Abstract
We conducted an open-label, steady-state pharmacokinetic (PK) study of drug-drug interactions between depot medroxyprogesterone acetate (DMPA) and twice-daily lopinavir (LPV) plus low-dose ritonavir (RTV) (LPV/r) among 24 HIV-infected women and compared the results to those for HIV-infected women receiving DMPA while on no antiretroviral therapy or on nucleosides only (n = 14 subjects from the control arm of AIDS Clinical Trials Group [ACTG] study 5093). The objectives of the study were to address the effect of LPV/r on DMPA and to address the effect of DMPA on LPV/r therapy. PK parameters were estimated using noncompartmental analysis with between-group comparisons of medroxyprogesterone acetate (MPA) PKs and within-subject comparisons of LPV and RTV PKs before and 4 weeks after DMPA dosing. Plasma progesterone concentrations were measured every 2 weeks after DMPA dosing through week 12. Although the MPA area under the concentration-time curve and maximum concentration of drug in plasma were statistically significantly increased in the study women on LPV/r compared to those in the historical controls, these increases were not considered clinically significant. There were no changes in LPV or RTV exposure after DMPA. DMPA was well tolerated, and suppression of ovulation was maintained. (This study has been registered at ClinicalTrials.gov under registration no. NCT01296152.)
INTRODUCTION
Human immunodeficiency virus (HIV) has profoundly affected the health of women worldwide, and now women represent over half (52%) of those living with HIV (1). Experience with combination antiretroviral therapy (cART) in HIV/AIDS gained over the past 30 years has led to the recognition of drug-drug interactions potentially associated with reduced or increased exposures to antiretroviral drugs resulting in either decreased efficacy or increased drug toxicity. This is especially true for medications known to be substrates and/or inducers or inhibitors of the hepatic cytochrome P450 (CYP) system (2, 3).
Globally, one of the most commonly used long-acting hormonal contraceptives is depot medroxyprogesterone acetate (DMPA), which is administered as an intramuscular injection of 150 mg every 3 months and which is used at a high rate in sub-Saharan Africa and Southeast Asia (4). Little is known about the use of DMPA in the setting of ritonavir (RTV)-enhanced protease inhibitors (PIs), commonly used in cART regimens. RTV is the most commonly used pharmacoenhancer and is known to be a potent inhibitor of CYP3A4, leading to elevated concentration of PIs and potentially other CYP3A4 substrates. Since both medroxyprogesterone acetate (MPA), the active metabolite of DMPA, and lopinavir (LPV), a PI, are metabolized through the CYP system, there may be significant drug-drug interactions and an increased risk of toxicities (3, 5). This may especially be a concern in women, since CYP3A4 activity differs by gender, with some reports suggesting that women have 40% greater CYP3A4 activity than men (6).
Developing countries have both a high prevalence of women with HIV and a high prevalence of DMPA use for contraception. The increasing use of cART, including LPV plus low-dose RTV (LPV/r), in developing country settings, underscores the importance of investigating the safety, efficacy, and potential drug-drug interactions between antiretroviral medications and DMPA. The objectives of this study were to assess the effect of twice-daily (b.i.d.) LPV/r on DMPA and the potential effect of DMPA on LPV/r as well as the safety and tolerance of this combination among HIV-infected women with HIV suppression.
(This work was presented in part at the 21st Conference on Retrovirus and Opportunistic Infections [CROI], 3 to 6 March 2014, Boston, MA.)
MATERIALS AND METHODS
Study design.
This study (ClinicalTrials.gov registration no. NCT01296152) was a 12-week, multicenter, open-label, nonrandomized, single-arm study of steady-state pharmacokinetic (PK) interactions between coformulated LPV/r (400/100 mg b.i.d.) in combination with 2 or more acceptable nucleoside reverse transcriptase inhibitors (NRTIs) and DMPA administered as an intramuscular injection in HIV-infected women. To evaluate the effect of LPV/r on the PKs of DMPA, we compared women on an LPV/r-based effective antiretroviral regimen with historical controls: HIV-infected women on no ART or only on nucleosides/nucleotides from a previous AIDS Clinical Trials Group (ACTG) study (ACTG study 5093 [A5093]). To evaluate the effect of DMPA on the PKs of LPV and RTV, subjects served as their own controls.
We chose to examine the use of LPV/r in women using DMPA for several reasons. First, LPV/r is one of the preferred PIs recommended for use during pregnancy and for prevention of mother-to-child transmission of HIV (7, 8) and is often continued postpartum. Additionally, LPV/r is frequently included in second-line antiretroviral (ARV) regimens for nonpregnant women in combination with nucleosides and/or integrase inhibitors (9). The twice-daily dosing schedule for LPV/r was chosen to provide a greater opportunity to uncover any drug-drug interactions.
Study population.
Subjects were nonpregnant, premenopausal, HIV type 1 (HIV-1)-infected women ≥13 years old who had been on a stable combination ARV regimen containing coformulated LPV at 400 mg plus ritonavir at 100 mg twice daily for at least the previous 14 days if they were not postpartum and for 30 days if they were postpartum. Furthermore, the subjects had not received DMPA within 180 days before study entry or any other hormonal therapies within 30 days of study entry. All subjects had plasma HIV-1 RNA levels of ≤400 copies/ml within 30 days of study entry, were required to continue the LPV/r twice-daily-based regimen without modifications for the 12 weeks of the study, and could not have initiated, stopped, or changed doses of medications that were CYP3A4 substrates within 30 days before study entry. Subjects were required to use a second, nonhormonal method of contraception (barrier method, nonhormonal intrauterine device, or sterilization) during the study. Women were excluded if they were breast-feeding, had had a bilateral oophorectomy, had a history of hypersensitivity to DMPA, or had any known contraindication for DMPA administration.
