi: 10.1128/AAC.44.5.1370-1374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arhin FF, Sarmiento I, Belley A, McKay GA, Draghi DC, Grover P, Sahm DF, Parr TR Jr, Moeck G. 2008. Effect of polysorbate 80 on oritavancin binding to plastic surfaces: implications for susceptibility testing. Antimicrob Agents Chemother 52:1597–1603. doi: 10.1128/AAC.01513-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arhin FF, Tomfohrde K, Draghi DC, Aranza M, Parr TR Jr, Sahm DF, Moeck G. 2008. Newly defined in vitro quality control ranges for oritavancin broth microdilution testing and impact of variation in testing parameters. Diagn Microbiol Infect Dis 62:92–95. doi: 10.1016/j.diagmicrobio.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Zhanel GG, Schweizer F, Karlowsky JA. 2012. Oritavancin: Mechanism of action. Clin Infect Dis 54(Suppl 3):S214–S219. doi: 10.1093/cid/cir920. [DOI] [PubMed] [Google Scholar]
- 15.Ambrose PG, Drusano GL, Craig WA. 2012. In vivo activity of oritavancin in animal infection models and rationale for a new dosing regimen in humans. Clin Infect Dis 54(Suppl 3):S220–S228. doi: 10.1093/cid/cis001. [DOI] [PubMed] [Google Scholar]
- 16.Belley A, Arhin FF, Sarmiento I, Deng H, Rose W, Moeck G. 2013. Pharmacodynamics of a simulated single 1,200-milligram dose of oritavancin in an in vitro pharmacokinetic/pharmacodynamic model of methicillin-resistant staphylococcus aureus infection. Antimicrob Agents Chemother 57:205–211. doi: 10.1128/AAC.01428-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunbar LM, Milata J, McClure T, Wasilewski MM. 2011. Comparison of the efficacy and safety of oritavancin front-loaded dosing regimens to daily dosing: an analysis of the SIMPLIFI trial. Antimicrob Agents Chemother 55:3476–3484. doi: 10.1128/AAC.00029-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corey GR, Kabler H, Mehra P, Gupta S, Overcash JS, Porwal A, Giordano P, Lucasti C, Perez A, Good S, Jiang H, Moeck G, O'Riordan W, SOLO I Investigators . 2014. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med 370:2180–2190. doi: 10.1056/NEJMoa1310422. [DOI] [PubMed] [Google Scholar]
- 19.The Medicines Company. 2014. Orbactiv package insert. The Medicines Company, Ville Saint Laurent, Quebec, Canada: http://www.orbactiv.com. Accessed 7 August 2014. [Google Scholar]
- 20.Jones RN, Mendes RE, Flamm RK, Sader HS, Stilwell MG. 2013. Interim use of vancomycin susceptibility testing results to predict activity of oritavancin, a new long acting lipoglycopeptide, poster D-583 Abstr 53rd Intersci Conf Antimicrob Agents Chemother, American Society for Microbiology, Washington, DC. [Google Scholar]
- 21.Vidaillac C, Parra-Ruiz J, Rybak MJ. 2011. In vitro time-kill analysis of oritavancin against clinical isolates of methicillin-resistant Staphylococcus aureus with reduced susceptibility to daptomycin. Diagn Microbiol Infect Dis 71:470–473. doi: 10.1016/j.diagmicrobio.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. 2008. M23-A3. Development of in vitro susceptibility testing criteria and quality control parameters, 3rd ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 23.Arias CA, Mendes RE, Stilwell MG, Jones RN, Murray BE. 2012. Unmet needs and prospects for oritavancin in the management of vancomycin-resistant enterococcal infections. Clin Infect Dis 54(Suppl 3):S233–S238. doi: 10.1093/cid/cir924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendes RE, Farrell DJ, Sader HS, Jones RN. 2012. Oritavancin microbiologic features and activity results from the surveillance program in the United States. Clin Infect Dis 54(Suppl 3):S203–S213. doi: 10.1093/cid/cir923. [DOI] [PubMed] [Google Scholar]
- 25.Mendes RE, Sader HS, Flamm RK, Farrell DJ, Jones RN. 2014. Oritavancin activity against Staphylococcus aureus causing invasive infections in U.S. and European hospitals: a 5-year international surveillance program. Antimicrob Agents Chemother 58:2921–2924. doi: 10.1128/AAC.02482-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendes RE, Sader HS, Flamm RK, Jones RN. 2014. Activity of oritavancin tested against uncommonly isolated Gram-positive pathogens responsible for documented infections in hospitals worldwide. J Antimicrob Chemother 69:1579–1581. doi: 10.1093/jac/dku016. [DOI] [PubMed] [Google Scholar]
- 27.Turnidge J, Kahlmeter G, Kronvall G. 2006. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological values. Clin Microbiol Infect 12:418–425. doi: 10.1111/j.1469-0691.2006.01377.x. [DOI] [PubMed] [Google Scholar]