We used as historical controls HIV-infected women who participated in a previous ACTG study (A5093) and were in the A5093 control arm (they received no ARV medications or regimens with nucleoside reverse transcriptase inhibitors only). The sample size in each arm was calculated to detect a difference in the MPA area under the concentration-time curve (AUC) between arms of 40% or greater, assuming the use of the t test for two independent samples to compare log-transformed AUCs, fixing type I and type II error rates at 0.05 and 0.10, respectively, and assuming a between-subject coefficient of variation (CV) for the MPA AUC of 40%. Under these assumptions, a difference in the MPA AUC of 40% or greater would be detected with a sample size of 12 subjects per arm. To provide protection against potential adherence and/or assay problems, a buffer of two additional subjects per arm was added for this small exploratory study. Thus, the control arm had a sample size of 14 subjects from A5093.
ACTG 5283 (the study reported here) was powered to evaluate both the effect of LPV/r on the MPA AUC and the effect of DMPA on the LPV AUC. The study required 20 evaluable subjects to provide at least an 80% power to detect a 40% difference in the MPA AUC between subjects receiving LPV/r and subjects not receiving LPV/r (n = 14 in the control group) and a 30% difference in the LPV AUC between before and after DMPA administration, using the nonparametric Wilcoxon test with a type I error of 0.05. The sample size of 20 evaluable subjects was calculated by considering a 95% relative efficiency in case of violation of the underlying distributional assumption of normality compared to that obtained by the t test. To provide protection against potential adherence and/or assay problems, three additional subjects were enrolled.
Study procedures.
Blood samples for MPA and progesterone concentration determinations were obtained before the DMPA injection (day 0) and at weeks 2, 4, 6, 8, 10, and 12 after DMPA administration. Ovulation was assumed to have occurred if progesterone concentrations were >5 ng/ml on week 2 or later. MPA concentrations of ≥0.1 ng/ml were assumed to suppress ovulation (10). Lopinavir and ritonavir concentrations were measured at both week 0 (day 0, before DMPA injection) and week 4 (after DMPA injection); intensive sampling for determination of lopinavir and ritonavir concentrations was performed, with blood collected at time zero (predosing) and 0.5, 1, 2, 3, 4, 5, 6, 8, and 10 h postdosing.
Subjects received a single 150-mg DMPA injection (Pfizer) in the gluteal region after confirmation of a negative pregnancy test and after the single blood sample was drawn at study entry. Follow-up study visits occurred at 2, 4, 6, 8, 10, and 12 weeks after DMPA administration.
Subjects completed a self-report questionnaire at the baseline to assess potential factors affecting adherence, including drug and alcohol use. Subjects who were tobacco smokers agreed not to change their pattern of tobacco use over the 12-week study. Alcohol was prohibited during the 24 h before and during the 10-h ARV PK studies on day 0 and week 4. Adherence to ARV medications was assessed at each study visit using a self-report questionnaire developed by the AIDS Clinical Trials Group (11). Study participants were asked to abstain from the consumption of any grapefruit product or supplement for the duration of the study. This protocol was approved by the institutional review boards at each participating AIDS Clinical Trials Group and International Maternal, Pediatric, Adolescent AIDS Clinical Trials (IMPAACT) site; each patient provided written informed consent prior to enrollment.
New symptoms, signs, or laboratory abnormalities were graded using the standardized Division of AIDS (DAIDS) Table for Grading Severity of Adult Adverse Experiences (http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf, clarification 2009). Signs and symptoms were graded as mild, moderate, severe, or life threatening, with mild symptoms usually requiring no therapy, moderate symptoms requiring no therapy or only outpatient treatment, and severe symptoms requiring medical intervention and possible treatment in the hospital. All signs and symptoms of any grade, laboratory abnormalities thought to be related to the study medication (DMPA), and any signs or symptoms of grade 2 or higher were recorded for the other medications. All adverse events reported were reviewed by the team on monthly calls and assigned causality as definitely, probably, possibly, unlikely, or not related to the primary study treatment (DMPA).
PK and statistical data analyses.
There were two primary objectives. One was to evaluate the effect of LPV/r on the PK parameter the AUC of MPA. This objective was evaluated by comparing the MPA PK parameter obtained from the study subjects (to whom DMPA was administered with LPV/r) with the MPA PK parameter obtained from the historical controls (A5093 historical controls [to whom DMPA was administered without LPV/r]) using the nonparametric Wilcoxon rank sum test. The other primary objective was to evaluate the effect of DMPA on the PK parameter the AUC of LPV. This objective was evaluated by testing intrasubject changes in the LPV PK parameter from study day 0 (before DMPA was administered) to study week 4 (4 weeks after DMPA was administered) using the nonparametric Wilcoxon signed-rank test. Other PK parameters of MPA and LPV evaluated were the minimum concentration (Cmin), maximum concentration (Cmax), time to Cmax (Tmax), apparent clearance (CL/F), and half-life (t1/2). All the PK parameters were estimated using standard noncompartmental methods. The relationships of MPA PK parameters with each subject's baseline characteristics were examined using the nonparametric Spearman's correlation coefficient. All statistical analyses were performed using SAS, version 9.2 (SAS Institute Inc., Cary, NC, USA).
Drug concentration assays.
LPV and RTV concentrations were analyzed using high-pressure liquid chromatography at the ACTG Pharmacology Support Laboratory as previously described (12). The lower limit of quantification (LLQ) and upper limit of quantification (ULQ) for LPV were 50 and 8,000 ng/ml, respectively. The LLQ and ULQ for RTV were 50 and 4,000 ng/ml, respectively. Drug concentrations below the LLQ were assigned a value of half the LLQ. By design, samples were not drawn for LPV and RTV concentration determination at 12 h postdosing. Instead, the predosing LPV and RTV concentrations were imputed for the 12-h time points, relying on the theory of no difference in the concentration between times of 0 and 12 h for steady-state twice-daily dosing. During the course of LPV analysis, 32 sets of quality controls at four concentrations (150, 1,500, 3,000, and 7,000 ng/ml) were analyzed along with the clinical samples. The mean accuracy and precision (mean percentage of the nominal value ± CV) were 92.9% ± 4.8%, 101.8% ± 4.9%, 98.7% ± 4.4%, and 96.8% ± 5.2%, respectively. During RTV analysis, 40 sets of quality controls at three concentrations (150, 750, and 3,500 ng/ml) were analyzed. The mean accuracy and precision were 97.1% ± 5.4%, 100.2% ± 5.9%, and 97.9% ± 6.3%, respectively.
MPA concentrations for both the subjects in this study and the historical controls were measured at the Pharmaceutical Product Development (PPD) Laboratory using high-pressure liquid chromatography–mass spectroscopy with an LLQ of 0.02 ng/ml and a ULQ of 5 ng/ml. The mean interassay precision (CV) for MPA was 6%, and the mean bias was −1.8325%. After week 0, MPA concentrations that were below the LLQ were replaced by concentrations that were half the LLQ. Progesterone concentrations were determined by an enzyme multiplied immunoassay technique (EMIT) at a central ACTG processing laboratory. Concentrations of >5 ng/ml were considered presumptive for ovulation.
Virologic and immunologic markers.
We assessed HIV RNA levels and CD4+ cell counts to evaluate the impact of the combination of LPV/r and DMPA on these standard markers of virologic and immunologic responses to therapy. HIV RNA plasma levels were determined in laboratories certified by the DAIDS Virology Quality Assurance Program (13) using the Roche AmpliPrep/Cobas TaqMan HIV-1 monitor assay (Roche Molecular Systems, Pleasanton, CA) and the Abbott RealTime HIV-1 assay. CD4+ cell counts were determined using standard flow cytometric methods in laboratories certified by the DAIDS Quality Assurance Program (14). Samples for HIV RNA quantification were obtained at screening, the baseline (day 0), and 2, 4, 8, and 12 weeks after DMPA administration.
CD4+ cell counts were obtained at scheduled visits at weeks 0, 4, and 12. The nonparametric Wilcoxon signed-rank test was used to evaluate the changes between the counts at the baseline and those at weeks 4 and 12. DMPA PK data from 14 HIV-infected women that participated in A5093 and that were receiving no ARV medications or taking an NRTI only were used as historical controls for this study, and their HIV RNA levels and CD4+ cell counts, collected using a study design identical to that used in the present study, were included.
RESULTS
Demographic characteristics.
Twenty-five eligible women were enrolled between June 2011 and October 2012 at 13 domestic sites overall, which included 5 AIDS Clinical Trial Group clinical research sites (CRSs) and 8 National Institute of Child Health and Human Development (NICHD)-funded IMPAACT CRSs. One participant was not evaluable for PK analysis due to noncompliance, leaving 24 total evaluable women in this study. The median age was 32 years, and the age range was from 15 to 47 years. Fourteen participants (58%) were black non-Hispanic, 6 (25%) were Hispanic (regardless of race), 3 (13%) were white non-Hispanic, and 1 (4%) was Native American. No participants had a history of intravenous drug use. The median CD4+ cell count at the baseline (the time of study entry, day 0) was 622 cells/μl (range, 326 to 1,367 cells/μl). The median weight at study entry (day 0) was 68 kg (range, 43 to 110 kg), and the median body mass index (BMI) was 28.2 kg/m2 (range, 17.7 to 40.4 kg/m2). The baseline characteristics are shown in Table 1.
TABLE 1.
Baseline characteristics of study group and historical controls
| Characteristic | Study groupa | Historical controlsb |
|---|---|---|
| Median (range) age (yr) at baseline | 32 (15–47) | 33 (22–46) |
| Median (range) body wt (kg) at baseline | 68.0 (43.5–110.0) | 67.9 (51.8–107.5) |
| Median (range) BMI (kg/m2) at baseline | 28.2 (17.7–40.4) | 23.3 (18.1–34.4) |
| No. (%) of subjects with the following race/ethnicity: | ||
| White | 3 (13) | 1 (7) |
| Black | 14 (58) | 11 (79) |
| Hispanic | 6 (25) | 2 (14) |
| Native American | 1 (4) | 0 |
| Median (range) CD4+ cell count (no. of cells/μl) | 622 (326–1,367) | 704 (328–1,255) |
| No. (%) of subjects with the following HIV-1 RNA level (no. of copies/ml): | ||
| <400 | 24 (100) | 6 (46) |
| 400–10,000 | 0 (0) | 5 (39) |
| >10,000 | 0 (0) | 2 (15) |
Subjects from this study (ACTG study 5283). The subjects received LPV/r b.i.d. plus two NRTIs (n = 24).
Historical controls from A5093. The controls received no ARV therapy or NRTIs only (n = 14). For the historical controls, BMI was based on data for 12 subjects (2 subjects did not have height documented) and the baseline viral load was based on data for 13 subjects.
Fourteen HIV-infected women who participated as controls in ACTG 5093 and were on DMPA but on no ARV medications or were on NRTIs only were used as historical controls. Their median age was 33 years (range, 22 to 46 years). Seventy-nine percent of the participants were black non-Hispanic, 14% were Hispanic, 7% were white, and there were no participants who were Native Americans. The median CD4+ cell count was 704 cells/μl (range, 328 to 1,255 cells/μl). At study entry the median weight was 67.9 kg (range, 51.8 to 107.5 kg), and the median BMI was 23.3 kg/m2 (range, 18.1 to 34.4 kg/m2). The characteristics of the historical controls were similar to those of the study group (ACTG 5283), except that they had higher HIV loads since they were not on combination antiretroviral medications (Table 1).
Lopinavir and ritonavir pharmacokinetics.
The PK parameters for lopinavir and ritonavir estimated before and 4 weeks after DMPA was administered are presented in Tables 2 and 3, respectively. LPV and RTV exposures according to the AUC and other PK parameters were not altered by DMPA.
TABLE 2.
LPV PK parameters in ACTG study 5283 subjects before and after DMPA administrationa
| Parameter | AUC0–12 h (ng · h/ml) |
Cmin (ng/ml) |
Cmax (ng/ml) |
Tmax (h) |
CL/F (liters/h) |
t1/2 (h) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Without DMPA | With DMPA | Without DMPA | With DMPA | Without DMPA | With DMPA | Without DMPA | With DMPA | Without DMPA | With DMPA | Without DMPA | With DMPA | |
| Mean | 95,254.14 | 101,125.05 | 5,471.21 | 6,082.08 | 10,646.67 | 11,010.42 | 3.42 | 3.21 | 4.69 | 4.26 | 13.81 | 15.03 |
| SD | 28,217.87 | 29,553.53 | 2,708.57 | 2,247.51 | 2,602.51 | 2,859.41 | 1.64 | 1.56 | 1.91 | 1.21 | 8.49 | 11.31 |
| Median | 98,046.46 | 97,948.29 | 5,630.00 | 5,700.00 | 10,750.00 | 10,950.00 | 3.00 | 3.00 | 4.08 | 4.08 | 11.76 | 12.64 |
| IQR (Q1, Q3) | 81,563.58, 106,116.46 | 84,116.75, 113,629.33 | 4,645.00, 6,700.00 | 4,585.00, 6,810.00 | 9,220.00, 12,350.00 | 9,300.00, 12,300.00 | 2.00, 4.00 | 2.00, 4.00 | 3.77, 4.90 | 3.52, 4.77 | 8.03, 18.94 | 9.73, 15.56 |
| Range (minimum, maximum) | 38,347.88, 158,899.17 | 56,489.42, 192,989.17 | 25.00, 10,600.00 | 3,630.00, 13,900.00 | 4,760.00, 16,000.00 | 6,290.00, 18,700.00 | 1.00, 8.00 | 1.00, 8.00 | 2.52, 10.43 | 2.07, 7.08 | 1.60, 33.67 | 5.15, 63.98 |
| P valueb | 0.782 | 0.708 | 0.857 | 0.644 | 0.740 | 0.617 | ||||||
Data are for 24 subjects in each treatment group for all parameters. AUC0–12 h, area under the concentration-time curve over the dosing period of 12 h; Cmin, minimum concentration; Cmax, maximum concentration; Tmax, time to Cmax; CL/F, apparent clearance; t1/2, half-life; Q1, first quartile; Q3, third quartile.
P value by Wilcoxon signed-rank test of changes between before DMPA (without DMPA) and after DMPA (with DMPA).
TABLE 3.
RTV PK parameters in ACTG study 5283 subjects before and after DMPA administrationa
| Parameter | AUC0–12 h (ng · h/ml) |
Cmin (ng/ml) |
Cmax (ng/ml) |
Tmax (h) |
CL/F (liters/h) |
t1/2 (h) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Without DMPA (n = 24) | With DMPA | Without DMPA | With DMPA | Without DMPA | With DMPA | Without DMPA | With DMPA | Without DMPA | With DMPA | Without DMPA | With DMPA | |
| Mean | 5,609.01 | 5,453.02 | 209.29 | 207.68 | 913.67 | 892.17 | 3.67 | 2.83 | 20.98 | 21.53 | 6.45 | 5.94 |
| SD | 2,087.71 | 2,223.54 | 133.95 | 105.60 | 411.94 | 481.70 | 2.66 | 1.13 | 10.64 | 9.00 | 5.08 | 2.06 |
| Median | 5,176.79 | 5,014.73 | 181.00 | 194.50 | 884.00 | 726.00 | 3.00 | 3.00 | 19.32 | 19.94 | 4.56 | 6.32 |
| IQR (Q1, Q3) | 4,098.11, 7,451.07 | 3,648.21, 6,925.08 | 122.00, 281.00 | 112.00, 283.00 | 597.00, 1,080.00 | 586.50, 1,088.50 | 2.00, 4.50 | 2.00, 3.00 | 13.45, 24.40 | 14.48, 27.41 | 3.66, 8.07 | 4.31, 7.15 |
| Range (minimum, maximum) | 1,605.05, 9,858.86 | 2,325.59, 10,011.32 | 25.00, 527.00 | 68.90, 486.00 | 224.00, 2,070.00 | 268.00, 1,950.00 | 1.00, 12.00 | 1.00, 6.00 | 10.14, 62.30 | 9.99, 43.00 | 1.28, 22.59 | 2.23, 10.62 |
| P valueb | 0.598 | 1.000 | 0.541 | 0.140 | 0.216 | 0.768 | ||||||
Data are for 24 subjects in each treatment group for all parameters except t1/2 without DMPA, for which there were 23 subjects. AUC0–12 h, area under the concentration-time curve over the dosing period of 12 h; Cmin, minimum concentration; Cmax, maximum concentration; Tmax, time to Cmax; CL/F, apparent clearance; t1/2, half-life; Q1, first quartile; Q3, third quartile.
P value by Wilcoxon signed-rank test of changes between before DMPA (without DMPA) and after DMPA (with DMPA).
Pharmacodynamics of LPV/r.
The median baseline CD4+ cell count for the participants on LPV/r-based antiretroviral therapy was 622 cells/mm3, and the median CD4+ percentage at the baseline was 35. In the historical controls (women not taking antiretroviral therapy or on NRTIs only with CD4+ cell counts of >350 cells/mm3 at entry), the median CD4+ cell count was 704 cells/mm3, and the median CD4+ percentage at the baseline was 38. Women on LPV/r and women not taking ARV or NRTIs only (historical controls) had no significant changes in the median CD4+ cell count or CD4+ percentage at weeks 4 and 12 compared to the baseline values. Specifically, the median absolute CD4+ cell counts and percentages in women on LPV/r were 622 and 35% at the baseline, 717 and 37% at week 4, and 640 and 35% at week 12. Among the historical controls, the median absolute CD4+ cell counts and percentages were 704 and 38% at the baseline, 678 and 33% at week 4, and 665 and 37% at week 12.
All participants taking LPV/r had HIV-1 RNA levels below 400 copies/ml at the baseline and biweekly through week 8. By week 12, however, 3 participants (13%) had slight elevations in their HIV RNA levels, with values of 558, 566, and 5,101 copies/ml, respectively. The two subjects with 558 and 566 copies/ml reported on the ACTG self-report questionnaire that they had missed doses of antiretroviral medications. However, the third subject, whose HIV-1 RNA level rose to 5,101 copies/ml, appeared to be 100% adherent. Queries to the site could not identify a reason for the sudden rise in the HIV-1 RNA level for this subject. However, for this subject, the week 4 LPV AUC over the dosing period of 12 h (AUC0–12 h), Cmax, and Cmin were 100,375 ng · h/ml, 14,400 ng/ml, and 5,980 ng/ml, respectively, which were higher than the medians for the population. No LPV values for this subject were available at week 12.
DMPA pharmacokinetics.
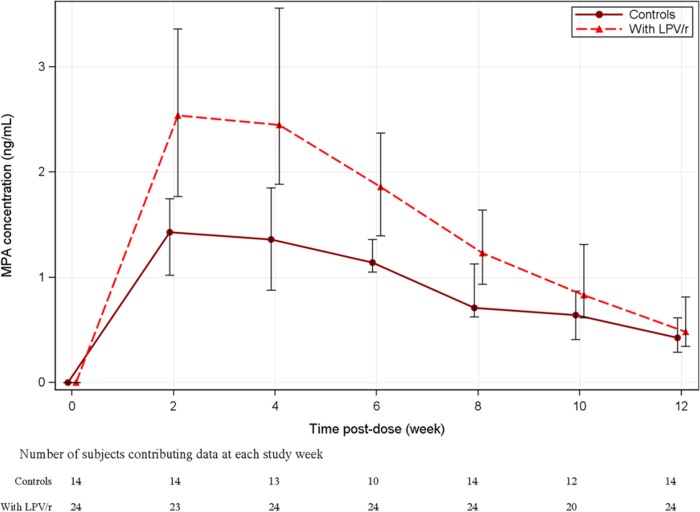
The median (interquartile range [IQR]) MPA concentration-time curve over the 12 weeks for women on LPV/r versus those not on LPV/r (historical controls from A5093) is presented in Fig. 1. The median MPA concentration was the highest at 2 weeks postdosing, and the median MPA concentration at 2 weeks was 78% higher when DMPA was administered with LPV/r (2.54 ng/ml [IQR, 1.77 to 3.36 ng/ml]) than when it was administered without LPV/r (1.43 ng/ml [IQR, 1.02 to 1.75 ng/ml]). The median MPA concentration at week 12 postdosing was similar between the study group (0.484 ng/ml [IQR, 0.347 to 0.815 ng/ml]) and the historical controls (0.427 ng/ml [IQR, 0.290 to 0.617 ng/ml]).
FIG 1.
Median MPA concentration-time curves over the 12 weeks of study for women on LPV/r versus those not on LPV/r (historical controls from A5093). Vertical bars represent the first and third quartiles.
The MPA PK parameters AUC over the dosing period of 12 weeks (AUC0–12 wk), Cmin, Cmax, Tmax, CL/F, and t1/2 for the study participants as well as for the historical controls are presented in Table 4. The MPA AUC0–12 wk and Cmax were significantly higher (46% and 66% increases in the median, respectively; P < 0.001) and the MPA CL/F was significantly lower when DMPA was coadministered with LPV/r than when it was administered without LPV/r. The MPA Cmin and t1/2 were not significantly different between the two groups. Linear regression analyses for the study participants showed that the participants' baseline body weight and BMI were significant predictors for MPA PK parameters AUC0–12 wk, Cmax, and CL/F; however, these baseline characteristics were not significant predictors of the MPA Cmin or t1/2. For the historical controls, neither body weight nor BMI was a significant predictor of MPA PK parameters. The differences in MPA PK parameters AUC0–12 wk, Cmax, and CL/F between the historical controls and the ACTG 5283 participants were also examined in models that adjusted for baseline body weight and (in a separate model) BMI and showed differences of similar magnitudes and levels of statistical significance (data not shown).
TABLE 4.
MPA PK parameters in study subjects and historical controlsa
| Parameter | AUC0–12 wk (ng · wk/ml) |
Cmin (ng/ml) |
Cmax (ng/ml) |
Tmax (wk) |
CL/F (liters/wk) |
t1/2 (wk) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | With LPV/r | Controls | With LPV/r | Controls | With LPV/r | Controls | With LPV/r | Controls | With LPV/r | Controls | With LPV/r | |
| Mean | 11.48 | 19.53 | 0.45 | 0.54 | 1.64 | 3.10 | 2.57 | 4.17 | 14,193.29 | 8,820.89 | 9.01 | 27.05 |
| SD | 3.10 | 6.51 | 0.24 | 0.36 | 0.56 | 1.35 | 0.94 | 2.76 | 4,645.24 | 4,251.24 | 11.04 | 107.59 |
| Median | 12.38 | 18.08 | 0.43 | 0.47 | 1.74 | 2.88 | 2.00 | 4.00 | 12,117.10 | 8,297.35 | 5.37 | 3.37 |
| IQR (Q1, Q3) | 8.88, 13.88 | 16.22, 24.10 | 0.29, 0.60 | 0.35, 0.74 | 1.02, 2.09 | 2.28, 4.04 | 2.00, 4.00 | 2.00, 4.00 | 10,808.03, 16,890.53 | 6,223.57, 9,250.23 | 3.04, 12.15 | 2.05, 5.42 |
| Range (minimum, maximum) | 6.03, 15.64 | 5.91, 32.33 | 0.04, 0.89 | 0.03, 1.43 | 0.88, 2.58 | 0.84, 5.88 | 2.00, 4.00 | 2.00, 12.00 | 9,588.34, 24,881.39 | 4,639.14, 25,385.74 | 1.54, 44.76 | 1.11, 508.53 |
| P valueb | <0.001 | 0.565 | <0.001 | 0.027 | <0.001 | 0.116 | ||||||
Data are for 14 control subjects and 24 subjects treated with LPV/r, except for t1/2 with LPV/r, for which there were 22 subjects. AUC0–12 wk, area under the concentration-time curve over the dosing period of 12 weeks; Cmin, minimum concentration; Cmax, maximum concentration; Tmax, time to Cmax; CL/F, apparent clearance; t1/2, half-life; Q1, first quartile; Q3, third quartile.
P value by Wilcoxon rank sum test.
Pharmacodynamics of DMPA.
No pregnancies occurred during the study. The progesterone concentration was followed every 2 weeks throughout the 12-week study and remained below 5 ng/ml both for the women on LPV/r and for the historical controls. For the women on LPV/r (ACTG 5283), the highest progesterone concentration was 1.2 ng/ml at week 2, and all subsequent progesterone concentrations were ≤0.8 ng/ml after week 2. At 12 weeks after administration of DMPA, MPA concentrations for two participants were below 0.1 ng/ml; however, the serum progesterone concentrations for these participants stayed below the lower limit of quantification (0.5 ng/ml).
Tolerability of DMPA.
DMPA was relatively well tolerated by the participants taking LPV/r. Menstrual irregularities with abnormal vaginal bleeding occurred in 6 (25%) of 24 evaluable subjects, with the irregularity for 1 subject being characterized as grade 3 due to its duration, because it lasted for 1 month. However, this subject did not require any specific treatment for the vaginal bleeding, which resolved spontaneously. All menstrual irregularities were considered possibly related to treatment with DMPA. There were no cases of anemia among the study patients or the historical controls during the study, suggesting no clinically important impact on hematocrit from abnormal vaginal bleeding.
DISCUSSION
Understanding potential drug-drug interactions between antiretroviral medications and hormonal contraceptives, such as DMPA, is of paramount importance to ensure effective antiretroviral therapy while meeting the need for efficient contraception in HIV-infected women. DMPA interactions with selected ARV medications, including nelfinavir, efavirenz, and nevirapine, have been previously studied and demonstrated that DMPA has no significant impact on the pharmacokinetic parameters of MPA or those of these ARV medications (15, 16). However, a knowledge gap existed regarding the impact of DMPA on the PKs of currently used PIs which are combined with ritonavir as a pharmacoenhancer.
In this steady-state pharmacokinetic study, we found that the concomitant use of LPV/r and DMPA resulted in a significant increase in MPA exposure. This increase in MPA exposure is likely caused by the inhibitory effect of LPV/r on CYP3A4 (17), of which MPA is considered to be a substrate (18). LPV/r is also known to inhibit membrane transporters, such as P-glycoprotein (P-gp) (19); however, the role of P-gp on the disposition of MPA administered by intramuscular injection is expected to be minimal.
Despite this increase in the MPA AUC, MPA was relatively well tolerated, and most of the adverse events reported were mild to moderate events that are known to occur in patients being treated with DMPA, such as irregular vaginal bleeding.
The persistence of a low progesterone concentration of <5 ng/ml throughout the 12 weeks of the study suggests that the concurrent use of twice-daily LPV/r and DMPA did not appear to have resulted in interference with the suppression of ovulation. Similarly, the administration of DMPA and the relatively high concentration of MPA did not affect the concentration of either LPV or RTV during the study. On the basis of these results, the use of LPV/r with DMPA appears to be safe and effective. The fact that the MPA concentrations at week 12 were not significantly different between the LPV/r-treated group and the historical controls suggests that there is no need to alter the dosing interval of DMPA.
We recognize limitations in our study, including the relatively short duration of observation, which precludes conclusions regarding long-term adverse events with more prolonged use of these medications from being made; in particular, higher MPA exposures could theoretically result in a higher incidence of MPA-related adverse events. This small study was powered to detected significant differences between the study group on LPV/r and the historical control group, and we were unable to match the two populations by BMI or other potentially important variables. It is also hard to extrapolate our results to other PIs which are used with lower doses of ritonavir for boosting and, hence, with which the same increases in the MPA AUC may not be seen.
One subject had an increase in the plasma HIV RNA level at week 12, despite reported excellent adherence at the time of the final intensive PK evaluation. Two other subjects also had increases in the plasma HIV RNA level at week 12, but they both acknowledged that they had not been taking their ARV medications. We would expect that missed doses of LPV/r might lead to lower LPV concentrations and rising HIV loads, ultimately increasing the risk for developing viral resistance. DMPA does not appear to influence the concentration of lopinavir or ritonavir when DMPA is taken concurrently with LPV/r, though missing LPV/r doses might result in less elevation of MPA exposure. No clear explanation for the viral load increase in the subject who reported excellent adherence was found.
This study demonstrates that the concurrent use of LPV/r and DMPA is safe and effective for HIV-infected women. Voluntary use of contraception by HIV-positive women who wish to prevent pregnancy continues to be an important strategy for the reduction of mother-to-child HIV transmission. Safe and effective contraceptive options that do not interfere with the efficacy of ARV medications are of critical importance for these women.
ACKNOWLEDGMENTS
We are grateful for the patients' commitment and participation in this study.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Office of the Global AIDS Coordinator, the National Institutes of Health (NIH), or the U.S. Department of State.
The research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health under award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701; grant 1U01AI069511 and CRC grant UL-1RR02460 to the University of Rochester; grant 1U01AI069471 and CRC grant UL 1TR000150 to Northwestern University; grant 1U01AI069513 to the Cincinnati, OH, CRS; grant 1U01AI069481 to the University of Washington; grant UM1-AI069423-08, CTSA grant 1UL1TR001111, and CFAR grant P30 AI50410 to the University of North Carolina Global CTU: Chapel Hill CRS; and UCSL PSL under NIH grant 1U01AI068636. Overall support for IMPAACT was provided by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), and UM1AI106716 (IMPAACT LC) with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH).
The following individuals assisted in conducting ACTG 5283: Becky Straub and Miriam Chicurel-Bayard, University of North Carolina Global CTU: Chapel Hill CRS (site 3201); Rachel K. Scott and Patricia Tanjutco, MedStar Washington Hospital Center (site 5023); Jenny Baer and Jennifer Forrester, Cincinnati, OH, CRS (site 2401); Mariam Aziz and Maureen McNichols, Rush University Medical Center/Ruth M. Rothstein Core Center (site 5083); Mary Adams and Christine Hurley, University of Rochester CRS (site 1101); Sheila Dunaway and Eric Helgeson, University of Washington ACTG CRS (site 1401); Donna McGregor, Northwestern University CRS (site 2701); Steven Zeichner and Connie Trexler, Children's National Medical Center (site 5015); Rodrigo Diaz-Velasco and Elvia Perez-Hernandez, San Juan Hospital (site 5031); Sharon Nachman, Denise Ferraro, and Erin Infanzon, State University of New York, Stony Brook, NICHD CRS (site 5040); and Patricia Riley and Sheila Bradford, Tulane University, New Orleans, LA, NICHD CRS (site 5095).
We declare no conflict of interest.
A. E. Luque and S. E. Cohn developed the original study design and wrote the manuscript, J.-G. Park and Y. Cramer performed the statistical analyses of the study results and assisted in the writing of the manuscript, J.-G. Park, A. Weinberg, E. Livingston, K. L. Klingman, F. Aweeka, and D. H. Watts provided valuable input to the protocol design and provided ongoing guidance on protocol eligibility and assigning causality to adverse events. The LPV and RTV analyses were performed in F. Aweeka's laboratory. All authors contributed to and approved the final version of the paper.
REFERENCES
- 1.Joint United Nations Programme on HIV/AIDS. 2010. Global report: UNAIDS report on the global AIDS epidemic, 2010, p v Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland. [Google Scholar]
- 2.Kashuba AD. 2005. Drug-drug interactions and the pharmacotherapy of HIV infection. Top HIV Med 13:64–69. [PubMed] [Google Scholar]
- 3.Flexner C. 1998. HIV-protease inhibitors. N Engl J Med 338:1281–1292. doi: 10.1056/NEJM199804303381808. [DOI] [PubMed] [Google Scholar]
- 4.Darroch JE, Singh S. 2013. Trends in contraceptive need and use in developing countries in 2003, 2008, and 2012: an analysis of national surveys. Lancet 381:1756–1762. doi: 10.1016/S0140-6736(13)60597-8. [DOI] [PubMed] [Google Scholar]
- 5.Yamazaki H, Shimada T. 1997. Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9, and 3A4 in human liver microsomes. Arch Biochem Biophys 346:161–169. doi: 10.1006/abbi.1997.0302. [DOI] [PubMed] [Google Scholar]
- 6.Harris RZ, Benet LZ, Schwartz JB. 1995. Gender effects in pharmacokinetics and pharmacodynamics. Drugs 50:222–239. doi: 10.2165/00003495-199550020-00003. [DOI] [PubMed] [Google Scholar]
- 7.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. 2009. Recommendations for use of antiretroviral drugs in pregnant HIV-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission, U.S. Department of Health and Human Services, Washington, DC: http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf Accessed 20 July 2014. [Google Scholar]
- 8.Pasley MV, Martinez M, Hermes A, d'Amico R, Nilius A. 2013. Safety and efficacy of lopinavir/ritonavir during pregnancy: a systematic review. AIDS Rev 15:38–48. [PubMed] [Google Scholar]
- 9.Second-Line Study Group, Boyd MA, Kumarasamy N, Moore CL, Nwizu C, Losso MH, Mohapi L, Martin A, Kerr S, Sohn AH, Teppler H, Van de Steen O, Molina JM, Emery S, Cooper DA. 2013. Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (Second-Line): a randomised, open-label, non-inferiority study. Lancet 381:2091–2099. doi: 10.1016/S0140-6736(13)61164-2. [DOI] [PubMed] [Google Scholar]
- 10.Mishell DR., Jr 1996. Pharmacokinetics of depot medroxyprogesterone acetate contraception. J Reprod Med 41(5 Suppl):381–390. [PubMed] [Google Scholar]
- 11.Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW. 2000. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care 12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 12.Gwaza L, Aweeka F, Greenblatt R, Lizak P, Huang L, Guglielmo BJ. 2013. Co-administration of a commonly used Zimbabwean herbal treatment (African potato) does not alter the pharmacokinetics of lopinavir/ritonavir. Int J Infect Dis 17:e857–e861. doi: 10.1016/j.ijid.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yen-Lieberman B, Brambilla D, Jackson B, Bremer J, Coombs R, Cronin M, Herman S, Katzenstein D, Leung S, Lin HJ, Palumbo P, Rasheed S, Todd J, Vahey M, Reichelderfer P. 1996. Evaluation of a quality assurance program for quantitation of human immunodeficiency virus type 1 RNA in plasma by the AIDS Clinical Trials Group virology laboratories. J Clin Microbiol 34:2695–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvelli T, Denny TN, Paxton H, Gelman R, Kagan J. 1993. Guideline for flow cytometric immunophenotyping: a report from the National Institute of Allergy and Infectious Diseases, Division of AIDS. Cytometry 14:702–715. doi: 10.1002/cyto.990140703. [DOI] [PubMed] [Google Scholar]
- 15.Cohn SE, Park JG, Watts DH, Stek A, Hitti J, Clax PA, Yu S, Lertora JJ, ACTG A5093 Protocol Team . 2007. Depo-medroxyprogesterone in women on antiretroviral therapy: effective contraception and lack of clinically significant interactions. Clin Pharmacol Ther 81:222–227. doi: 10.1038/sj.clpt.6100040. [DOI] [PubMed] [Google Scholar]
- 16.Watts DH, Park JG, Cohn SE, Yu S, Hitti J, Stek A, Clax PA, Muderspach L, Lertora JJ. 2008. Safety and tolerability of depot medroxyprogesterone acetate among HIV-infected women on antiretroviral therapy: ACTG A5093. Contraception 77:84–90. doi: 10.1016/j.contraception.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wyen C, Fuhr U, Frank D, Aarnoutse RE, Klaassen T, Lazar A, Seeringer A, Doroshyenko O, Kirchheiner JC, Abdulrazik F, Schmeisser N, Lehmann C, Hein W, Schömig E, Burger DM, Fätkenheuer G, Jetter A. 2008. Effect of an antiretroviral regimen containing ritonavir boosted lopinavir on intestinal and hepatic CYP3A, CYP2D6 and P-glycoprotein in HIV-infected patients. Clin Pharmacol Ther 84:75–82. doi: 10.1038/sj.clpt.6100452. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi K, Mimura N, Fujii H, Minami H, Sasaki Y, Shimada N, Chiba K. 2000. Role of human cytochrome P450 3A4 in metabolism of medroxyprogesterone acetate. Clin Cancer Res 6:3297–3303. [PubMed] [Google Scholar]
- 19.Storch CH, Theile D, Lindenmaier H, Haefeli WE, Weiss J. 2007. Comparison of the inhibitory activity of anti-HIV drugs on P-glycoprotein. Biochem Pharmacol 73:1573–1581. doi: 10.1016/j.bcp.2007.01.027. [DOI] [PubMed] [Google Scholar]